direction of Dr. Nanette Nascone-Yoder).
The liver is one of the many internal organs of the body that is left-right asymmetric in both anatomical location and morphology. In humans, the liver is located on the right side of the body cavity. Additionally, the right side of the liver is larger and consists of more lobes than the left. As many as 1 in 10,000 humans are born with defects in left-right
asymmetry that often involve severe anomalies in liver laterality including aberrant lobation, abnormal organ position, and hepatic duct and biliary tree malformations, yet the
mechanisms underlying the development of left-right asymmetries in the liver are unknown. In other asymmetric organs like the heart, lungs, stomach, and gastrointestinal tract, left-sided expression of Pitx2c, a homeobox transcription factor, is required for left-right
by
Mandy Ashton Womble
A dissertation submitted to the Graduate Faculty of North Carolina State University
in partial fulfillment of the requirements for the Degree of
Doctor of Philosophy
Comparative and Biomedical Sciences
Raleigh, North Carolina 2017
APPROVED BY:
_______________________________ _______________________________ Nanette Nascone-Yoder Troy Ghashghaei
Committee Chair
_______________________________ _______________________________
DEDICATION
To my grandmother, for being my guardian angel. To my mom and grandfather, for teaching
me that the sky is the limit. To my brothers and fathers, for making me stubborn,
hard-headed, and determined. To my friends, girlfriend, and life coach, for keeping me sane. To
BIOGRAPHY
Mandy Ashton Womble was born March 6, 1987 to Jeffrey Womble and Lori Chappell. She was raised by her mother, father, stepfather, Gene Chappell, and stepmother, Julie Womble. Mandy grew up in Clemmons, NC alongside her brothers, Matt Womble and Will Chappell. Her dream to become a veterinarian began at an early age, as she was always a lover of animals, biology, and science. Mandy attended West Forsyth High School where she was a scholar and athlete, participating in basketball, volleyball, and softball programs. During her senior year of high school, she was offered an opportunity to participate in an externship program at Clemmons Veterinary Clinic, where her desire to become a vet was solidified. Mandy moved to Raleigh, NC in 2005 to attend North Carolina State University to pursue a Bachelor of Science degree in Zoology. As an undergraduate, she was accepted into the University Honors Program, College of Agricultural and Life Sciences Honors Program, and the honors fraternity, Phi Sigma Pi. During her time at NCSU, Mandy continued to gain experience with animals and veterinary medicine by working at multiple animal hospitals in the area, volunteering at the Carolina Tiger Rescue and NC Zoo, and traveling to South Africa to work at the Center for Animal Rehabilitation and Education where she cared for orphaned, baby baboons. In her junior and senior years, she completed an undergraduate research project in the lab of Dr. Nanette Nascone-Yoder, receiving first place at the NCSU undergraduate research symposium and was published as second author in her first scientific journal. During her time in the lab, she gained an appreciation and love for the scientific process, cell biology, and developmental embryology. After graduating Summa Cum Laude from NCSU in the fall of 2008, Mandy gained employment at Duke University as a research assistant for one year in the laboratory of Brigid Hogan in the Cell Biology department. The next year, she worked in the laboratory of Dr. Garnett Kelsoe in Duke’s Immunology department where she was promoted to research analyst in the first four months and
published two more scientific papers. In 2011, Mandy was accepted into NCSU’s College of Veterinary Medicine. During the summer prior to vet school, she joined the vet school club, Turtle Rescue Team, a non-profit organization that rescues and treats injured wild
of officer roles including rehabilitation coordinator, captain, and is currently vice president. Participation in this club (and her trip to South Africa) sparked Mandy’s interest in wildlife medicine and launched a desire to work in a museum, aquarium, or wildlife center after graduation. Towards this goal, Mandy decided to apply to the dual DVM/PhD program in order to combine her love for veterinary medicine and research to gain an edge when
ACKNOWLEDGMENTS
“Each person holds so much power within themselves that needs to be let out. Sometimes
they just need a little nudge, a little direction, a little support, and a little coaching, and the
greatest things can happen.”
- Pete Carroll
First, I would like to thank my advisor, Dr. Nanette Nascone-Yoder. I entered your lab bright-eyed and bushy-tailed at the age of 19. Somehow you turned that crazy teenager into a scientist. You have always supported my many interests and have guided my research and future career. I have always described you as my “mom” away from home and I truly think
of you that way. You have been my shoulder to cry on, my therapist, and my constant support. Most importantly, you have taught me how to be a strong woman and a humble but confident scientist. Thank you for being an inspiring role model.
I also want to thank other members of the NCSU faculty, especially my committee members, Troy Ghashghaei, Jody Gookin, Phillip Sannes, and Ke Cheng for your advice, support, ideas, and mentorship. Dr. Lewbart, my vet school advisor, has been a constant springing board for ideas about my career and has always been available for solid advice. When people ask me what I want to be when I grow up, I typically say “Dr. Lewbart”. Thank you for being an inspiring role model and friend. I also would like to think my TA
supervisor, Colleen Grant, for inspiring my interest in teaching. Thank you to my undergraduate mentor, Dr. Roger Powell for guiding my graduate school decisions.
and Dr. Garnett Kelsoe, for instilling in me a love for research. Veterinarians, Dr. Allison Fox, Dr. Pete Gilyard, Dr. Harold Pierce, and Dr. Amy Neligon allowed me to gain experience in small animal practice.
I would like to thank my lab mates, friends, and family who have helped me remain sane and supported me over all of these years. Thank you to Jordan Ferguson, Melissa Pickett, Martha Alonzo-Johnsen, Nirav Amin, Mike Dush, and Adam Davis for supporting my research and providing valuable advice towards my project. To my friends, Jennifer Rodriguez, Denise Monkovich-Pell, Denise Hark, Adam Ward, Ryan Walters, and Chelsey Vanetten for your love and support. I would also like to express my gratitude and love to my girlfriend, Casey Snyder, for her loyalty and encouragement in the last year of my Ph.D.
Finally, I would like to thank my “Mama”, Lori Chappell. From an early age, you have taught me to have the “eye of the tiger”. You have supported and encouraged me
TABLE OF CONTENTS
LIST OF TABLES ... ix
LIST OF FIGURES ... x
CHAPTER 1: Introduction ... 1
1) The Establishment of Left-right Asymmetry in Development... 4
1.1) The initial left-right symmetry breaking event at the “node” ... 4
1.2) The transfer of left-right cues from the “node” to the lateral plate mesoderm (LPM) ... 7
1.3) The establishment of the midline barrier ... 9
1.4) The induction of left-right asymmetric organogenesis ... 10
1.5) The evolutionary conservation of LR asymmetry ... 11
1.6) The discovery and characterization of Pitx2 ... 12
2) Left-right Asymmetric Organogenesis ... 13
2.1) Heart looping ... 14
2.2) Asymmetric regression of blood vessels closely associated with the heart ... 20
2.3) Gut looping ... 21
2.4) Vascular and Lymphatic asymmetries in the dorsal mesentery... 29
2.5) Stomach Curvature ... 31
2.6) Asymmetric regression of the spleen ... 33
2.7) Lung Lobation ... 33
2.8) Liver lobation ... 34
2.9) Conclusions ... 37
3. Rationale and Hypothesis ... 37
References ... 45
CHAPTER 2: The left-right asymmetry of liver lobation is generated by Pitx2c-mediated asymmetries in the hepatic diverticulum ... 50
Introduction ... 51
Materials and Methods ... 52
Animals ... 52
Immunohistochemistry ... 53
RNA in situ hybridization ... 53
X. laevis Loss- and Gain-of-function experiments ... 54
Measurements and Statistics ... 54
Results ... 55
The liver diverticulum is left-right asymmetric ... 55
Cellular left-right asymmetries in the early liver diverticulum ... 56
Liver asymmetry is under the control of the left-right asymmetry pathway ... 57
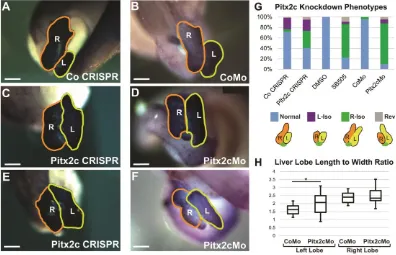
Pitx2c is required for left lobe morphogenesis ... 57
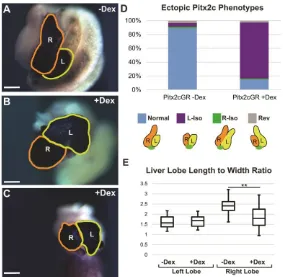
Ectopic R-sided Pitx2c induces heterotaxy ... 58
Pitx2c is sufficient to induce left-sided cell character ... 59
Discussion ... 60
Pitx2c is necessary and sufficient to induce left-sided epithelial characteristics ... 60
Ramifications for human health ... 63
References ... 81
CHAPTER 3: Discussion ... 86
1) Pitx2 induces left-right asymmetries in cell shape ... 86
1.1) Pitx2’s influence on Apical-Basal Cell Elongation and Apical Constriction ... 87
1.2) The role of cell adhesion in cell shape change ... 89
2) Pitx2c directs left-right asymmetrical epithelial remodeling ... 91
2.1) Cellular rearrangement ... 92
2.2) Interkinetic nuclear migration ... 94
3) Pitx2c expression in the mesoderm influences endodermal tissue architecture ... 97
3.1) Endodermal and Mesodermal interactions mediated by signaling ligands/receptors ... 98
3.2) Mesodermal and Endodermal interactions mediated by ECM components ... 99
3.3) Differential expression of Pitx2 in organ tissues layers ... 101
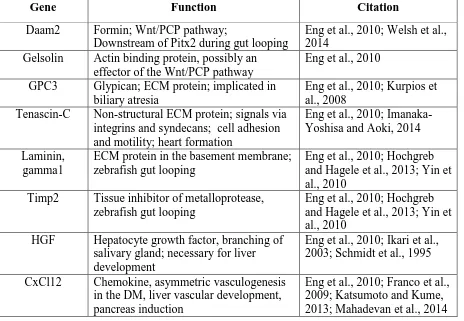
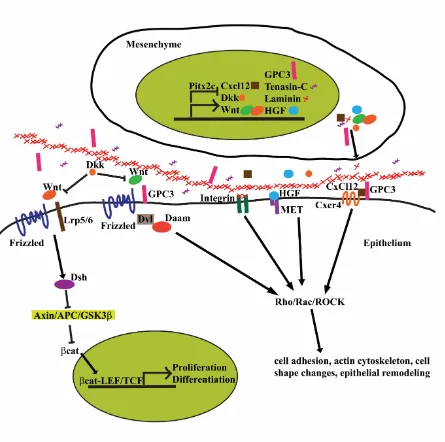
4) Downstream targets of Pitx2c during left-right liver development ... 103
4.1) Components of the Wnt signaling pathway ... 103
4.2) ECM components ... 106
4.3) Soluble Growth Factors ... 109
4.4) Chemokines ... 110
4.5) Conclusions ... 111
5) Ramifications for human health ... 112
References ... 115
CHAPTER 4: Conclusions and Future Directions ... 121
References ... 124
APPENDIX ... 127
Appendix A: Budgett’s frog (Lepidobatrachus laevis): a new amphibian embryo for developmental biology ... 128
Appendix B: Frogs as integrative models for understanding digestive organ development and evolution ... 170
Appendix C: Developmental Constraints on Endoderm Morphogenesis Underlie the Evolution of Gut Length ... 225
LIST OF TABLES
LIST OF FIGURES
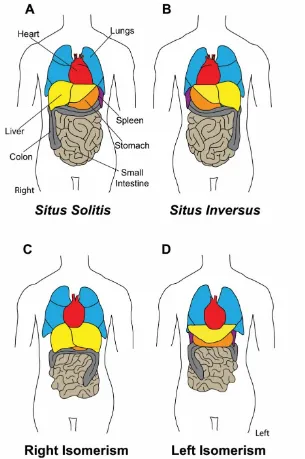
Figure 1- 1) LR asymmetric organ placement and morphology is vital for human health
... 41
Figure 1- 2) Four steps of initial LR symmetry breaking events during embryogenesis 42 Figure 1- 3) LR asymmetric organogenesis in the midgut and stomach ... 44
Figure 2- 1) Early development of LR asymmetry in the Xenopus liver ... 64
Figure 2- 2) Cellular level LR asymmetries in the early liver diverticulum ... 66
Figure 2- 3) The Nodal pathway is required for liver asymmetry ... 68
Figure 2- 4) Left-sided Pitx2c is required for LR liver asymmetry ... 69
Figure 2- 5) Knockdown of Pitx2c increases left-sided apical constriction and cell elongation ... 70
Figure 2- 6) Ectopic right-sided Pitx2c expression induces liver heterotaxia in the form of left isomerism ... 72
Figure 2- 7) Ectopic right-sided Pitx2c expression reduces apical constriction and cell elongation ... 73
Figure 2- 8) Model of the role of Pix2c in asymmetrical liver development ... 75
Supplementary Figure 2- 1) The left and right cells of the liver diverticulum originate from the left and right sides of the early embryo ... 76
Supplementary Figure 2- 2) Cell number and proliferation are not different between the left and right sides of the developing liver ... 77
Supplementary Figure 2- 3) Pitx2 is asymmetrically expressed in the left mesoderm surrounding the liver diverticulum ... 78
Supplementary Figure 2- 4) Pitx2c microinjection manipulation strategies ... 79
Supplementary Figure 2- 5) Ectopic right-sided Pitx2c expression decreases diverticulum length ... 80
Figure 3- 1) Possible downstream targets of Pitx2c ... 114
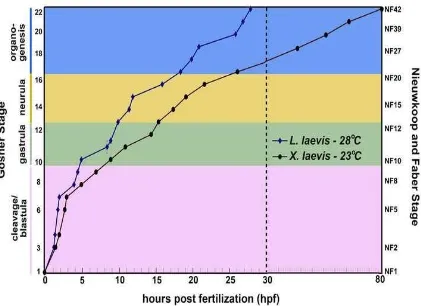
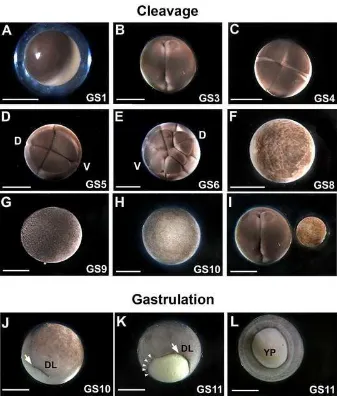
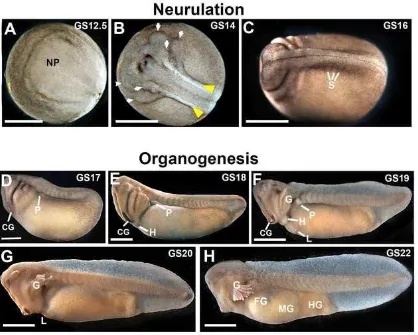
Figure A- 1) The schedule of Lepidobatrachus laevis embryonic development (versus Xenopus laevis) ... 152
Figure A- 2) Early cleavage and gastrulation patterns in L. laevis are similar to Xenopus ... 153
Figure A- 3) Neurulation and organogenesis in L. laevis ... 154
Figure A- 4) Craniofacial morphogenesis in L. laevis ... 155
Figure A- 5) Thermal tolerance of L. laevis and X. laevis embryos ... 157
Figure A- 6) L. laevis embryos are amenable to microinjection of exogenous reagents for fate-mapping and expression of synthetic mRNA ... 158
Figure A- 7) L. laevis explants are amenable to animal cap explant culture ... 159
Figure A- 8) Heart morphogenesis occurs at a larger scale in L. laevis ... 160
Figure B- 1) Wnt, RA, and FGF pattern the foregut ... 204
Figure B- 2) Intestine lengthening involves Hedghog- and Wnt/PCP-mediated endoderm cell polarization, rearrangement and epithelial differentiation ... 205
Figure B- 3) Altered RA signaling may have led to a novel foregut morphology ... 207
Figure B- 4) Endoderm morphogenesis in ancestral versus direct-developing frog species ... 208
Figure C- 1) Variation in tadpole feeding ecology correlates with variation in egg size (yolk reserves) and gut length ... 227
Figure C- 2) All endodermal cells contribute to the gut epithelium in exotrophic and terrestrial, delayed herbivorous tadpoles, regardless of egg size ... 229
Figure C- 3) The innermost endoderm cells do not contribute to the gut epithelium in rapidly-developing, exotrophic carnivorous tadpoles, regardless of egg size ... 231
Figure C- 4) Endoderm rearrangement increases epithelial surface area, but not length, in endotrophic (direct developing) tadpoles ... 233
Figure C- 5) Sonic Hedgehog signaling increases intestinal surface area and reduces apoptosis in cannibalistic tadpoles ... 235
Figure C- 6) Changes in endoderm morphogenesis may underlie anuran gut evolution ... 237
Figure D- 1) Gene Expression patterns of transverse cross sections at NF 35.36 ... 240
Figure D- 2) Gene expression patterns of frontal sections at NF 37.38. ... 243
Figure D- 3) Gene expression patterns of transverse cross sections at NF 37.38 ... 246
Figure D- 4) Gene expression patterns of frontal sections at NF 39 ... 249
Figure D- 5) Gene expression patterns of transverse cross sections at NF 39.40 ... 252
Figure D- 6) Gene expression patterns of frontal sections at NF 40.41 ... 254
Figure D- 7) Gene expression patterns of transverse cross sections at NF 40.41 ... 257
CHAPTER 1: Introduction
As many as 1 in 10,000 human infants are born with defects in left-right asymmetry termed heterotaxia (Lin et al., 2000). The normal positioning of the organs within the body cavity is called situs solitus (Fig. 1.1A). The complete reversal of all organ placement and morphology is called situs inversus (Fig. 1.1B). Situs inversus occurs in 1 in 8,500 human births (Basu and Brueckner, 2008; Brueckner, 2007). These individuals are relatively normal and can live their entire lives without knowledge of their condition because the complete body axis is reversed and therefore functions correctly. Problems occur in cases of heterotaxy when one or more organs have defects in LR asymmetry that affect the function of the organ or the connection of that organ with other organs, vasculature, lymphatics, or ducts. Infants with heterotaxy syndrome have a fatal prognosis in 90-99% of cases (Lee et al., 2014).
present with intermittent biliary cirrhosis and require additional treatment and surgery throughout their lives (Kerkeni et al., 2015). The association of liver heterotaxia with other organ laterality defects suggests a common development program.
Research over the past several decades has given us insight into the etiology of heterotaxia including knowledge about initial left-right axis determination, cellular level mechanisms of LR organ morphogenesis, and genes required for this process like the
transforming growth factor beta (TGF-growth factor, Nodal and its downstream target, the
Paired like homeodomain 2 (Pitx2) that is expressed on only the left side of many organs as they develop. Researchers are just beginning to understand the LR differences in tissue morphogenesis including cell shape changes, cell migration, and tissue remodeling that are required for organ laterality in the stomach, heart, and midgut; however, the cellular level differences required for the development of LR asymmetries in liver lobation are unknown.
Left-sided expression of Pitx2 is required for stomach curvature, intestinal rotation, heart looping, lung laterality, and asymmetric vasculature development (Davis et al., 2008; Davis et al., 2017; Kitamura et al., 1999; Mahadevan et al., 2014). Pitx2 is expressed on the left side of the septum transversum mesenchyme (STM), a mesodermal tissue that surrounds the liver during development; however, it is unknown whether Pitx2 is required for
1) The Establishment of Left-right Asymmetry in Development
The establishment of LR asymmetry consists of 4 major steps: (1) The initial symmetry breaking event in or near the embryonic “node” (Fig. 1.2A), (2) The transfer of these LR cues to the lateral plate mesoderm (LPM) by the asymmetric expression of the signaling molecule Nodal (Fig. 1.2B), (3) The establishment of a midline barrier (Fig. 1.2C), and (4) the induction of LR asymmetric organogenesis (Fig. 1.2D) (Ibanes and Izpisua Belmonte, 2009; Raya and Izpisua Belmonte, 2006; Shiratori et al., 2006; Yamamoto et al., 2003).
1.1) The initial left-right symmetry breaking event at the “node”
In all vertebrate species studied including zebrafish, Xenopus, chick, rabbit, and mouse, the presence of a LR organizer or node is required for LR axis determination:
Kupffer’s vesicle in zebrafish, Spemann’s Organizer in Xenopus, Henson’s node in chick, the
posterior notochord in rabbits, and the node in mice (Basu and Brueckner, 2008; Blum et al., 2014; Ibanes and Izpisua Belmonte, 2009; Raya and Izpisua Belmonte, 2006; Shiratori et al., 2006). Although the timing and geometry of the LR organizer varies among species, all involve a shift from symmetrical expression of the TGF- growth factor, Nodal, to left-sided
determinants that initiate left-limited Nodal expression (Basu and Brueckner, 2008, Blum et al., 2014).
The first evidence that cilia were required for the establishment of the LR axis came with the discovery of a correlation between human disorders involving ciliogenesis or ciliary function and LR defects. As many as 50% of patients with Kartagener syndrome or primary ciliary dyskinesia, a disorder caused by immotile cilia, have defects in LR asymmetrical organ development (Basu and Brueckner, 2008). Additionally, 13 independent mouse mutations that affect ciliary biogenesis and function also cause LR defects (Basu and
Brueckner, 2008). For example, mice with defects in microtubule associated motor proteins in which no cilia are present [kinesin-like protein (Kif3a/b) mutants], or cilia are immotile [left-right dynein (Lrd) mutants], have abnormalities in LR asymmetric organogenesis (Basu and Brueckner, 2008; Shiratori et al., 2006; Wagner and Yost, 2000).
position of the cilium is established by the localization of the basal body within the cell (Shiratori et. al., 2006).
All of the vertebrate LR organizers studied have motile primary cilia in the node except for the chick (Basu and Brueckner, 2008). It is possible that avian species have a different mechanism than “nodal flow” to establish asymmetric Nodal expression but it is
more likely that researchers have not observed nodal cilia in the chick yet due to the imaging constraints of the species (Basu and Brueckner, 2008). In other vertebrates, the direction of cilia beating is conserved although the beating frequency varies, most likely due to the constraints of each embryonic strategy. In rabbits, the cells of the node itself do not have motile cilia but cells at the posterior segment of the notochord do have cilia and are
surrounded by Nodal expression (Basu and Brueckner, 2008). Fluid flow has been observed
in Xenopus, rabbit, zebrafish, and mouse (Basu and Brueckner, 2008). In Xenopus, increasing
the viscosity of the fluid induces LR defects, possibly by preventing normal fluid flow. Morpholino knockdown of the genes involved in cilia motility and assembly in zebrafish also causes LR defects (Basu and Brueckner, 2008). In general, nodal flow generates a LR
difference within the node that is subsequently amplified as this signal is transferred to the left LPM (Shiratori et al., 2006).
maternally derived H+/K+ ATPase subunit transcripts (Ibanes and Izpisua Belmonte, 2009;
Raya and Izpisua Belmonte, 2006). In chicks, the first evidence is LR differences in ion fluxes or membrane voltage potential as the left side is more depolarized than the right. This is due to differences in the activity of the H+/K+ ATPase pump as pharmacological inhibition results in organ heterotaxia (Raya and Izpisua Belmonte, 2006). This mechanism is also found in zebrafish, urochordates, and echinoderms (Duboc et al., 2005; Raya and Izpisua Belmonte, 2006). Therefore, no model of initial symmetry breaking is the same for all animals.
1.2) The transfer of left-right cues from the “node” to the lateral plate mesoderm (LPM)
In all vertebrates, the initial asymmetric left-sided Nodal expression surrounding the embryonic LR organizer or node is required for subsequent left-sided Nodal expression in the LPM, a tissue located in the lateral region of the embryo at somite stages that eventually contributes to the mesenchyme of visceral organs (Raya and Izpisua Belmonte, 2006; Saijoh et al., 2003; Shiratori et al., 2006; Yamamoto et al., 2003). Ablation of Nodal in the region surrounding the LR organizer eliminates Nodal expression in the LPM (Shiratori et al., 2006). However, how the initial Nodal signal is transferred from the node to the LPM is still unknown.
paraxial mesoderm between the node and the LPM. Caronte induces Nodal within the left LPM (Shiratori et al., 2006). It is unknown whether a similar mechanism exists in other vertebrates because a Caronte-like BMP antagonist has not been found (Shiratori et al., 2006). Interestingly, however, in Xenopus, the chemical inhibition of Smad1 and Smad5 (BMP effectors) causes Nodal to be expressed bilaterally in the LPM, hinting that a similar mechanism is yet to be discovered.
There is evidence in mice that growth differentiation factor 1 (GDF-1), a
TGF-related protein, is required for the transfer of the Nodal signal from the node to the LPM (Raya and Izpisua Belmonte, 2006; Shiratori et al., 2006). GDF-1 is expressed in the perinodal region, in a similar expression pattern to Nodal. GDF1 mutant mice do not have Nodal expression in the LPM and the organs of these animals are heterotaxic. GDF-1 is also expressed in the LPM prior to Nodal expression, suggesting that GDF-1 may make the LPM competent for Nodal signaling (Shiratori et al., 2006). Although these interesting correlations have been found between GDF-1 and Nodal expression in the LPM, additional signaling molecules must be involved as GDF-1 alone cannot activate Nodal signaling when overexpressed in cultured cells or frog embryos (Shiratori et al., 2006).
are species specific. What remains conserved between all vertebrates studied is that left-sided asymmetric expression of Nodal within the LPM is required for the establishment of the midline barrier and subsequent LR asymmetrical organogenesis.
1.3) The establishment of the midline barrier
1.4) The induction of left-right asymmetric organogenesis
Nodal expression in the left LPM is required for LR visceral organ development. Nodal mutants are heterotaxic; stomach curvature is randomized, spleens are reduced in size, the lung is double right-sided, heart looping is randomized with transposition of the great arteries, atrial-septal defects, and common atrial chambers, there are abnormalities in liver lobation including clefts in the medial lobes, and gastrointestinal tract coiling is disorganized (Saijoh et al., 2003). Although expression of Nodal within the LPM is a transient event, only lasting from the 2-6 somite stages in mice, a signaling cascade is initiated that sets up the entire LR internal body plan (Basu and Brueckner, 2008; Ibanes and Izpisua Belmonte, 2009; Shiratori et al., 2006). Nodal induces the expression of the homeobox transcription factor, Pitx2, in the left LPM (Basu and Brueckner, 2008; Ibanes and Izpisua Belmonte et al., 2009; Raya and Izpisua Belmonte, 2006; Shiratori et al., 2006). Pitx2 expression is maintained, even after Nodal expression disappears, by the homeobox transcription factor, NK2 homeobox 5 (Nkx2.5) (Basu and Brueckner, 2008). Pitx2 is the only known downstream target of Nodal signaling still expressed during organogenesis and is also required for LR organ development as Pitx2 deficient mice phenocopy Nodal mutants in visceral organ heterotaxia (Gage et al., 1999; Kitamura et al., 1999; Lin et al., 1999; Liu et al., 2001; Lu et al., 1999; Raya and Izpisua Belmonte, 2006; Shiratori et al., 2006).
The downstream cellular mechanism by which Pitx2 directs organ laterality is just starting to be uncovered. Research over the past few decades has shown that left-sided Pitx2 expression is required for cell shape changes, epithelial remodeling, and/or cell
al., 2017). However, the mechanism by which Pitx2 confers morphological laterality in other organs like the lung, heart, spleen, and liver is still unknown.
1.5) The evolutionary conservation of LR asymmetry
The role of Nodal signaling and the left-sided expression of Pitx2 is well established in vertebrates. Interestingly, the LR asymmetry pathway is a conserved feature of all
chordates as amphioxi (cephalochordates) and ascidians (urochordates) also show left-sided Nodal and Pitx2 expression (Duboc et al., 2005). The Nodal signaling cascade can even be found in lophotrochozoans as mollusks and snails require the asymmetric expression of a Nodal homolog for shell coiling (Grande and Patel, 2009). The Nodal signal was lost in ecdysozoans as no Nodal-like protein has been found (Blum et al., 2014). Drosophila instead utilizes actin filament based systems to orchestrate gut rotation (Okumura et al., 2008). Homologs of Nodal, Pitx2, and Lefty are found asymmetrically expressed in echinoderms, however, this expression is on the right side, not the left (Blum et al., 2014; Duboc et al., 2005). Adult echinoderms are radially symmetrical but evolved from a bilateral ancestor and have bilateral larvae. These larvae are LR asymmetric as the imaginal disc or rudiment is on the left side of the embryo (Duboc et al., 2005). Interestingly, Sry-related HMG box E (Sox E), a protein related to Sry-related HMG box 9 (Sox 9), is expressed in the left coelomic pouch (Duboc et al., 2005). Inhibition of Nodal signaling eliminates right sided Nodal, Pitx2, and Lefty expression while Sox E expression becomes bilateral (Duboc et al., 2005).
The conservation of Nodal signaling across multiple animal Phyla suggests that Nodal evolved as a LR determination gene before the split between Protostomes and Deuterostomes and that the role of Pitx2 as a downstream effector of Nodal evolved before the separation of echinoderms and chordates. It is very interesting that the expression of the Nodal pathway is right sided in echinoderms and left sided in chordates (Duboc et al., 2005). This could raise interesting questions as to whether our definition of left versus right in echinoderms is backwards or whether they have a complete inversion of the DV axes (Duboc et al., 2005).
1.6) The discovery and characterization of Pitx2
Pitx2 was first discovered for its role in Axenfeld-Riegar syndrome (ARS). ARS is an autosomal dominant, haploinsufficiency disorder characterized by a variety of birth
defects in eye, tooth, pituitary, umbilicus, facial structure, intestinal tract, and abdominal wall development (Hjait and Semina, 2005). Thirty different mutations in the Pitx2 gene located at chromosomal locus 4q25 are associated with ARS; mostly point mutations in the
the protein with a nonfunctional homeodomain (Hjait and Semina, 2005). Pitx2a, b, and c are expressed widely throughout the body, particularly in the craniofacial region, eye, oral ectoderm, pituitary, body wall, central nervous system, and internal organs while Pitx2d is only found in the craniofacial region (Hjait and Semina, 2005, Liu et al., 2003). Pitx2c is the only isoform that has an asymmetrical expression, found on the left side of visceral organs (Schweickert et al., 2000). In cell culture, each isoform has different gene promoter targets so they most likely have diverged functions (Cox et al., 2002; Hjait and Semina, 2005). Pitx2c but not Pitx2a or Pitx2c is required for asymmetric organ morphogenesis as misexpression induces heterotaxia of the lungs, heart, spleen, and gut (Liu et al., 2001; Kitamura et al., 1999; Schweickert et al., 2000; Shiratori et al., 2006). In human heterotaxy patients, LR congenital liver and biliary defects are often linked with defects of the other visceral organs, therefore, it is likely that Pitx2 is also required for LR liver development, although this has never been described.
2) Left-right Asymmetric Organogenesis
the heart, gut, and stomach, 2) LR differences in organ size and branching as in the lungs and liver, and 3) unilateral regression of organs like the spleen and structures like blood vessels (Shiratori et al., 2006). Cellular level mechanisms of LR asymmetrical development have recently been discovered during heart looping, vasculature development, stomach curvature, and midgut rotation. Left-sided expression of Pitx2 is required for LR development in the heart, vasculature, lungs, spleen, intestine, and stomach (Liu et al., 2001; Kitamura et al., 1999; Schweickert et al., 2000; Shiratori et al., 2006). Downstream cellular level mechanisms of Pitx2 have been described during vasculature, stomach, and midgut development but have yet to be discovered in other organ systems including the lungs and heart (Davis et al., 2008; Davis et al., 2017; Mahadevan et al., 2014; Muller et al., 2003). Knowledge pertaining to the mechanisms of LR development and their link to Pitx2 will be valuable when determining how liver laterality is established. This section of the introductory chapter reviews
mechanisms of LR development during organogenesis in a variety of different model organisms.
2.1) Heart looping
The heart is the first organ to develop with LR asymmetric morphology. There are 5 phases of cardiac development that are crucial for the proper form and function of the heart. The first phase (“pre-looping”) is when cells are bilaterally specified to form the heart fields.
2009; Manner, 2009). The second phase is “dextral looping” and occurs as the straight tube
forms a C-shaped loop with the convex portion towards the right side of the embryo (Manner, 2009). The third phase is the “early phase of S-looping” when the arterial and venous poles shorten, causing the C-shape to transform into an S-shape and the ventricular bend to move from a position cranial to the atria to a more caudal position (Manner, 2009). During phase four, the “late S-phase”, the proximal outflow tracts move leftward (Manner,
2009). At this time, the arterial trunk anlage develops at the distal portion of the outflow tract and the atrial and ventricular portions of the heart balloon outward (Manner, 2009). During phase five, “cardiac septation”, septal structures first appear, dividing the heart lumens and arterial trunks into left and right compartments (Manner, 2009). The proper establishment of LR asymmetry is vital for all phases of cardiac morphogenesis.
The earliest evidence of LR asymmetry during heart morphogenesis is in gene expression patterns in the primary heart field prior to fusion at the midline (Ramsdell, 2005). Extracellular matrix (ECM) proteins exhibit LR asymmetries in expression levels; Fibrillin-2 is higher on the right side while heart specific lectin-associated matrix protein-1 (hLamp-1) and Flectin are expressed higher on the left (Ramsdell, 2005). In fact, the asymmetric expression of Flectin is maintained even after initial heart tube fusion (Ramsdell, 2005). In addition to gene expression asymmetries, cardiomyocyte differentiation and myofibril formation begin slightly sooner in the right early heart field (Ramsdell, 2005).
anterior portion of the heart is larger on the right side while the posterior portion is larger on the left as cells derived from the right and left heart fields contribute to the anterior and posterior regions differently (Ramsdell, 2005). A larger proportion of cells originating from the right heart field contribute to the posterior or inflow region (Ramsdell, 2005). Fate-mapping experiments in Xenopus laevis reveal that, later in development, the left side contributes to the inner curvature and the ventral face of the loop while the right side contributes to the outer curvature and dorsal portion of the loop (Gormley and Nascone-Yoder, 2003). The localization of the left-sided cells correlates to the expression pattern of Pitx2, indicating that the Nodal-Pitx2 pathway may be involved in patterning of these regions (Gormley and Nascone-Yoder, 2003).
Pitx2 is expressed in the entire left side of the heart throughout early heart
In zebrafish, the adult heart is relatively simple, consisting of sinus venous, atrium, ventricle, and bulbous arteriosus segments (Hu et al., 2000). During development, there are two asymmetric events: a leftward displacement of the developing heart termed “heart jogging”, and the formation of the c-shaped curvature, “dextral looping” (Manner, 2009). In
zebrafish, like other vertebrates, the heart is derived from bilaterally symmetrical mesoderm populations that undergo organized, collective movements towards the ventral embryonic midline where they fuse together to form the cardiac cone (Bakkers et al., 2009; Smith et al., 2008). The ECM protein, Fibronectin, is crucial for this midline migration as natter mutants that lack Fibronectin develop cardia bifidia due to a failure of coordinated cell movements towards the midline (Bakkers et al., 2009). Fibronectin is important in providing the correct path of migration (Bakkers et al., 2009). After the fusion of these cells at the midline, jogging occurs due to displacement of the heart cone to the left side of the embryo (Bakkers et al., 2009). Both the mesocardium and endocardium, formed from different precursor
populations, are displaced left at the same time although the endocardium population is dispensable in this asymmetric displacement as cloche mutants that lack endothelial cells still display cardiac jogging (Bakkers et al., 2009).
Following the initial left-ward displacement of the heart, dynamic cell rearrangements in the cardiac cone contribute to the dextral looping or c-shaped curvature that follows
migration behaviors of specific regions of the heart tube itself contribute to the directionality of rotation (Bakkers et al., 2009; Smith et al., 2008). By tracking individual cells during this migration process, Smith et al. (2008) found that all myocardial cells were linearly displaced to the left and anterior but that the speed and consistency of movement depended on whether the cells were anteriorly or posteriorly derived. Posterior cells did not move at a constant speed but rather started at the same speed as anterior cells before doubling their speed, eventually having a higher displacement rate (Bakkers et al., 2009; Smith et al., 2008). The anterior cells moved at a constant speed the entire time (Bakkers et al., 2009; Smith et al., 2008). The inconsistencies of cell movement causes the cardiac cone to rotate in a clockwise direction when observed from a dorsal view (Bakkers et al., 2009; Smith et al., 2008).
Bmp4 is expressed asymmetrically in the left LPM during cardiac looping events (Bakkers et al., 2009; Smith et al., 2008). This asymmetric expression is required for dextral looping as bmp4 mutants have reduced heart rotation and no cardiac jogging (Bakkers et al., 2009; Smith et al., 2008). During the cell migration that initiates dextral looping events, bmp4 mutants have irregular cellular movements with a high meandering index (Bakkers et al., 2009; Smith et al., 2008). Interestingly, the addition of Bmp soaked beads directs the path of cell migration towards the Bmp signal (Bakkers et al., 2009; Smith et al., 2008).
Therefore, Bmp instructs migration by acting as a directional cue while Has2 acts to create the rotational events by affecting differential speeds of migrating cells (Bakkers et al., 2009; Smith et al., 2008).
The downstream mechanism by which BMP and Has2 act to coordinate both cardiac jogging and dextral looping is still unknown but may require the actions of the actin
2.2) Asymmetric regression of blood vessels closely associated with the heart
Many of the blood vessels that connect to the heart are asymmetric in both location and function. For example, the superior and inferior vena cava return deoxygenated blood from the body to the left atrium. The pulmonary artery and veins bring blood to and from the lungs for the oxygenation process. The aorta takes freshly oxygenated blood from the right ventricle to the rest of the body. Additionally, many vessels and capillary beds must provide blood and oxygen to the heart tissue itself. The proper separation and formation of each of these vessels is vital for the proper functioning of the heart.
2.3) Gut looping
Like the heart, the midgut also loops in a stereotypical direction required for proper gastrointestinal tract function. In chick and mice, the primitive gut tube first arises during body folding events as the endoderm forms an elongated cylinder that becomes segregated into the foregut, midgut, and hindgut (Davis et al., 2008). As the midgut elongates, a hairpin loop develops in the small intestine (Davis et al., 2008). This loop eventually extends outside of the body cavity and undergoes a 90 degree counterclockwise rotation before retracting back inside the body and rotating another 180 degrees counterclockwise (Burn and Hill, 2009; Davis et al., 2008). These rotation events are required in order to fit the entire length of the intestine inside the body cavity. The LR signaling pathway is required for proper midgut looping as Nodal and Pitx2 mutants display disorganized gut coiling (Saijoh et al., 2003). Research over the past several decades using chick, mouse, zebrafish, and Xenopus has given us a glimpse into the mechanisms required for midgut coiling.
both compartments of the DM are downstream of Pitx2 and required for midgut looping (Burn and Hill, 2009; Davis et al., 2008; Kurpios et al., 2008; Welsh et al., 2014).
LR differences in cellular architecture downstream of the Pitx2 pathway causes the DM to adopt a trapezoidal shape, tilting the gut tube to the left (Davis et al., 2008; Kurpios et al., 2008; Welsh et al., 2014). In the epithelial compartment, differences in cell shape
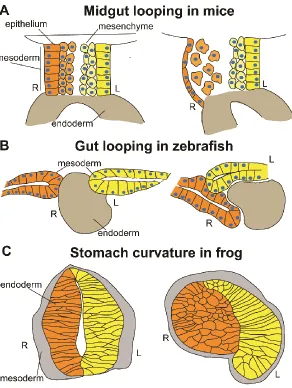
contribute to this leftward tilt as cells on the left side are more columnar in morphology while cells on the right are flattened and cuboidal (Fig. 1.3A) (Davis et al., 2008; Kurpios et al., 2008). Additionally, cells on the left side are more polarized than cells on the right, as indicated by higher levels of actin staining on the apical border and the presence of a basement membrane; right-sided actin is more diffuse and no basement membrane is apparent (Davis et al., 2008; Kurpios et al., 2008). Pitx2, Isl1, and Ncad expression are required for these cellular asymmetries as ectopic right-sided expression of any of these genes causes the right sided cells to become more columnar in shape, preventing the normal tilting of the DM (Davis et al., 2008; Kurpios et al., 2008; Plageman Jr. et al., 2011). In Pitx2 mutant mice, in which Isl1and Ncad expression is eliminated, cells on the left side become shorter and more cuboidal, similar to normal right-sided morphology (Davis et al., 2008; Kurpios et al., 2008; Plageman Jr. et al., 2011). Ncad, expressed asymmetrically on the left side of the DM, cooperates with Shroom3 to regulate left-sided cell shape (Plageman Jr. et al., 2011). Shroom3 induces apical F-actin and Myosin II, increasing apical constriction and cell elongation on the left side (Plageman Jr. et al., 2011). Interestingly, cell shape changes occur only on the right side, (i.e., both sides of the epithelium have a columnar shape prior to the initial tilting process), indicating that Shroom3 and Ncad interactions may act
In the mesenchymal compartment, there are no LR differences in cell shape, however; there are differences in cellular condensation. Left-sided cells are more densely packed together than right-sided cells (Fig. 1.3A) (Davis et al., 2008; Kurpios et al., 2008). Pitx2 and Ncad are required for this difference in mesenchymal compaction as ectopic right sided expression of either gene induces cell condensation (Davis et al., 2008; Kurpios et al., 2008). The left versus right composition of ECM proteins accounts for this difference. The left mesenchyme compartment consists of more Glycosaminoglycans (GAGs) while the right has more Hyaluronic acid (HA) (Kurpios et al., 2008). HA is a big protein that occupies a larger volume of space than GAGs, therefore, asymmetric expression of HA in the right
mesenchymal compartment would cause expansion of the right side (Kurpios et al., 2008). Downstream of Pitx2, Ncad is expressed asymmetrically within the DM on the left side. This asymmetric expression is required for LR differences in mesenchymal compaction as Ncad alters ECM production of HA and GAGs. Ectopic expression of Ncad in the right DM induces mesenchymal cells to become more densely packed due to an increase in GAGs and a decrease in HA (Kurpios et al., 2008). Additionally, the expression of a dominant negative Ncad on the left side increases HA and decreases GAGs, causing mesenchyme cells to adopt a sparse organization (Kurpios et al., 2008). Therefore, asymmetric expression of Ncad in the left mesenchymal compartment downstream of Pitx2 inhibits the deposition of HA, instead providing a favorable environment for GAGs.
Daam2, typically polymerize unbranched F-actin to form stress fibers critical for cytoskeletal rearrangements, cell polarity, and adhesion (Welsh et al., 2014). Ectopic expression of Daam2 on the right side of the DM induces mesenchymal condensation due to an increase in cell contacts as a result of an expansion in the length of cell junctions (Welsh et al., 2014). Conversely, Daam2 mutants have disrupted left-sided condensation due to a loss of F-actin and abnormal intercellular adhesion as a result of a decrease in the length of cell junctions (Welsh et al., 2014). Interestingly, Daam2 physically interacts with a cell to cell adhesion protein, -catenin, as they co-immunoprecipitate together (Welsh et al., 2014). Therefore,
Daam2 downstream of Pitx2 directs the formation and size of cadherin based junctions required for left-sided mesenchymal condensation through its effect on F-actin organization and possibly through its physical interaction with cell adhesion proteins (Welsh et al., 2014).
In the chick and mouse, asymmetries in the DM are required for midgut looping, however; in zebrafish and frog, there is no dorsal mesentery. In zebrafish, asymmetries in cell movements in the LPM orchestrate gut laterality. In this species, the gut originates from a solid rod of endodermal cells found at the ventral midline (Horne-Badovinac et al., 2003; Yin et al., 2010). Looping of the gut occurs at 26 hours post fertilization as the gut tube curves to the left (Horne-Badovinac et al., 2003). The LPM surrounding the developing gut arise from two distinct left and right populations. Both sides of the LPM have a highly polarized epithelium, however, asymmetries exist in the morphology and position of cells (Horne-Badovinac et al., 2003; Yin et al., 2010).
dorsal side (Horne-Badovinac et al., 2003; Yin et al., 2010). Before the onset of gut looping, the LPM is symmetrical, however, during looping, the right side undergoes ventrolateral migration while the left side moves dorsal to the endoderm (Fig. 1.3B) (Horne-Badovinac et al., 2003; Yin et al., 2010). Cell tracking experiments show that more ventrally located cells of the right LPM roll dorsally, moving the entire LPM on this side to a more ventrolateral position (Horne-Badovinac et al., 2003; Yin et al., 2010). Thus, the right-sided movement of the LPM actively pushes the gut leftward (Horne-Badovinac et al., 2003; Yin et al., 2010).
Gut displacement is autonomous to the LPM as bonnie and clyde mutants (bon) that lack endoderm, still have asymmetric migration of the LPM (Horne-Badovinac et al., 2003). Interestingly, the proper polarity of cells within the LPM compartment is vital to this event as embryos with defects in nagie oko, a gene required for epithelial polarity, have defects in gut looping (Horne-Badovinac et al., 2003). In these mutants, the epithelial structure of the LPM is disrupted and the ventrolateral migration of the right LPM is perturbed as these cells migrate dorsally, similar to the normal pattern of the left LPM (Horne-Badovinac et al., 2003). Proper epithelial polarity is likely necessary for coordinated and directional cell migration events required for gut laterality.
the migratory path of rearranging cells (Yin et al., 2010). Normally, Laminin expression is degraded in the direction of cell movement; however, in Hand2 mutants, Laminin persists, preventing proper migration (Yin et al., 2010). Hand2 regulates Laminin deposition by regulating the activity of matrix metalloproteases (MMPs) (Yin et al., 2010). MMPs are proteolytic enzymes that aide in the remodeling of the ECM by cleaving ECM proteins like Laminin (Yin et al., 2010). Hand2 mutants have reduced MMP proteolytic activity due to the increased expression of MMP inhibitors Timp2a and b (Yin et al., 2010).
Hand2 is only expressed in the ventral portion of the LPM and this expression is regulated by Bmp2 as ectopic expression of Bmp2b induces Hand2 expression throughout the LPM (Yin et al., 2010). Bmp itself is restricted to the ventral portion of the LPM due to the expression of Bmp antagonists in the somites (Yin et al., 2010). Inhibition of Bmp also eliminates Hand2 expression (Yin et al., 2010). Hand2 mutants, as well as embryos treated with Bmp inhibitors, have gut laterality defects (Yin et al., 2010). Therefore, Hand2 downstream of Bmp2b regulates cellular rearrangements by altering the activity of MMP through the expression of MMP inhibitors, creating a path of migration and subsequent cellular rearrangement by remodeling the ECM of the LPM to initiate gut looping events (Yin et al., 2010). Gastrointestinal laterality in zebrafish is downstream of the Nodal signaling pathway as Nodal knockdowns display abnormalities in gut looping and have randomized LPM migration (Horne-Badovinac et al., 2003). However, it is unknown whether the Pitx2 pathway influences the expression of polarity genes, or BMP and Hand2 to
The model frog Xenopus laevis also lacks a well-defined DM region. However, midgut looping is not a consequence of LR differences in cellular migration of the LPM but rather due to differences in cellular rearrangements within the endoderm of the gut tube itself (Muller et al., 2003). During the peak of midgut curvature, the right side of the gut tube becomes two times longer than the left, inducing a right-sided convex surface as opposed to the concave left side (Muller et al., 2003). This differential LR gut elongation is driven by Pitx2c as ectopic expression induces midgut malrotation (Muller et al., 2003). It is likely that Pitx2c influences cell shape and cell rearrangement patterns, ultimately orchestrating
differential convergent extension and radial intercalation movements that are required for the process of gut elongation, but this is not well understood (Reed et al., 2009).
The morphological mechanism by which midgut looping is established varies
2.4) Vascular and Lymphatic asymmetries in the dorsal mesentery
The proper development and placement of the vascular system that supplies the gastrointestinal tract is important for providing blood supply to the intestine as well as transporting materials absorbed to the liver for detoxification. Arteries connect to the dorsal portion of the gut from the aorta while veins located ventrally drain into the hepatic portal system (Mahadevan et al., 2014). This segregation of arteries and veins is vital as defects in this process induce portosystemic shunts and metabolic imbalances as the material drained from the gut bypasses the detoxification system within the liver (Mahadevan et al., 2014). Many of the arteries that supply the gastrointestinal tract develop within the dorsal mesentery, the tissue that suspends the gut tube from the body wall and directs intestinal rotation (Mahadevan et al., 2014). Interestingly, vascular patterning develops asymmetrically from the endothelial plexus within the DM as the forming arterial cords are present only on the left side within the densely packed mesenchyme (Mahadevan et al., 2014). Prior to intestinal rotation, the endothelial plexus is bilaterally symmetric in both the left and right mesenchymal compartments of the DM (Mahadevan et al., 2014). At the first appearance of cellular asymmetries within the DM that are required for intestinal rotation, the endothelial cells leave the right side and cross over to the left (Mahadevan et al., 2014). As the gut tilts leftward, endothelial cords are only found on the left side. Pitx2, expressed in the left mesenchyme surrounding the endothelial cords, is required for this asymmetrical regression as Pitx2 deficient mice have decreased arterial cord formation (Mahadevan et al., 2014).
deficient for either gene display defective DM arteriogenesis (Mahadevan et al., 2014). Cxcl12 is expressed asymmetrically in the left side of the dorsal mesentery in the tissue that surrounds endothelial cells while Cxc4 is expressed bilaterally within the endothelial cells themselves (Mahadevan et al., 2014). Pitx2 is required for asymmetric Cxcl12 expression as ectopic right-sided Pitx2 induces ectopic Cxcl12 expression and also causes the vasculature to development bilaterally, preventing right-sided vascular regression (Mahadevan et al., 2014). Additionally there are binding sites for Pitx2c in the promoter region of Cxcl12 (Mahadevan et al., 2014). Asymmetric Cxcl12 expression, induced by Pitx2c, likely acts as a chemotactic agent, guiding the Cxc4+ endothelial cells to the left side (Mahadevan et al., 2014).
Asymmetric vascular development also initiates asymmetric lymphatic development within the DM (Mahadevan et al., 2014). As the arterial cords are regressing to the left side, lymphatic precursors also move to the left (Mahadevan et al., 2014). The Pitx2-Cxcl12 pathway is required for this asymmetry as both mutations in Pitx2 and inhibition of Cxcl12 causes the lymph vessels to not develop (Mahadevan et al., 2014). Therefore, Pitx2 is
required to set up the proper location of both the vasculature and the lymphatic system within the dorsal mesentery of the gastrointestinal tract (Mahadevan et al., 2014). The mechanism by which both vascular and lymphatic precursors cells migrate within the mesenchyme or how much the mechanics of this migration are also influenced by Pitx2 is unknown.
interplay between liver lobation and vasculature infiltration could suggests that LR
asymmetries also exist during blood vessel development as they intermingle with the liver parenchyma. It is unknown if any asymmetries exist in liver associated vasculature
development although portosystemic shunts and preduodenal portal veins are commonly associated with heterotaxy syndrome.
2.5) Stomach Curvature
The earliest event of LR asymmetrical morphogenesis in the gastrointestinal tract is the bulging of the foregut to the left to create the J-shaped curvature of the stomach with the longer, “greater” curvature on the left and the shorter, “lesser curvature” on the right (Davis
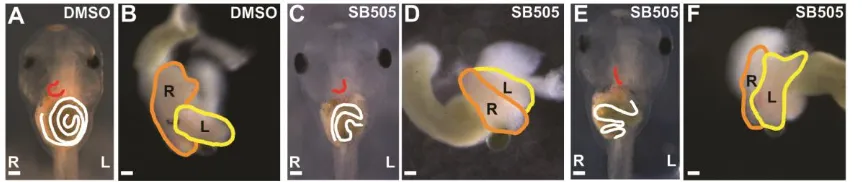
et al., 2017; Burn and Hill, 2009). Pitx2c is expressed in the left endoderm and mesoderm of
the Xenopus stomach and this expression is required for LR asymmetric stomach curvature as
mutations of Pitx2c cause reversed or midline stomach orientation (Davis et al., 2017). Research published just this year reveals downstream mechanisms of Pitx2c during stomach curvature.
2017). The Nodal-Pitx2c pathway is required for differential elongation as chemical
inhibition of Nodal and misexpression of Pitx2c in Xenopus causes the left wall to increase in cell layers, with abnormal architecture due to failed cellular rearrangement that causes a straightened stomach as the left wall fails to thin (Davis et al., 2017). Knock-down of Pitx2c in either the mesodermal or endodermal layer also affects adjacent, non-targeted tissue suggesting reciprocal signaling between the two compartments (Davis et al., 2017).
Interestingly, cellular mechanisms upstream of midgut looping, in the DM, occur extrinsic to the gut tube whereas both midgut looping and stomach curvature in the frog requires a mechanism intrinsic to the organ itself (Davis et al., 2008; Davis et al., 2017). Although Pitx2 is required for both stomach curvature and intestinal looping, the cellular level morphological result is contrasting as Pitx2 drives tissue expansion in the left stomach wall and tissue condensation in the mesenchymal compartments of the dorsal mesentery of the midgut (Davis et al., 2008; Davis et al., 2017). Additionally, in the midgut of the frog, the right wall of the epithelium elongates instead of the left, opposite to events during stomach curvature (Muller et al., 2003). Therefore, the role of Pitx2 during LR asymmetric
2.6) Asymmetric regression of the spleen
The spleen is asymmetrically located on the left side of the body cavity. In fact, the placement of the spleen is commonly used for the diagnosis of heterotaxia. The presence of bilateral spleen indicates left-isomerism while the absence of the spleen or splenic hypoplasia indicates right isomerism. During spleen development in X. laevis, there is initially a bilateral pool of precursor cells indicated by the expression of Nkx2.5 (Burn and Hill, 2009; Patterson et al., 2000). The left sided precursors develop into the spleen while the right sided
precursors adopt a different developmental fate and are incorporated into other organs due to the loss of Nkx2.5 (Burn and Hill, 2009; Patterson et al., 2000). The Nodal-Pitx2 pathway is required for this process as Nodal and Pitx2 mutants have a reduction in spleen size (i.e. right isomerism) or loss of the spleen (Burn and Hill, 2009; Patterson et al., 2000). Pitx2 most likely acts to maintain Nkx2.5 expression in left precursor cells, however, more research is needed into the cellular mechanisms of asymmetric splenic development (Burn and Hill, 2009; Patterson et al., 2000).
2.7) Lung Lobation
(Kitamura et al., 1999) while, in humans, the right lung has three lung lobes and the left lung has two (Gray, 1918). The Nodal-Pitx2 pathway is required for this LR asymmetrical
morphology as Pitx2 mutant mice show lung right isomerism (i.e., both the left and right lungs have four lobes) (Gage et al., 1999; Kitamura et al., 1999; Lin et al., 1999; Liu et al., 2001; Lu et al., 1999; Shiratori et al., 2006). However, the underlying cellular mechanism of LR lung morphogenesis is unknown. Knowledge about the development of the liver
including cellular level mechanism of differential lobation will also give us clues about the formation of lung laterality.
2.8) Liver lobation
The role of Pitx2 in LR asymmetrical organogenesis is just beginning to be elucidated in organs like the stomach and midgut, however, the role of Pitx2 in orchestrating liver laterality is unknown. The liver is LR asymmetric in both location within the body (on the right side) and morphology (the right lobe of the liver is generally larger than the left) (Abdel-Misih and Bloomston, 2010; Gray, 1918). In humans, the right side of the liver consists of four lobes, the right medial, right lateral, quadrate, and caudate, while the left side consists of two lobes, left medial and lateral (Gray, 1918).
ventral midline (Weiss et al., 2016), however, the mechanisms that produce left-right asymmetries in the shape and lobation patterns of the liver are not well understood.
In zebrafish, there is no LR asymmetry within the liver itself (no differences in LR lobal morphology), however, the placement of the liver with respect to the gastrointestinal tract displays laterality as the liver always buds off of the left side of the developing foregut (Cayuso et al., 2016). Future hepatoblasts are initially symmetrically located at the
embryonic midline (Cayuso et al., 2016). Shortly after initial specification, the liver bud shifts to the left side of the midline due to cell shape changes and cellular migration patterns (Cayuso et al., 2016). During active liver bud formation and leftward outgrowth, hepatoblasts become elongated and change their orientation towards anterior-leftward outgrowth, moving in a consistent and coordinated direction, exchanging positions with their neighbors (Cayuso et al., 2016). Anteriorly positioned cells move posterior and leftward while posterior cells move anterior and leftward before a second phase of more consistent, angular displacement to the left (Cayuso et al., 2016). Cytoskeletal components like filamentous actin (F-actin) are required for this process as F-actin depolymerizing drugs cause hepatoblasts to remain at the midline (Cayuso et al., 2016). Hepatoblasts have distinct F-actin rich cellular protrusions including flat, sheet like protrusions (lamellipodia) and thin extensions (filopodia) (Cayuso et al., 2016). Lamellipoda are typically seen at the leading edge of migrating cells while
left side moves dorsal to the underlying liver endoderm while the right moves ventrolaterally (Cayuso et al., 2016). This suggests that signaling factors within the mesoderm and
endoderm coordinate zebrafish liver laterality.
In fact, EphrinB1 in the endoderm interacts with the Ephrin receptor, Ephb3b, expressed asymmetrically in the right lateral plate mesoderm to orchestrate leftward
hepatoblast migration (Cayuso et al., 2016). Ephrin B1 in the endoderm regulates hepatoblast directional migration through its interaction with EphB3b controlling the polarity and
movement of the LPM (Cayuso et al., 2016). Ephb3b actually repels hepatoblasts inducing their leftward movement into the liver bud (Cayuso et al., 2016).
2.9) Conclusions
Although the final shape and geometry of LR asymmetric organs varies widely based on the position within the body cavity and the functions required, many factors of LR
asymmetric morphogenesis remain conserved. An initial symmetry breaking event during neurulation at the organizer or “node” sets up the embryonic LR axes by cilia driven
left-ward flow that induces the left-sided expression of Nodal (Basu and Brueckner, 2008; Blum, 2014; Ibanes and Izpisua Belmonte, 2009; Raya and Izpisua Belmonte, 2006; Shiratori et al., 2006). Nodal expression, limited to the left LPM by the midline barrier, is vital for LR organ development (Brennan et al., 2002; Saijoh et al., 2003; Shiratori et al., 2006; Yamamoto et al., 2003). During organogenesis, a variety of morphogenetic events are responsible for laterality including LR asymmetric migration/cell rearrangement events, modifications to the actin cytoskeleton, cell shape changes, and the distribution of ECM proteins. Importantly, the homeobox transcription factor, Pitx2, downstream of the initial Nodal signal, is the driving force behind these asymmetrical events during morphogenesis in many organs and model systems studied. However, additional downstream mechanisms by which Pitx2 orchestrates these asymmetries are still relatively unknown. Additionally, the role of Pitx2 in the LR asymmetric development of many organs like the liver have yet to be elucidated.
3. Rationale and Hypothesis
is larger than the left and the two lobes have different morphologies. As many as 1 in 10,000 humans are born with defects in LR asymmetry that often involve severe anomalies in liver laterality including hepatic duct and biliary tree malformations, yet the mechanism by which this asymmetry develops and the genes involved are largely unknown (Lin et al., 2000).
In other LR asymmetric organs, LR differences in cell rearrangements, cell shape changes, reorganization of the actin cytoskeleton, and redistribution of ECM proteins orchestrate laterality. For example, cellular rearrangements in the cardiac cone orchestrated by cytoskeletal lamellipodial extensions are required for cardiac jogging and dextral heart looping in zebrafish (Bakkers et al., 2009, Smith et al., 2008). During X. laevis midgut looping, asymmetries in cellular rearrangements of the gut endoderm initiate gut curvature (Muller et al., 2013). In mice, asymmetries in ECM composition and cell shape in the dorsal mesentery create the leftward tilt required to initiate midgut rotation (Davis et al., 2008). In this context, cells on the right side of the DM become cuboidal and flattened in shape while cells on the left are more elongated and apically constricted due to apical accumulation of F-actin and Myosin II (Davis et al., 2008; Plageman Jr. et al., 2011). In zebrafish, liver
morphology develops is unknown. It is likely that mechanisms employed by other LR asymmetric organs to initiate laterality are also used for LR liver development.
Pitx2c, a homeobox transcription factor, expressed on the left side of many LR asymmetric organs, is required for proper asymmetric morphogenesis of the heart, lung, gastrointestinal tract, and stomach (Gage et al., 1999; Kitamura et al., 1999; Lin et al., 1999; Liu et al., 2001; Lu et al., 1999; Raya and Izpisua Belmonte, 2006; Shiratori et al., 2006). Pitx2 is required for apical F-actin and Myosin II accumulation that drives cell shape changes in the dorsal mesentery of the gut (Plagemen Jr. et al., 2011). Pitx2 is also required for
cellular rearrangements during stomach curvature (Davis et al., 2017).
Pitx2c is expressed on the left side of the septum transversum mesenchyme, a mesodermal tissue that surrounds the developing liver, however, the requirement for Pitx2c in LR asymmetric liver development is unknown. I hypothesize that left-limited Pitx2c is required for LR asymmetrical development of the liver and that Pitx2c orchestrates these LR differences through asymmetries in cellular level epithelial morphogenesis including cell shape changes and cellular rearrangements.
In the model frog Xenopus laevis, the right liver lobe is larger and more elongate while the left liver lobe is small and spherical. Using the experimental amenability of
Xenopus, and a combination of chemical and molecular techniques, I tested my hypothesis
with the following specific aims:
Specific Aim 2: Determine the requirement for left-limited Pitx2c expression during LR asymmetrical development of the liver.
Specific Aim 3: Determine the cellular level mechanism by which Pitx2c orchestrates LR asymmetries in the liver.
My results reveal surprising contrasts between mechanisms that shape LR liver morphology and those that generate laterality in other organs like the gastrointestinal tract and stomach. I describe asymmetries in cell shape and behavior downstream of the
Figure 1- 2) Four steps of initial LR symmetry breaking events during embryogenesis The establishment of LR asymmetry during embryonic development consists of four major steps. A) The initial LR symmetry breaking event at the embryonic node or LR organizer.
Cilia driven leftward flow of embryonic fluid induces the left-sided expression of Nodal surrounding the node. B) This initial LR cue is transferred to the left LPM. Left-sided asymmetric expression of Nodal migrates from the perinodal region to the left LPM where
Nodal expression expands anteriorly and posteriorly. C) The establishment of the midline barrier. Nodal induces the expression of Lefty, a gene required to restrict Nodal expression to
the left side of the embryo by maintaining a midline barrier. D) The induction of LR asymmetric organogenesis. Nodal induces the expression of Pitx2 in the left LPM. The expression of Pitx2 is maintained during organogenesis where it surrounds the left side of organ primordia throughout development. A: anterior, P: posterior, R: right, L: left, LPM:
Figure 1- 3) LR asymmetric organogenesis in the midgut and stomach
During midgut looping in mice, cell shape changes in both the mesenchymal and epithelial compartments of the dorsal mesentery induce curvature. Cells in the left epithelium remain columnar in morphology while those on the right become flattened and cuboidal. In the left mesenchymal compartment, cells remain compact and close together while those in the right mesenchyme become sparse and less condensed. B) During zebrafish gut looping,
asymmetric migration of the LPM pushes the gut tube to the left to initiate curvature. The cells of the right LPM undergo ventrolateral migration while the cells of the left LPM move dorsal to the endoderm. C) During asymmetric stomach development, cellular level
References
Abdel-Misih, S.R.Z., and Bloomston, M. (2010). Liver Anatomy. Surg Clin North Am. 90, 643-653.
Amand, T.R.S., Ra, J., Zhang, Y., Hu, Y., Baber, S.I., Qiu, M., and Chen, Y. (1998). Cloning and expression pattern of chicken Pitx2: a new component in the SHH signaling pathway controlling embryonic heart looping. Biochem. Biophys. Res. Commun. 247, 100-105.
Applegate, K.E., Goske, M.J., Pierce, G., and Murphy, D. (1999) Situs-revisited: imaging of the heterotaxy syndrome. Radiographics. 19.
Bakkers, J., Verhoeven, M.C., and Abdelilah-Seyfried, S. (2009). Shaping the zebrafish heart: from left-right axis specification to epithelial tissue morphogenesis. Dev Biol. 330, 213-220.
Basu, B., and Brueckner, M. (2008). Chapter six cilia: multifunctional organelles at the center of vertebrate left–right asymmetry. Curr. Top. Dev. Biol. 85, 151-174.
Blum, M., Schweickert, A., Vick, P., Wright, C.V.E., and Danilchik, M.V. (2014). Symmetry breakage in the vertebrate embryo: When does it happen and how does it work? Dev. Biol. 393, 109-123
Brennan, J., Norris, D.P., and Robertson, E.J. (2002). Nodal activity in the node governs left-right asymmetry. Genes Dev. 16, 2339-2344.
Brueckner, M. (2007). Heterotaxia, congenital heart disease, and primary ciliary dyskinesia. Circulation 115, 2793-2795.
Burn, S.F., and Hill, R.E. (2009). Left-right asymmetry in gut development: what happens next? Bioessays 10, 1026-1037.
Burton, E.C., Olson, M., and Rooper, L. (2014). Defects in laterality with emphasis on heterotaxy syndrome with asplenia and polysplenia: an autopsy case series at a single institution. Pediatr. Dev. Pathol. 17, 250-264.
Cayuso, J., Dzementsei, A., Fischer, J.C., Karemore, G., Caviglia, S., Bartholdson, J., Wright, G.J., and Ober, E.A. (2016). EphrinB1/EphB3b coordinate bidirectional epithelial-mesenchymal interactions controlling liver morphogenesis and laterality. Dev. Cell. 39, 316-328.
Cox, C.J., Espinoza, H.M., McWilliams, B., Chappell, K., Morton, L., Hjalt, T.A.,
Semina, E.V., and Amendt, B.A. (2002). Differential regulation of gene expression by Pitx2 isoforms. Journal of Biological Chemistry. 277, 25001-25010.
Davis, A., Amin, N.M., Johnson, C., Bagley, K., Ghashghaei, T., and Nascone-Yoder, N. (2017) Stomach curvature is generated by left-right asymmetric gut morphogenesis.
Development.
Davis, N.M., Kurpios, N.A., Sun, X., Gros, J., Martin, J.F., and Tabin, C.J. (2008). The chirality of gut rotation derives from left-right asymmetric changes in the architecture of the dorsal mesentery. Developmental cell 15, 134-145.
Duboc, V., Rottinger, E., Lapraz, F., Besnardeau, L., and Lepage, T. (2005). Left-right asymmetry in the sea urchin embryo is regulated by nodal signaling on the right side. Dev. Cell. 9, 147-58.
Gage, P.J., Suh, H., and Camper, SA. (1999). Dosage requirement of Pitx2 for development in multiple organs. Development. 126, 4643-4651.
Gormley, J.P., and Nascone-Yoder, N.M. (2003). Left and right contributions to the
Xenopus heart: implications for asymmetric morphogenesis. Dev. Genes Evol. 213, 390-398.
Gottschalk, I., Stressig, R., Ritgen, J., Herberg, U., Breuer, J., Vorndamme, A., Strizek, B., Willruth, A., Geipel, A., Gembruch, U., and Berg, C. (2016). Extracardiac anomalies in prenatally diagnosed heterotaxy syndrome. Ultrasound Obstet. Gynecol. 47, 443-449.
Grande, C., and Patel, N.H. (2009). Nodal signaling is involved in left-right asymmetry in snails. Nature. 457, 1007-1011.
Gray, H. (1918). Anatomy of the Human Body. Philadelphia: Lea & Febiger, Bartleby.com, 2000. www.bartleby.com/107/.
Hjalt, T.A., and Semina, E.V. (2005). Current molecular understanding of Axenfeld-Rieger Syndrome. Expert Rev. Mol. Med. 7, 1-17.