Influence of Bath Composition on Tensile Ductility
in Electrodeposited Bulk Nanocrystalline Nickel
Isao Matsui
*, Yorinobu Takigawa, Tokuteru Uesugi and Kenji Higashi
Department of Materials Science, Osaka Prefecture University, Sakai 599-8531, Japan
We investigated the influence of bath composition on characteristics in an electrodeposited bulk nanocrystalline Ni (nc-Ni). This study was aim to establish the fabrication process of bulk nc-Ni with good tensile property. The bulk nc-Ni was electrpdeposited from a sulfate bath and a sulfamate bath. The nc-Ni was evaluated for microstructure and mechanical properties, such as microhardness, tensile strength. As a result, bulk nc-Ni obtained from a sulfamate bath exhibited enhanced tensile property (ultimate tensile strength of 1006 MPa and ductility of 8.8%) compared to that obtained from a sulfate bath. Dimple pattern was observed in fracture surface of bulk nc-Ni obtained from a sulfamate bath. However, dimple pattern and brittle fracture surface were observed in bulk nc-Ni obtained from a sulfate bath. We consider the ductility of electrodeposited nc-Ni was influenced by internal stress generated from electrodepostion. These experiments and analyses suggest that it is important to control internal stress on fabrication of bulk nc-Ni with high tensile strength and good ductility.
[doi:10.2320/matertrans.M2010279]
(Received August 20, 2010; Accepted November 4, 2010; Published December 15, 2010)
Keywords: nanocrystal, electrodepositon, nickel, tensile ductility
1. Introduction
Nanocrystalline materials (grain size is smaller than 100 nm) have received worldwide attention due to their extremely high strength with respect to their microcrystalline counterparts.1–4) Additionally, nanocrystalline materials are characterized by a large volume fraction of grain boundaries and triple junctions.5)When the grain size is reduced to the nanometer range, the dislocation density within the grain is decreased in particular for materials with average grain sizes less than 30 nm.6) It would appear that dislocation activity might play roles in the deformation of the nanocrystalline materials. In the past, there has been considerable research on electrodeposited nanocrystalline Ni (nc-Ni) and nc-Ni alloys. However, there were no research into the connection between electrodeposition parameters and tensile property of nc-Ni and nc-Ni alloys. So, reported data vary widely especially in ductility. In our previous work, tensile testing of thin film nc-Ni-W did not also show a notable plastic deformation.7) It should be considered that this might be the result of problems in the sample fabrication. So, our laboratory has optimized fabrication process of bulk Ni-W in order to obtained nc-Ni-W that displays a plastic deformation. In particular, we have introduced an electroforming system to fabricate bulk Ni-W alloy with sufficient thickness and homogeneity.8–10) As a result, homogeneous bulk nc-Ni-W was obtained with a thickness of 2.5 mm in which to meet these requirement the W-concentration was controlled to standard deviation of about 1.0 at%.11)However, that as-fabricated nc-Ni-W was warped and brittle. We consider the brittleness of nc-Ni-W as consequence of the influence of internal stress generated from the electrodepostion. In this study, the influence of internal stress was investigated on plastic deformation of bulk nc-Ni. For this purpose, bulk nc-Ni was electrodeposited from a sulfate bath and a sulfamate bath in order to obtained bulk nc-Ni with different internal stress.
2. Experimental Procedure
[image:1.595.304.550.350.390.2]The bulk nc-Ni was produced using a sulfate bath and a sulfamate bath. The sulfate bath compositions were deter-mined by reference to an electroforming system8–11) and listed in Table 1. The sulfamate bath was a similar direct current technique as that of Nakamaruet al.12)The chemical compositions of the sulfamate bath were listed in Table 2 and given a detailed description in reference.13) The main components of our electrodeposition system were a power supply and an electrolyte containing 1 L beaker. The bath was constantly stirred by a magnetic stirrer. Sulfur nickel chips were used as anode, and the chips were put in a titanium basket. The cathode was chemical polished copper plate. The pH values of the solutions were kept4:00:1by adding a sulfamic acid solution. When bulk nanocrystalline Ni was electrodeposited, masking shield was used in order to make current distribution of cathode uniform. By installation of
Table 1 Bath composition of sulfate bath.
Chemicals Concentration Purpose Nickel sulfate 63.0 g/L Ni source Ammonia solution 25% pH adjuster
Table 2 Bath composition of sulfamate bath. Chemicals Concentration Purpose Nickel sulfamate 300 g/L Ni source
Nickel chloride 5.0 g/L Passivation inhibitor Propionic acid 20.0 g/L Complexing agent Amidosulfuric
acid 1.0 mol/L pH adjuster Sodium
hydroxide 1.0 mol/L pH adjuster
*Graduate Student, Osaka Prefecture University
[image:1.595.304.550.435.532.2]masking shield, bath flow around the cathode was sup-pressed, and it was in much the same state as unstirred. With the general system setup and baseline chemical bath described previously, three series of experiments were carried out under the following conditions.
2.1 Nc-Ni fabricated by sulfamate bath
For the first series of experiments, the bath composition listed in Table 2 was used, and electrodeposition was run at a temperature of 50C and current density of 22–50 mA/cm2.
In addition, electrodeposition was run without stirring at current density of 50 mA/cm2in order to investigate stirring effect. This experiment was run in order to assess the availability of sulfamate bath as the nc-Ni fabrication process.
2.2 Bulk nc-Ni fabricated by sulfate bath
For the second series of experiments, the bath composition listed in Table 1 was used, and electrodeposition was run at a temperature of 70C and current density of 6.8 mA/cm2.
The pH values of the solutions were kept 6.3 by adding aqueous ammonia. This experiment was run in order to obtain bulk nc-Ni with high internal stress to form the strongly-warped tensile specimens.
2.3 Bulk nc-Ni fabricated by sulfamate bath
For the third series of experiments, the bath composition listed in Table 2 was used, and electrodeposition was run at a temperature of 50C and current density of 30 mA/cm2. This experiment was run in order to obtain bulk nc-Ni with low internal stress to form the flat tensile specimens.
After deposition, the grain size of the as-deposited samples was determined by X-ray diffraction (XRD) and Scherrer’s equation. The microstructure was observed by transmission electron microscopy (TEM). Dog-bone specimens were machined by electrical discharge machining from the as-deposited plates. Tensile tests were performed at strain rate of 110 3s 1 and room temperature using 15mm 6:25mm1:4mm (lengthwidththickness) dog-bone shaped specimens obtained from sulfamate bath and
30mm6:25mm1:0mm specimens obtained from sul-fate bath. Yield stress was calculated using nominal stress-strain curve measured by a stress-strain gauge. Elongation of the specimen was measured by change of the gauge length. The fracture surfaces were examined using scanning electron microscopy (SEM). Microhardness of the specimen was determined using micro Vickers hardness tester with a load of 500 g for 10 s.
3. Results
3.1 Nc-Ni fabricated by sulfamate bath
The nc-Ni was deposited using a sulfamate bath at different current density with stirring for 9 h. Sample were obtained from depositing at current density of (a) 22 mA/cm2, (b) 30 mA/cm2, (c) 50 mA/cm2, (d) 50 mA/cm2 without stirring. The XRD patterns of the each sample show a single-phase with fcc structure in Fig. 1. The XRD pattern of samples (a), (b), (c) showed strong (111) peak. In contrast, the XRD pattern of the sample (d) showed strong (200) peak.
The grain size of each samples were calculated from XRD (111) peaks and Scherrer’s equation: the samples (a), (b), (c) with grain size of about 40 nm, the sample (d) with grain size of about 65 nm. These results show the sulfamate bath is available to produce nc-Ni and, moreover, agitation in sulfamate bath profoundly affects characteristics of electro-deposited nc-Ni.
Figure 2 shows microhardness of nc-Ni deposited using a sulfamate bath for 9 h. The samples (a), (b), (c) with grain size of about 40 nm exhibited microhardness of about 420 Hv, and the sample (d) with grain size of 60 nm exhibited a microhardness of about 310 Hv.
3.2 Microstructure of bulk nc-Ni
The bulk nc-Ni was obtained from a sulfate bath and a sulfamate bath. Tensile specimens fabricated by a sulfate bath were strongly-warped in Fig. 3(a). In contrast, speci-mens fabricated by a sulfamate bath were near flat shape in Fig. 3(b). The strongly-warped tensile specimens are called sample W. The near flat tensile specimens are called sample F. The XRD patterns of the each sample were shown in Fig. 4. The grain size of bulk nc-Ni calculated from a XRD (111) peak and Scherrer’s equation was listed in Table 3.
Fig. 1 The XRD pattern of nc-Ni deposited by sulfamate bath at current density of (a) 22 mA/cm2, (b) 30 mA/cm2, (c) 50 mA/cm2, (d) 50 mA/
cm2without stirring for 9 h.
Fig. 2 The microhardness of nc-Ni deposited by sulfamate batn at current density of (a) 22 mA/cm2, (b) 30 mA/cm2, (c) 50 mA/cm2, (d) 50 mA/
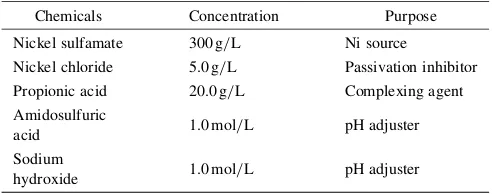
[image:2.595.322.528.72.233.2] [image:2.595.322.529.293.452.2]The microstructure of sample F was examined using TEM. Figure 5 shows bright filed TEM images for the as-deposited sample F. The grain size of about 50–100 nm can be observed in sample F.
3.3 Tensile property of bulk nc-Ni
The nominal stress-strain tensile curves are shown in Fig. 6. Sample W with grain size of about 60 nm exhibited tensile strength of about 900 MPa and ductility of about
Fig. 3 Photograph of as-deposited bulk nc-Ni obtained from the (a) sulfate bath and (b) sulfamate bath.
[image:3.595.322.528.70.235.2]Fig. 4 The XRD patterns of the sample W fabricated by a sulfate bath and the sample F fabricated by a sulfamate bath.
Table 3 Mechanical properties of the bulk nc-Ni obtained from sulfate bath and sulfamate bath measured from tensile tests at strain rate of
110 3s 1.
Bath Dimensions (mm)
Grain size (nm)
"f
(%)
0:2
(MPa)
UTS (MPa)
Hardness (Hv)
Sulfate 306:250:99 58 2.9 597 884 271
Sulfate 306:250:97; Sample W 58 3.1 639 929 295
-Salfamate 156:251:42; Sample F 61 8.8 639 1006 275
Salfamate 156:251:49 71 4.8 626 920 257
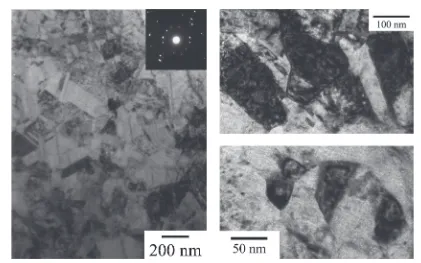
[image:3.595.76.261.72.272.2] [image:3.595.45.550.345.421.2] [image:3.595.85.506.439.702.2]3.0%. Sample F with grain size of about 60 nm exhibited tensile strength of about 1000 MPa and ductility of 8.8%. Figure 7 shows fracture surface for the sample W and the sample F. The dimple pattern which was shown in ductile failure was observed for each sample. In comparison with the dimple pattern of sample W, the dimple of sample F was formed deeply and finely. Also, flat fracture part of varying size which was similar to fracture surface shown
in brittle failure were observed in the sample W. In the sample F, the flat fracture part was not observed. These results on fracture surface were corresponding to the differ-ence in the tensile ductility behavior of sample W and sample F, respectively. Table 3 summarizes the mechanical proper-ties of the bulk nc-Ni obtained from a sulfate bath and a sulfamate bath.
4. Discussions
Initially, we attempted to produce nc-Ni using a sulfamate bath. Previously, there has been considerable research on electrodeposited nc-Ni.14–25) Most of these investigations were used sulfate electrolyte as a fabrication process of nc-Ni. In general, electrodepositon bath based nickel sulfate lead to larger internal stress than that based nickel sulfamate in the coatings.12)Problem with bulk nc-Ni deposits is that they suffer from relatively high internal stress, which can give rise to warped shape and crack occurrence in the electrodeposits.26)So, strongly-warped specimens generated from electrodeposition could be expected to have a large volume fraction of defect and exhibit no plastic deform-ability. To obtain a low internal stress specimen with flat shape, it is important to investigate fabrication of bulk nc-Ni using a sulfamate bath. In this study, electrodepositon was performed using a sulfamate electrolyte with propionic acid under different current density and stirring. As a result, it was confirmed that sulfamate bath produced nc-Ni exhibited grain size of about 40–60 nm and microhardness of about 300–400 Hv. These results show that the sulfamate bath is available to produce nc-Ni. In addition, it is expected that sulfamate bath is available to produce nc-Ni with different grain size, microhardness by controlling a current density, agitation, pH, and bath temperature like a Watts bath.27–29)
Secondly, bulk nc-Ni was fabricated by a sulfate bath and a sulfamate bath in order to investigate influence of internal stress on tensile property of bulk nc-Ni. The sample W and sample F were compared on average grain size, orientation and internal stress. The sample W and sample F exhibited the same value of grain size calculated from XRD (111) peak and Scherrer’s equation in Table 3. The similar XRD patterns with stronger (111) and (200) peaks were observed for the sample W and sample F in Fig. 4. So, difference of grain size and orientation for each sample had little direct influence on tensile property of bulk nc-Ni in this experiment. In contrast, there was clear difference in internal stress between the sample W and sample F. The sample W had so large internal stress that specimen was warped in Fig. 3(a). The sample F had low internal stress, and it was not be warped in Fig. 3(b). As a result of tensile test, the sample F exhibited higher ductility than the sample W. Moreover, the flat fracture part which was similar to fracture surface shown in brittle failure were observed in the sample W. It is considered that high internal stress which provided a warped specimen introduced a lot of defect into the sample W. In reported data, fabrication process of nc-Ni exhibited good ductility of over 5% was added some gloss agents, surfactants that acted as stress relievers.18,20–22,24,25) Therefore, internal stress is one of the important factors that have the effect on tensile
Fig. 6 Nominal stress-strain tensile curves of the sample W obtained from a sulfate bath and (b) the sample F obtained from a sulfamate bath measured by strain gauge (maximum measured value is 5.0%).
Fig. 7 SEM images of fracture surfaces for (a) the sample W deformed at strain rate of110 3s 1to elongation of 3.1% and (b) the sample F
[image:4.595.64.273.69.222.2] [image:4.595.56.284.283.635.2]property of electrodesposited nc-Ni. It is suggested that internal stress which is generated from electrodepostion was necessary to suppress on investigating tensile property of electrodeposited nc-Ni.
5. Conclusions
The bulk nanocrystalline Ni was produced by a sulfate bath and a sulfamate bath. The following conclusions can be obtained:
(1) The bulk nc-Ni fabricated by sulfamate bath exhibited ultimate tensile strength of 1006 MPa and good ductility of 8.8%.
(2) The bulk nc-Ni obtained from sulfamate bath exhibited lower internal stress than that obtained from sulfate bath. (3) These experiments and analyses suggest that internal stress is important parameters on ductility of bulk nc-Ni.
Acknowledgements
This work was supported by Grant-in-Aid for Scientific Research (C) (19560735) and (22560726) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
REFERENCES
1) T. Yamasaki, N. Odab, H. Matsuoka and T. Fukami: Mater. Sci. Eng. A
449–451(2007) 833–835.
2) H. Wei, G. D. Hibbard, G. Palumbob and U. Erb: Scr. Mater.57(2007) 996–999.
3) G. J. Fan, L. F. Fu, H. Choo, P. K. Liaw and N. D. Browning: Acta Mater.54(2006) 4781–4792.
4) L. Lu, Y. Shen, X. Chen, L. Qian and K. Lu: Science304(2004) 422– 426.
5) B. Y. C. Wu, P. J. Ferreira and C. A. Schuh: Metall. Mater. Trans. A36
(2005) 1927–1936.
6) D. Farkas and W. A. Curtin: Mater. Sci. Eng. A412(2005) 316–322. 7) A. Giga, Y. Kimoto, Y. Takigawa and K. Higashi: Scr. Mater. 55
(2006) 143–146.
8) Y. Kimoto, S. Wakayama, A. Fujii, Y. Takigawa and K. Higashi: Mater. Trans.48(2007) 1483–1491.
9) Y. Kimoto, A. Giga, T. Ohkubo, Y. Takigawa, K. Hono and K. Higashi: Mater. Trans.48(2007) 996–1000.
10) S. Wakayama, Y. Kimoto, Y. Takigawa, T. Uesugi and K. Higashi: Mater. Sci. Forum561–565(2007) 1375–1378.
11) A. Fujii, Y. Kimoto, S. Wakayama, Y. Takigawa, T. Uesugi and K. Higashi: Adv. Mater. Res.26–28(2007) 691–694.
12) Y. Nakamaru, Y. Wakuda, K. Tashiro, M. Watanabe and H. Honma: J. Surface Finish. Soc. Jpn.59/2(2008) 133–137.
13) I. Matsui, Y. Takigawa, T. Uesugi and K. Higashi: Mater. Sci. Forum
654–656(2010) 1114–1117.
14) F. Ebrahimi, G. R. Bourne, M. S. Kelly and T. E. Matthews: Nanost. Mater.11(1999) 343–350.
15) C. A. Schuh, T. G. Nieh and H. Iwasaki: Acta Mater.51(2003) 431– 443.
16) U. Erb: Nanost. Mater.6(1995) 533–538.
17) M. Thuvander, M. Abraham, A. Cerezo and G. D. W. Smith: Mater. Sci. Technol.17(2001) 961–970.
18) F. D. Torre, H. V. Swygenhoven and M. Victoria: Acta Mater. 50
(2002) 3957–3970.
19) D. H. Jeong, F. Gonzalez, G. Palumbo, K. T. Aust and U. Erb: Scr. Mater.44(2001) 493–499.
20) H. Li and F. Ebrahimi: Appl. Phys. Lett.84(2004) 4307–4309. 21) F. Ebrahimi and Z. Ahmed: Mater. Charact.49(2002) 373–379. 22) C. Gu, J. Lian, Z. Jiang and Q. Jiang: Scr. Mater.54(2006) 579–
584.
23) C. Gu, J. Lian and Q. Jiang: Scr. Mater.57(2007) 233–236. 24) X. Shen, J. Lian, Z. Jiang and Q. Jiang: Mater. Sci. Eng. A487(2008)
410–416.
25) M. J. N. V. Prasad, S. Suwas and A. H. Chokshi: Mater. Sci. Eng. A503
(2009) 86–91.
26) I. Mizushima, P. T. Hans, N. Hansen and M. A. J. Somers: Electro-chemi. Acta51(2006) 6128–6134.
27) E. Moti, M. H. Shariat and M. E. Bahrololoom: Mater. Chem. Phys.111
(2008) 469–474.
28) A. M. Rashidi and A. Amadeh: Surf. Coat. Technol.202(2008) 3772– 3776.
29) J. X. Kang, W. Z. Zhao and G. F. Zhang: Surf. Coat. Technol.203