metal-organic papers
m602
Alexander BricenÄoet al. [Ba(C5H4O4)(H2O)4] DOI: 10.1107/S1600536802017099 Acta Cryst.(2002). E58, m602±m605 Acta Crystallographica Section EStructure Reports Online
ISSN 1600-5368
The two-dimensional coordination
polymer tetraaquamesaconatobarium(II),
[Ba(C
5H
4O
4)(OH
2)
4]
nAlexander BricenÄo,aJose Miguel
Delgadoband Graciela DõÂaz de
Delgadob*
aCentro de QuõÂmica, Instituto Venezolano de
Investigaciones CientõÂficas (IVIC), Apartado 21827, Caracas 1020-A, Venezuela, and bFacultad de Ciencias, Departamento de
QuõÂmica, Universidad de Los Andes, Apartado 40 La Hechicera, MeÂrida 5201, Venezuela
Correspondence e-mail: diaz@ciens.ula.ve
Key indicators
Single-crystal X-ray study
T= 293 K
Mean(C±C) = 0.007 AÊ H-atom completeness 34%
Rfactor = 0.046
wRfactor = 0.132
Data-to-parameter ratio = 19.0
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2002 International Union of Crystallography Printed in Great Britain ± all rights reserved
The structure of [Ba(C5H4O4)(OH2)4]n consists of
two-dimensional rectangular grid layers, built up from the self-assembly of zigzag chains of edge-sharing {BaO9} polyhedra
linked by bridging bidendate mesaconate anions (C5H4O42ÿ)
through different coordination modes of the carboxylate groups. The polymer layers form a three-dimensional network via OÐH O hydrogen-bond interactions between the coordinated water molecules and the O atoms of the carboxylate groups.
Comment
Unsaturated carboxylic acids and their derivatives display interesting reactivity patterns when they are heated and/or irradiated in the solid state (DõÂaz de Delgadoet al., 1991; Case & Foxman, 1994; Velaet al., 2000; Xiaoet al., 2000; Odaniet al., 2001). The nature of the reactions in the solid state is deter-mined by the control that the crystalline lattice exerts. In general, the preorganization of molecules and the orientation of double bonds in the crystal determine the nature of the reaction products. For example, we have reported that, when barium hydrogen itaconate monohydrate is heated to about 473 K, an unusual isomerization to barium citraconate occurs in the solid state as a consequence of the arrangement of the molecules in the starting material (BricenÄoet al., 1999).
In this work, we report the X-ray diffraction study of barium mesaconate tetrahydrate, (I), and its thermal behavior. This study is part of a systematic investigation of the struc-ture±reactivity relationships in the solid state of metal complexes of,-unsaturated carboxylic acids. In spite of the simplicity of mesaconic acid (methylmaleic acid), and its similarity to maleic and fumaric acids (cis- and
dioic acids, respectively), the structural chemistry of its salts and complexes is unknown. Only the structure of potassium mesaconate (Gupta & Yadav, 1975) has been reported, as indicated by a search in the Cambridge Structural Database (Version 5.23; Allen & Kennard, 1993).
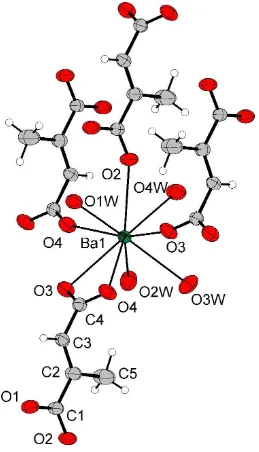
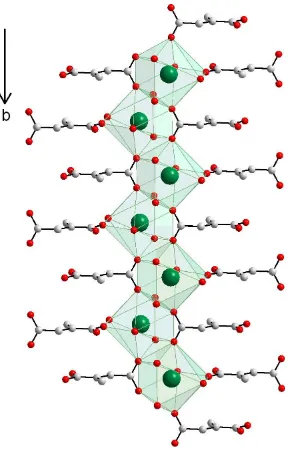
In the structure of (I), the Ba atoms are coordinated by nine O atoms, as shown in Fig. 1. Five of these O atoms come from four different mesaconate anions and four O atoms come from water molecules. The BaÐO distances range from 2.683 (3) to 2.967 (4) AÊ. The disposition of the O atoms around the Ba atom can be described as a distorted monocapped rectangular antiprism. Edge-sharing polyhedra form unidimensional zigzag chains along the b axis, which are linked through bridging bidendate mesaconate anions (Fig. 2). The carboxylate groups display different coordination modes. The
O1/C1/O2 carboxylate acts as monodentate group through the O2 atom, while O3/C4/O4 displays a combination of sym-metrical chelating and monoatomicanti±antibridging modes, allowing the coordination to three different Ba atoms. The dihedral angle between the planes containing the carboxylate groups is approximately 89.8 (1). These coordination modes
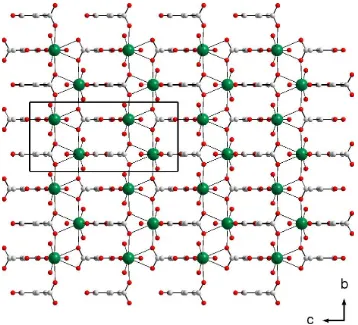
induce self-assembly of the chains of barium polyhedra with the mesaconate anions, as well as their arrangement in a parallel and alternate head-to-tail fashion, and produce two-dimensional rectangular grid layers parallel to the bc plane (Fig. 3). The polymer layers interact through extensive hydrogen bonds between water molecules and carboxylate O atoms: O1W O3Wi 2.836 (3), O1W O2Wii 2.833 (2) and
O2W O1iii 2.807 (3) AÊ [symmetry codes: (i) 1
2ÿx, 12+y, 1
2ÿz; (ii)12ÿx,ÿ21+y,12ÿz; (iii)ÿ1 +x, y, z], among others,
along thecaxis.
The disposition of the double bonds in neighboring mol-ecules is parallel, and a series of in®nite contacts along theb axis is observed. An analysis of the distances for such contacts indicates that there are contacts C2 C2iv [symmetry code:
Acta Cryst.(2002). E58, m602±m605 Alexander BricenÄoet al. [Ba(C5H4O4)(H2O)4]
m603
metal-organic papers
Figure 1
Coordination environment of the Ba atom in [Ba(C5H4O4)(OH2)4]n.
Displacement ellipsoids are shown at the 50% probability level.
Figure 2
metal-organic papers
m604
Alexander BricenÄoet al. [Ba(C5H4O4)(H2O)4] Acta Cryst.(2002). E58, m602±m605(iv)ÿx+ 1,ÿy+ 1,ÿz+ 1] along this direction at a distance of 4.168 (2) AÊ. In spite of these favorable structural char-acteristics for reactivity in the solid state, thermal analysis (TGA and DSC) and FT±IR spectra indicate that the material does not react upon heating. It is stable between 473 and 613 K, which corresponds to the temperature range between dehydration and decomposition of the organic ligand.
It is worth noting that the analysis of the results using PLATON(Spek, 1990) suggested the existence of a unit cell (2) with half of the volume of the experimentally determined cell, (1). They are related by the following transformation matrix: [a,b,c]2 = [12,0,12/0,1,0/1,0,0][a,b,c]1. The dimensions of
the proposed smaller cell are:a= 8.323 (1),b= 7.478 (1),c= 8.893 (1) AÊ, = 105.92 (1), space group P2
1/m. PLATON
also indicated pseudo-B centering and the possibility of maintaining cell 1 but in space group P21/m. Solution and
re®nement of the structure in cell 1 using space groupsP21/m
andP2/mwere not successful.
A careful examination of the data set collected showed that 1125 out the 2446 unique re¯ections can only be accounted for using the larger cell. Even though the majority of the super-structure re¯ections are rather weak, 376 of those re¯ections haveFo> 4(Fo). It may be noticed that the majority of the
atoms haveycoordinates equal or close to1
4, and that only a
few of the O atoms are clearly removed from such a position. After the reduction of the unit cell proposed byPLATON, the atoms withycoordinates equal or close to1
4are assigned to
special positions withy= 1
4. In the case of the Ba atom, the
value obtained for itsy-coordinate in the structure re®nement carried out in the larger cell [y = 0.24927 (2)] is statistically different from 1
4. Also, after the reduction, in order to
accommodate the number of O atoms in the small unit cell in general positions, they should have half of the occupancy factors indicated by the multiplicity of their positions.
Because of the substructure±superstructure relationship that exists between cells 1 and 2, we believe that the experi-mentally determined unit cell (cell 1) should used in order to properly describe the structure of the compound under study. Sub-superstructure relationships have been observed in other metal dicarboxylates, for example lithium hydrogen maleate dihydrate (DõÂaz de Delgadoet al., 1993).
Experimental
The title compound was prepared by reaction of mesaconic acid (C5H6O4) and BaCO3 in a 1:1 ratio in water. The mixture was maintained under continuous stirring for 24 h. The resulting solution was ®ltered and allowed to evaporate slowly at ambient temperature. After 2±3 weeks, colorless crystals suitable for X-ray analysis formed. The IR spectra were recorded from KBr discs, using a PE-1725X FT± IR spectrometer. IR (cmÿ1):(br, OÐH) 3495,(w, C C) 1627,(s, C Oasym) 1501, (s, C Osym) 1389±1335. In order to study the reactivity in the solid state, samples of approximately 80 mg of the material were placed in a reactor connected to a vacuum line and heated at 473, 543, 613 and 823 K. In each case, once the desired temperature was reached, the temperature was kept constant for 10 min. Thermogravimetric analyses (TG and DTG) and differential scanning calorimetry measurements (DSC) were performed in a Dupont 951 Thermal Analyzer and a Dupont 990 cell, under a dynamic dry nitrogen atmosphere at a ¯ow rate of 50 ml sÿ1and a heating rate of 20 K minÿ1. The temperature range was 298±873 K. TGA and DSC data: weight loss to step 1: 21.29% (calculated 21.34%), 343±443 K,endo; step 2: 20.20% (calculated 20.16%), 673± 783 K,endo.
Crystal data
[Ba(C5H4O4)(H2O)4]
Mr= 337.49 Monoclinic,P21=n a= 8.893 (1) AÊ
b= 7.478 (1) AÊ
c= 16.582 (2) AÊ = 105.13 (1)
V= 1064.5 (2) AÊ3
Z= 4
Dx= 2.106 Mg mÿ3
Dm= 2.07 (2) Mg mÿ3
Dmmeasured by neutral buoyancy in CHCl3/CH3I
MoKradiation Cell parameters from 25
re¯ections = 20.0±35.0 = 3.75 mmÿ1
T= 293 (2) K Prism, colorless 0.600.350.28 mm
Data collection
NicoletP3/F(Crystal Logic) diffractometer
±2scans
Absorption correction: scan (Northet al., 1968)
Tmin= 0.220,Tmax= 0.350 4858 measured re¯ections 2446 independent re¯ections 1478 re¯ections withI> 2(I)
Rint= 0.026 max= 27.5
h=ÿ11!11
k=ÿ9!9
l= 0!21
3 standard re¯ections every 97 re¯ections intensity decay: <0.5%
Re®nement
Re®nement onF2
R[F2> 2(F2)] = 0.046
wR(F2) = 0.133
S= 1.18 2446 re¯ections 129 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0728P)2 + 0.0708P]
whereP= (Fo2+ 2Fc2)/3 (/)max= 0.001
max= 2.06 e AÊÿ3 min=ÿ2.88 e AÊÿ3
Extinction correction:SHELXL97 Extinction coef®cient: 0.045 (2)
Figure 3
Table 1
Selected geometric parameters (AÊ,).
Ba1ÐO3i 2.683 (3)
Ba1ÐO4ii 2.685 (3)
Ba1ÐO2 2.796 (4)
Ba1ÐO1W 2.838 (4)
Ba1ÐO3iii 2.853 (3)
Ba1ÐO4iii 2.855 (3)
Ba1ÐO2W 2.861 (3)
Ba1ÐO3W 2.862 (3)
Ba1ÐO4W 2.967 (4)
C1ÐO2 1.250 (6)
C1ÐO1 1.278 (6)
C1ÐC2 1.495 (7)
C2ÐC3 1.352 (6)
C2ÐC5 1.523 (6)
C3ÐC4 1.492 (5)
C4ÐO4 1.248 (4)
C4ÐO3 1.253 (4)
O2ÐC1ÐO1 122.8 (4)
O2ÐC1ÐC2 117.1 (4)
O1ÐC1ÐC2 120.1 (4)
C3ÐC2ÐC1 121.1 (4)
C3ÐC2ÐC5 120.6 (5)
C1ÐC2ÐC5 118.2 (5)
C2ÐC3ÐC4 121.6 (4)
O4ÐC4ÐO3 122.4 (4)
O4ÐC4ÐC3 119.0 (3)
O3ÐC4ÐC3 118.6 (3)
Symmetry codes: (i) 1ÿx;ÿy;ÿz; (ii) 1ÿx;1ÿy;ÿz; (iii)xÿ1
2;12ÿy;12z.
Two unassigned extremes of residual density, positive 2.06 e AÊÿ3 (0.17, 0.25, 0.20) and negativeÿ2.88 e AÊÿ3(0.13, 0.25, 0.12), were observed approximately 0.7 and 0.9 AÊ from atom Ba1. The water H atoms were not located; all other H atoms were re®ned as riding.
Data collection:COLLECTinUCLA Crystallographic Package (Strouse, 1988); cell re®nement:LEASTin UCLA Crystallographic Package; data reduction: REDUCE in UCLA Crystallographic Package; program(s) used to solve structure:SHELXS97 (Sheldrick, 1997); program(s) used to re®ne structure:SHELXL97 (Sheldrick, 1997); molecular graphics: DIAMOND(Brandenburg, 1996±1998); software used to prepare material for publication:SHELXL97 and PLATON(Spek, 1990).
This work was possible thanks to grants LAB-98000821 and S1-95000398 from FONACIT-Venezuela. AB is grateful for support from the Instituto Venezolano de Investigaciones Cienti®cas (IVIC) and FONACIT.
References
Allen, F. H. & Kennard, O. (1993).Chem. Des. Autom. News,8, 1, 31±37. Brandenburg, K. (1996±1998). DIAMOND. Version 2.0h. Crystal Impact
GbR, Bonn, Germany.
BricenÄo, A., DõÂaz de Delgado, G., RamõÂrez, B., VelaÂzquez, W. O. & Bahsas, A. (1999).J. Chem. Crystallogr.29, 785±791.
Case, C. B. & Foxman, B. M. (1994).Inorg. Chim. Acta,222, 239±343. DõÂaz de Delgado, G., Wheeler, K. A., Snider, B. B. & Foxman, B. M. (1991).
Angew. Chem. Int. Ed. Engl.30, 420±422.
DõÂaz de Delgado, G., Mora, A. J. & Delgado, J. M. (1993).Acta Cryst.A49 (Suppl.), 233.
Gupta, M. P. & Yadav, S. R. P. (1975).Z. Kristallogr.141, 151.
North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968).Acta Cryst.A24, 351± 359.
Odani, T., Matsumoto, A., Sada, K. & Miyata, M. (2001).Chem. Commun.pp. 2004±2005.
Sheldrick, G. M. (1997). SHELXL97 and SHELXS97. University of GoÈttingen, Germany.
Spek, A. L. (1990).Acta Cryst.A46, C-34.
Strouse, C. E. (1988). UCLA Crystallographic Package. University of California, Los Angeles, USA.
Vela, M. J., Buchholz, V., Enkelmann, V., Snider, B. B. & Foxman, B. M. (2000).
Chem. Commun.pp. 2225±2226.
Xiao, J., Yang, M., Lauher, J. W. & Fowler, F. W. (2000).Angew. Chem. Int. Ed. Engl.39, 2132±2135.
Acta Cryst.(2002). E58, m602±m605 Alexander BricenÄoet al. [Ba(C5H4O4)(H2O)4]
m605
supporting information
sup-1
Acta Cryst. (2002). E58, m602–m605supporting information
Acta Cryst. (2002). E58, m602–m605 [doi:10.1107/S1600536802017099]
The two-dimensional coordination polymer tetraaquamesaconatobarium(II),
[Ba(C
5H
4O
4)(OH
2)
4]
nAlexander Briceño, José Miguel Delgado and Graciela Díaz de Delgado
S1. Comment
Unsaturated carboxylic acids and their derivatives display interesting reactivity patterns when they are heated and/or
irradiated in the solid state (Díaz de Delgado et al., 1991; Case et al., 1994; Vela et al., 2000; Xiao et al., 2000; Odani et
al., 2001). The nature of the reactions in the solid state is determined by the control that the crystalline lattice exerts. In general, the preorganization of molecules and the orientation of double bonds in the crystal determine the nature of the
reaction products. For example, we have reported that when heating barium hydrogen itaconate monohydrate at about
473 K, an unusual isomerization to barium citraconate in the solid state occurs as a consequence of the arrangement of
the molecules in the starting material (Briceño et al., 1999).
In this work, we report the X-ray diffraction study of barium mesaconate tetrahydrate, (I), and its thermal behavior. This
study is part of a systematic investigation on the structure–reactivity relationships in the solid state of metal complexes of
α,β-unsaturated carboxylic acids. In spite of the simplicity of mesaconic acid (methylmaleic acid), and of its similarity with maleic and fumaric acids (cis and trans butenedioic acids, respectively), the structural chemistry of its salts and
complexes is unknown. Only the structure of potassium mesaconate (Gupta & Yadav, 1975) has been reported, as
indicated by a search in the Cambridge Structural Database (Allen & Kennard, 1993).
In the structure of (I), the Ba atoms are coordinated to nine O atoms, as shown in Fig. 1. Five of these O atoms come
from four different mesaconate anions and four O atoms come from water molecules. The Ba—O distances range from
2.683 (3) to 2.967 (4) Å. The disposition of the O atoms around the Ba atom can be described as a distorted monocapped
rectangular antiprism. Edge-sharing polyhedra form unidimensional zigzag chains along the b axis, which are linked
through bridging bidendate mesaconate anions (Fig. 2). The carboxylate groups display different coordination modes.
The O1/C1/O2 carboxylate acts as monodentade group through the O2 atom, while O3/C4/O4 displays a combination of
symmetrical chelating and monoatomic anti–anti bridging modes, allowing the coordination to three different Ba atoms.
The dihedral angle between the planes containing the carboxylate groups is approximately 89.8 (1)°. These coordination
modes induce self-assembly of the chains of barium polyhedra with the mesaconate anions, as well as their arrangement
in a parallel and alternate head-to-tail fashion, and produce two-dimensional rectangular grid layers parallel to the bc
plane (Fig. 3). The polymer layers interact through extensive hydrogen bonds between water molecules and carboxylate
O atoms: O1W···O3Wi 2.836 (3) Å, O1W···O2Wii 2.833 (2) Å and O2W···O1iii 2.807 (3) Å [symmetry codes: (i) 1/2 − x,
1/2 + y, 1/2 − z; (ii) 1/2 − x, −1/2 + y, 1/2 − z; (iii) −1 + x, y, z], among others, along the c direction.
The disposition of the double bonds in neighboring molecules is parallel, and a series of infinite contacts along the b
direction is observed. An analysis of the distances among such contacts indicates that there exist a group of infinite
contacts between the atoms C2···C2iv [symmetry code: (iv) −x + 1, −y + 1, −z + 1] along this direction at a distance of
supporting information
sup-2
Acta Cryst. (2002). E58, m602–m605DSC) and FT–IR spectra indicate that the material does not react upon heating. It is stable between 473 and 613 K, which
corresponds to the temperature range between dehydration and decomposition of the organic ligand.
It is worth noting that the analysis of the results using PLATON (Spek, 1990) suggested the existence of a unit cell (2)
with half of the volume of the experimentally determined cell, (1). They are related by the following transformation
matrix: [a,b,c]2 = [1/2,0,1/2/0,1,0/-1,0,0][a,b,c]1. The dimensions of the proposed smaller cell are: a = 8.323 (1), b =
7.478 (1), c = 8.893 (1) Å, β = 105.92 (1)°, space group P21/m. PLATON also indicated pseudo-B centering and the
possibility of maintaining cell 1 but in space group P21/m. Solution and refinement of the structure in cell 1 using space
groups P21/m and P2/m were not successful.
A careful examination of the data set collected showed that 1125 out the 2446 unique reflections, can only be accounted
for using the larger cell. Even though the majority of the superstructure reflections are rather weak, 376 of those
reflections have Fo > 4σFo. It may be noticed that the majority of the atoms have y coordinates equal or close to 1/4, and
that only a few of the O atoms are clearly distant from such a position. After the reduction of the unit cell proposed by
PLATON, the atoms with y coordinates equal or close to 1/4 are assigned to special positions with y = 1/4. In the ase of the Ba atom, the value obtained for its y-coordinate in the structure refinement carried out in the larger cell [y =
0.24927 (2)] is statistically different from 1/4. Also, after the reduction, in order to accomodate the number of O atoms in
the small unit cell in general positions, they should have half of the occupancy factors indicated by the multiplicity of
their positions.
Because of the substructure–superstructure relationship that exists between cells 1 and 2, we believe that the
experimentally determined unit cell (cell 1) should used in order to properly describe the structure of the compound
under study. Sub-superstructure relationships have been observed in other metal dicarboxylates, for example lithium
hydrogen maleate dihydrate (Díaz de Delgado et al., 1993).
S2. Experimental
The title compound was prepared by reaction of mesaconic acid (C5H6O4) and BaCO3 in a 1:1 ratio in water. The mixture
was maintained under continuous stirring for 24 h. The resulting solution was filtered and allowed to evaporate slowly at
ambient temperature. After 2–3 weeks, colorless crystals suitable for X-ray analysis formed. The IR spectra were
recorded from KBr discs, using a PE-1725X FT—IR spectrometer. IR (cm−1): υ(br, O—H) 3495, υ(w, C═C) 1627, υ(s,
C═Oasym) 1501, υ(s, C═Osym) 1389–1335. In order to study the reactivity in solid state, samples of approximately 80 mg
of the material were placed in a reactor connected to a vacuum line an heated at 473, 543, 613 and 823 K. In each case,
once the desired temperature was reached, the temperature was kept constant for 10 min. Thermogravimetric analyses
(TG and DTG) and differential scanning calorimetry measurements (DSC) were perfomed in a Dupont 951 Thermal
Analyzer and a Dupont 990 cell, under a dynamic dry nitrogen atmosphere at a flow rate of 50 ml seg−1 and a heating rate
of 20 K min−1. The temperatue range was 298–873 K. TGA and DSC data: weight loss to step 1: 21.29% (calculated
21.34%), 343–443 K, endo; step 2: 20.20% (calculated 20.16%), 673–783 K, endo.
S3. Refinement
Two unassigned extremes of residual density, positive 2.06 e/A3 (0.17, 1/4, 1/5) and negative −2.88 e/A3 (0.13, 1/4, 0.12),
supporting information
[image:7.610.177.436.70.522.2]sup-3
Acta Cryst. (2002). E58, m602–m605Figure 1
Coordination enviroment of the Ba atom in [Ba(C5H4O4)(OH2)4]n. Displacement ellipsoids are shown at the 50%
supporting information
[image:8.610.163.453.68.518.2]sup-4
Acta Cryst. (2002). E58, m602–m605Figure 2
supporting information
[image:9.610.127.485.69.394.2]sup-5
Acta Cryst. (2002). E58, m602–m605Figure 3
Projection of the structure of [Ba(C5H4O4)(OH2)4]n onto the bc plane.
tetraaquomesaconatebarium(II)
Crystal data
[Ba(C5H4O4)(H2O)4]
Mr = 337.49 Monoclinic, P21/n
Hall symbol: -P 2yn
a = 8.893 (1) Å
b = 7.478 (1) Å
c = 16.582 (2) Å
β = 105.13 (1)°
V = 1064.5 (2) Å3
Z = 4
F(000) = 648
Dx = 2.106 Mg m−3
Dm = 2.07 (2) Mg m−3
Dm measured by Neutral buoyancy in
CHCl3/CH3I
Mo Kα radiation, λ = 0.71070 Å Cell parameters from 25 reflections
θ = 20.0–35.0°
µ = 3.75 mm−1
T = 293 K Prism, colorless 0.60 × 0.35 × 0.28 mm
Data collection
Nicolet P3/F (Crystal Logic) diffractometer
Radiation source: normal-focus sealed tube Graphite monochromator
τ–2τ scans
Absorption correction: ψ scan (North et al., 1968)
Tmin = 0.220, Tmax = 0.350
4858 measured reflections 2446 independent reflections 1478 reflections with I > 2σ(I)
Rint = 0.026
θmax = 27.5°, θmin = 2.4°
supporting information
sup-6
Acta Cryst. (2002). E58, m602–m605k = −9→9
l = 0→21
3 standard reflections every 97 reflections intensity decay: <0.5
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.046
wR(F2) = 0.133
S = 1.18 2446 reflections 129 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(F
o2) + (0.0728P)2 + 0.0708P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 2.06 e Å−3
Δρmin = −2.88 e Å−3
Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4
Extinction coefficient: 0.045 (2)
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Ba1 0.21293 (2) 0.249273 (16) 0.166617 (10) 0.0224 (2)
C1 0.5437 (5) 0.2496 (3) 0.0509 (3) 0.0286 (8)
C2 0.5626 (6) 0.2498 (3) −0.0361 (3) 0.0321 (9)
C3 0.7056 (5) 0.2502 (3) −0.0504 (3) 0.0286 (8)
H3 0.7933 0.2500 −0.0052 0.034*
C4 0.7258 (5) 0.2509 (3) −0.1369 (2) 0.0268 (8)
C5 0.4164 (7) 0.2510 (5) −0.1085 (4) 0.057 (2)
H5A 0.4454 0.2471 −0.1604 0.074*
H5B 0.3581 0.3581 −0.1063 0.074*
H5C 0.3537 0.1486 −0.1046 0.074*
O1 0.6636 (5) 0.2501 (2) 0.1134 (3) 0.0380 (10)
O2 0.4076 (5) 0.2492 (2) 0.0587 (3) 0.0384 (10)
O3 0.7329 (4) 0.1041 (4) −0.17209 (17) 0.0386 (7)
O4 0.7329 (4) 0.3971 (4) −0.17193 (17) 0.0391 (7)
O1W 0.5357 (4) 0.2496 (2) 0.2489 (2) 0.0351 (7)
O2W −0.0654 (3) 0.4583 (5) 0.1388 (2) 0.0471 (8)
O3W −0.0659 (3) 0.0409 (4) 0.1385 (2) 0.0446 (8)
supporting information
sup-7
Acta Cryst. (2002). E58, m602–m605Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Ba1 0.0237 (3) 0.0231 (3) 0.0207 (3) 0.00033 (7) 0.00595 (14) 0.00038 (6)
C1 0.0261 (18) 0.035 (2) 0.026 (2) −0.0022 (12) 0.0098 (15) −0.0018 (11)
C2 0.027 (2) 0.043 (2) 0.027 (2) −0.0024 (13) 0.0090 (17) −0.0008 (12)
C3 0.033 (2) 0.030 (2) 0.0236 (17) 0.0010 (12) 0.0095 (15) 0.0001 (10)
C4 0.0279 (19) 0.0275 (19) 0.0262 (18) 0.0001 (11) 0.0089 (15) −0.0006 (10)
C5 0.031 (3) 0.109 (6) 0.029 (3) −0.0013 (19) 0.006 (2) −0.0004 (17)
O1 0.0252 (19) 0.059 (3) 0.0308 (19) 0.0013 (10) 0.0098 (15) −0.0009 (9)
O2 0.0265 (19) 0.063 (3) 0.0277 (19) −0.0013 (10) 0.0106 (16) 0.0015 (9)
O3 0.064 (2) 0.0233 (13) 0.0357 (14) 0.0038 (13) 0.0254 (13) −0.0012 (10)
O4 0.064 (2) 0.0234 (13) 0.0376 (14) −0.0031 (13) 0.0259 (14) 0.0012 (10)
O1W 0.0333 (17) 0.0457 (19) 0.0266 (16) 0.0013 (9) 0.0084 (13) 0.0015 (8)
O2W 0.0363 (16) 0.0426 (17) 0.059 (2) −0.0006 (13) 0.0071 (14) −0.0077 (14)
O3W 0.0240 (14) 0.0429 (17) 0.064 (2) 0.0004 (12) 0.0069 (13) 0.0095 (14)
O4W 0.0342 (18) 0.077 (3) 0.0333 (17) 0.0012 (11) 0.0089 (14) 0.0005 (10)
Geometric parameters (Å, º)
Ba1—O3i 2.683 (3) C2—C3 1.352 (6)
Ba1—O4ii 2.685 (3) C2—C5 1.523 (6)
Ba1—O2 2.796 (4) C3—C4 1.492 (5)
Ba1—O1W 2.838 (4) C3—H3 0.9300
Ba1—O3iii 2.853 (3) C4—O4 1.248 (4)
Ba1—O4iii 2.855 (3) C4—O3 1.253 (4)
Ba1—O2W 2.861 (3) C4—Ba1vi 3.231 (4)
Ba1—O3W 2.862 (3) C5—H5A 0.9600
Ba1—O4W 2.967 (4) C5—H5B 0.9600
Ba1—C4iii 3.231 (4) C5—H5C 0.9600
Ba1—Ba1iv 4.5946 (5) O3—Ba1i 2.683 (3)
Ba1—Ba1v 4.5946 (5) O3—Ba1vi 2.853 (3)
C1—O2 1.250 (6) O4—Ba1ii 2.685 (3)
C1—O1 1.278 (6) O4—Ba1vi 2.855 (3)
C1—C2 1.495 (7)
O3i—Ba1—O4ii 160.00 (15) O2—Ba1—Ba1iv 111.71 (6)
O3i—Ba1—O2 83.44 (8) O1W—Ba1—Ba1iv 75.03 (5)
O4ii—Ba1—O2 83.43 (8) O3iii—Ba1—Ba1iv 32.75 (5)
O3i—Ba1—O1W 80.48 (9) O4iii—Ba1—Ba1iv 77.50 (6)
O4ii—Ba1—O1W 80.40 (9) O2W—Ba1—Ba1iv 68.57 (6)
O2—Ba1—O1W 65.80 (11) O3W—Ba1—Ba1iv 121.55 (7)
O3i—Ba1—O3iii 112.34 (6) O4W—Ba1—Ba1iv 125.23 (4)
O4ii—Ba1—O3iii 67.91 (8) C4iii—Ba1—Ba1iv 55.35 (4)
O2—Ba1—O3iii 134.64 (11) O3i—Ba1—Ba1v 35.11 (5)
O1W—Ba1—O3iii 75.00 (10) O4ii—Ba1—Ba1v 141.75 (6)
O3i—Ba1—O4iii 67.91 (8) O2—Ba1—Ba1v 111.68 (6)
supporting information
sup-8
Acta Cryst. (2002). E58, m602–m605O2—Ba1—O4iii 134.64 (11) O3iii—Ba1—Ba1v 77.55 (6)
O1W—Ba1—O4iii 75.04 (10) O4iii—Ba1—Ba1v 32.80 (5)
O3iii—Ba1—O4iii 45.15 (10) O2W—Ba1—Ba1v 121.58 (7)
O3i—Ba1—O2W 133.12 (11) O3W—Ba1—Ba1v 68.73 (6)
O4ii—Ba1—O2W 66.89 (10) O4W—Ba1—Ba1v 125.61 (4)
O2—Ba1—O2W 123.45 (10) C4iii—Ba1—Ba1v 55.31 (4)
O1W—Ba1—O2W 143.20 (8) Ba1iv—Ba1—Ba1v 108.935 (12)
O3iii—Ba1—O2W 77.34 (10) O2—C1—O1 122.8 (4)
O4iii—Ba1—O2W 101.55 (10) O2—C1—C2 117.1 (4)
O3i—Ba1—O3W 67.03 (10) O1—C1—C2 120.1 (4)
O4ii—Ba1—O3W 132.98 (11) C3—C2—C1 121.1 (4)
O2—Ba1—O3W 123.41 (10) C3—C2—C5 120.6 (5)
O1W—Ba1—O3W 143.46 (8) C1—C2—C5 118.2 (5)
O3iii—Ba1—O3W 101.61 (10) C2—C3—C4 121.6 (4)
O4iii—Ba1—O3W 77.49 (10) C2—C3—H3 119.2
O2W—Ba1—O3W 66.09 (12) C4—C3—H3 119.2
O3i—Ba1—O4W 92.55 (7) O4—C4—O3 122.4 (4)
O4ii—Ba1—O4W 92.12 (7) O4—C4—C3 119.0 (3)
O2—Ba1—O4W 54.75 (12) O3—C4—C3 118.6 (3)
O1W—Ba1—O4W 120.54 (10) O4—C4—Ba1vi 61.5 (2)
O3iii—Ba1—O4W 153.30 (8) O3—C4—Ba1vi 61.5 (2)
O4iii—Ba1—O4W 153.67 (8) C3—C4—Ba1vi 171.4 (3)
O2W—Ba1—O4W 78.53 (9) C2—C5—H5A 109.5
O3W—Ba1—O4W 78.64 (9) C2—C5—H5B 109.5
O3i—Ba1—C4iii 90.39 (7) H5A—C5—H5B 109.5
O4ii—Ba1—C4iii 90.48 (7) C2—C5—H5C 109.5
O2—Ba1—C4iii 141.32 (13) H5A—C5—H5C 109.5
O1W—Ba1—C4iii 75.52 (10) H5B—C5—H5C 109.5
O3iii—Ba1—C4iii 22.69 (6) C1—O2—Ba1 147.6 (4)
O4iii—Ba1—C4iii 22.60 (6) C4—O3—Ba1i 151.4 (2)
O2W—Ba1—C4iii 87.95 (10) C4—O3—Ba1vi 95.9 (2)
O3W—Ba1—C4iii 88.01 (10) Ba1i—O3—Ba1vi 112.14 (9)
O4W—Ba1—C4iii 163.93 (12) C4—O4—Ba1ii 151.4 (2)
O3i—Ba1—Ba1iv 141.70 (6) C4—O4—Ba1vi 95.9 (2)
O4ii—Ba1—Ba1iv 35.16 (6) Ba1ii—O4—Ba1vi 112.03 (9)