GC-MS ANALYSIS OF
ALBIZIA LEBBECK
BENTH
Md. Nazneen Bobby1, Wesely Edward Gnanaraj2, Johnson Marimuthu Alias Antonysamy3*, Anto Arockia Raj Adaikalam4, Vinnarasi Jamesraj4
1
Research and Development Department, Bharathiyar University, Coimbatore – 641 046,
Tamil Nadu, India.
2
Department of Botany, Arignar Anna Government Arts College, Namakkal – 637 002,
Tamil Nadu, India.
3
Centre for Plant Biotechnology, St. Xavier’s College (Autonomous), Palayamkottai, Tamil
Nadu, India – 627 002.
4
Department of Chemistry, St. Xavier’s College (Autonomous), Palayamkottai, Tamil Nadu,
India – 627 002.
ABSTRACT
Objective: The present study was aimed to characterize the chemical profile present in the petroleum ether, metahnolic, ethylacetate extract
of leaves of Albizia lebbeck Benth by using GCMS analysis and
predict their biological activity using PASS. Methods: 10 g powdered sample is soaked with 30 mL methanol, petroleum ether and ethyl
acetate overnight and filtered through an ash less filter paper with
sodium sulphate (2 g). The extract is concentrated to 1 mL by bubbling
nitrogen into the solution. 2 μL of the extracts of leaves of A. lebbeck
were employed for GC-MS analysis. The detection employed the NIST
Ver.2.0-Year 2005 library and Wiley library. The biological activities
are predicted based on Dr. Duke’s Phytochemical and Ethnobotanical
Databases and Pass prediction. Results: The petroleum ether and metahnolic, extract of A. lebbeck leaves revealed the presence of 23
different compounds whereas ethyl acetate extract of A. lebbeck showed the presence of 25
different compounds. PASS analysis predicted various kinds of biological properties for the
identified compounds. Conclusion: It paves the way for the development of several treatment regimens based on different extracts. Further work is needed to isolate and identify these
bioactive compounds.
Volume 4, Issue 11, 1284-1304. Research Article ISSN 2277– 7105
Article Received on 03 Sep 2015,
Revised on 23 Sep 2015, Accepted on 13 Oct 2015
*Correspondence for
Author
Johnson Marimuthu
Alias Antonisamy
Centre for Plant
Biotechnology, St. Xavier’s College
(Autonomous),
Palayamkottai, Tamil
KEYWORDS: GC-MS; PASS; Phytochemistry; Bio-efficacy.
INTRODUCTION
Since time immemorial, Indian traditional medicines (ITM) have been widely exercised to
prevent and cure human disease. Because of its effective therapeutic performance and less
side effects, the herbal based medicines have attracted considerable attention in the global
pharmaceuticals. Due to the momentous growth of the use of this herbal medicine, it is
essential to develop a high standard of quality control in identifying the herbals and produce a
tool to assess the active components in raw plant materials to guarantee the quality, identity,
reliability and accuracy.[1] In the ancient times, the quality of the plant based drugs was not an
issue when the herbal medicines were dispensed by the local medical practitioners for their
patients. However, the quality of the plant parts based medicine has taken front seat with the
commercialization of plant drugs. The quality of a plant product cannot be assured without
assuring the quality of the raw material. It is also required to ensure quality products are
in-process control, quality control of the finished product, good manufacturing practice (GMP)
controls and process validation.[2] The World Health Organization is playing an outstanding
role in the quality maintenance of plant based drugs. To identify the medicinal plants and
active principles of the plants / plant parts, chromatographic fingerprint techniques provide a
meaningful quality control method.[3] Due to their accuracy on the integral chemical
characterization of medicinal plants with a quantitative and qualitative degree of reliability
and focus on identifying and assessing the stability of the plants, it has been attracted more
and more people's attention.[4] ITM considers chromatography as an effective means to
control ITM quality with several published reports on chromatographic fingerprinting of
medicinal plants.[5-7] In the recent years, Gas Chromatography Mass Spectrometry (GC-MS)
is strongly recognized as a tool / method to identify the metabolites profiles /
phyto-constituents of medicinal plants.[8] Owing to the development of GC or LC/MS, analysis of
small amounts of chemicals has become easier and more cost-effective.[9]
Albizia lebbeck Benth is widely distributed in India and is also found in South Africa and
Australia. Traditionally, the barks are used in toothache and diseases of the gum. Decoction
of the leaves and barks are protective against bronchial asthma and other allergic disorders.
Barks and seeds are astringent and are given in piles and diarrhoea. Ethanolic and methanolic
extracts of pods possesses anti-protozoal, anti-fertility activity, hypoglycemic and anticancer
anti-ovulatory, anti-inflammatory, antimicrobial and anti-tubercular activities.[14-17] The plant also
contains saponins, macrocyclic alkaloids, anthraquinone glycosides, tannins, and flavonols.
The saponin constituents of Albizia so far described are echinocystic acid glycosides.[18, 19]
The Albizia saponins A, B and C were isolated from the barks of A. lebbeck.[20] The
tri-O-glycoside flavonols kaempferol and quercetin were identified from the leaves of A.
lebbeck.[21] Albizia hexoside, a new hexaglycosylated saponin was isolated from leaves of A.
lebbeck. [22] Misra et al.[23] isolated N-demethyl budmunchiamines from A. lebbeck seeds and
Maa et al [24] confirmed the tannin presence in A. lebbeck. Bobby et al.[25] reported the
alkaloids HPTLC profile of A. lebbeck. With this background, the present study was aimed to
characterize the chemical profile present in the petroleum ether, metahnolic, ethylacetate
extract of A. lebbeck leaves by using GCMS analysis and predicted their biological activity
using PASS.
MATERIALS AND METHODS
Collection and processing of plant material
Healthy, disease free plants of Albizia lebbeck Benth were collected from the natural habitats
at Rasipuram, Namakkal, Tamil Nadu, India. The samples were washed thoroughly in
running tap water to remove soil particles and adhered debris and finally washed with sterile
distilled water. Leaves were separately cut, shade dried, ground into fine powder and stored
in air tight polythene bags until use.
GC-MS analysis
10 g of each powdered sample is soaked with 30 mL methanol, petroleum ether and ethyl
acetate overnight and filtered through ash less filter paper with sodium sulphate (2 g). The
extract is concentrated to 1 mL by bubbling nitrogen into the solution. The extract contained
both polar and non-polar phytocomponents. 2 μL of the petroleum ether, metahnolic,
ethylacetate extract of leaves were employed for GC-MS analysis.[8] The Clarus 500 GC used
in the analysis employed a fused silica column packed with Elite-1 [100% dimethyl poly
siloxane, 30 nm × 0.25 nm ID × 1µm df] and the components were separated using Helium as
carrier gas at a constant flow of 1 ml/min. The 2 µL sample extract injected into the
instrument was detected by the Turbo gold mass detector (Perkin Elmer) with the aid of the
Turbo mass 5.1 software. During the 36th minute GC extraction process, the oven was
maintained at a temperature of 1100C with 2 minutes holding. The injector temperature was
500 MS, were also standardized (Inlet line temperature: 2000C; Source temperature: 2000C).
Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 45 to 450 Da.
The MS detection was completed in 36 minutes.
Identification of components
The relative percentage amount of each component was calculated by comparing its average
peak area to the total areas. The detection employed the NIST (National Institute of Standards
and Technology) Ver.2.0-Year 2005 library and Wiley library. Interpretation of GC-MS was
conducted using the database of NIST having more than 62,000 patterns. The spectrum of the
unknown component was compared with the spectrum of the known components stored in
the NIST library. The name, molecular weight and structure of the components of the test
materials were ascertained.
Biological activity Prediction
The biological activities are predicted based on Dr. Duke’s Phytochemical and
Ethnobotanical Databases and Pass prediction. Biological activity is the result of chemical
compound's interaction with biological entity. In clinical study, biological entity is
represented by human organism. In preclinical testing it is the experimental animals (in vivo)
and experimental models (in vitro). Biological activity depends on peculiarities of compound
(structure and physico-chemical properties), biological entity (species, sex, age, etc.), mode
of treatment (dose, route, etc.). Any biologically active compound reveals wide spectrum of
different effects. Some of them are useful in treatment of definite diseases but the others
cause various side and toxic effects. Total complex of activities caused by the compound in
biological entities is called the "biological activity spectrum of the substance". Biological
activity spectrum of a compound presents every activity despite of the difference in essential
conditions of its experimental determination. The biological activity spectrum of PASS is
designed according to the algorithm specified below: For the compound under prediction
structural descriptors are generated. For each activity the following values are calculated:
Then the results are validated and predicted. In case when the probabilities for more than 400
different activities are estimated simultaneously and the ideal training set should include all
referenced biologically active compounds from literature, the best estimate of prediction's
quality can be calculated by leave one out cross validation. Each of the compounds is
subsequently removed from the training set and the prediction of its activity spectrum is
carried out on the basis of the remaining part of the training set. The result is compared to the
known activity of a compound and the maximal error of prediction (MEP) is calculated
through all compounds and activities.
RESULTS
The results pertaining to GC-MS analysis leads to the identification of a number of
compounds from the GC fractions of the different parts leaf of methanolic extract of A.
lebbeck. These compounds were identified through mass spectrometry attached with GC.
These observations may be due to the nature of biological active components and the stronger
extraction capacity of ethanol could have produced a number of active constituents
responsible for antibacterial activity.
The petroleum ether extract of A. lebbeck leaves revealed the presence of 23 different
compounds (Table 1; Fig. 1). Of which, the major components were
(6'(2Chloroacetoxy)3'(2, 2dimethyl1, 3dioxolan4ylmethoxy) spiro (isobenzofuran 1(3H), 9' (9'H)xanthen)
-3-one [0.58%]; E-3, 4-Dinitro-3-hexene [0.86%]; Ricinoleic acid [0.83%]; 2-Hexadecen-1-ol,
3, 7, 11, 15-tetramethyl-, [R-[R*, R*-(E)]]- (CAS) [3.70%];
1-Cyclohexyl-5-methyl-1H-tetrazole [0.68%]; 1-(2-Hydroxyethoxy) tridecane [3.33%]; Heptane, 1,1-dichloro-(CAS)
[10.58%]; Furan, 2,5-dimethyl- (CAS) [0.99%]; N-[7-Methoxycarbonyl)heptyl]
phenanthridinium chloride [3.41%]; (1RS, 2RS, 3RS, 4RS)-1, 2:3, 4-Diepoxycyclopentane
[1.27%]; 4, 6, 8-Trimethyl-decanone [1.04%]; Isothiocyanatocyclohex-4-ene-1, 1, 2,
2-tetracarbonitrile [13.49%]; Hexadecane, 1-chloro- (CAS) [1.37%];
E/Z-4-Bromo-3-methyl-2-buten-1-ol [1.33%]; (meso)-3,4-dihydroxymethyl-3,4-dimethylhexane [0.57%];
(3S)-3,6-Dihydro-6-(t-butyl)-5,8-dimethoxy-1-vinyl-4, 7-diaza-spiro [2.5%]; octane[1.35%];
Hexacosane (CAS) [13.61%]; 1H-1, 2, 4-Triazole-3, 5-dicarbaldehyde [15.64%]; 3',
5-di-o-acetyl-2'-deoxy-2"-ethoxycarbonyl-8, 2'-methylene-cycloadenosine [0.67%]. The above said
compounds were analyzed using PASS prediction for their biological activity. The PASS
analysis predicted the biological activities were tabulated in table 1. Some of the important
antidiabetic, antihelmintic, antiseborrheic, immunostimulant, anticoagulant, insecticide and
are used in skin diseases treatment, urologic disorders treatment, sickle-cell anemia treatment,
alzheimer's disease treatment, alopecia treatment, gaucher disease treatment,
hepatoprotectant, antipruritic, ophthalmic drug, cytoprotectant, atherosclerosis treatment and
[image:6.595.36.564.234.734.2]menopausal disorders treatment (Table 1).
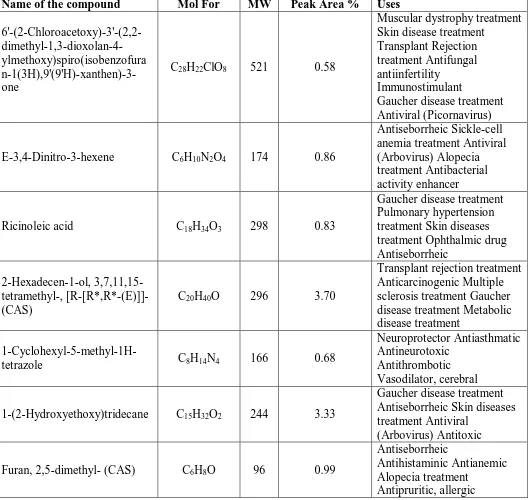
Table 1: Phytocomponents and biological activity of Albizia lebbeck leaves Petroleum ether extract.
Name of the compound Mol For MW Peak Area % Uses
6'-(2-Chloroacetoxy)-3'-(2,2- dimethyl-1,3-dioxolan-4-ylmethoxy)spiro(isobenzofura n-1(3H),9'(9'H)-xanthen)-3-one
C28H22ClO8 521 0.58
Muscular dystrophy treatment Skin disease treatment
Transplant Rejection treatment Antifungal antiinfertility
Immunostimulant
Gaucher disease treatment Antiviral (Picornavirus)
E-3,4-Dinitro-3-hexene C6H10N2O4 174 0.86
Antiseborrheic Sickle-cell anemia treatment Antiviral (Arbovirus) Alopecia treatment Antibacterial activity enhancer
Ricinoleic acid C18H34O3 298 0.83
Gaucher disease treatment Pulmonary hypertension treatment Skin diseases treatment Ophthalmic drug Antiseborrheic
2-Hexadecen-1-ol, 3,7,11,15-tetramethyl-, [R-[R*,R*-(E)]]- (CAS)
C20H40O 296 3.70
Transplant rejection treatment Anticarcinogenic Multiple sclerosis treatment Gaucher disease treatment Metabolic disease treatment
1-Cyclohexyl-5-methyl-1H-tetrazole C8H14N4 166 0.68
Neuroprotector Antiasthmatic Antineurotoxic
Antithrombotic Vasodilator, cerebral
1-(2-Hydroxyethoxy)tridecane C15H32O2 244 3.33
Gaucher disease treatment Antiseborrheic Skin diseases treatment Antiviral
(Arbovirus) Antitoxic
Furan, 2,5-dimethyl- (CAS) C6H8O 96 0.99
Antiseborrheic
Antihistaminic Antianemic Alopecia treatment
Heptane, 1,1-dichloro- (CAS) C7H14Cl2 168 10.58
Gaucher disease treatment Antiviral (Arbovirus) Skin diseases treatment
Antiprotozoal (Leishmania) Inflammatory Bowel disease treatment Antimutagenic
4,6,8-Trimethyl-2-decanone C13H26O 198 1.04
Antiseborrheic Multiple sclerosis treatment Autoimmune disorders treatment Vascular (periferal) disease treatment Gaucher disease treatment
N-[7-(Methoxycarbonyl)heptyl]phen anthridinium chloride
C22H26 ClNO2 371 3.41
Antinephritic Vasodilator, cerebral Pulmonary hypertension treatment Skin diseases treatment Multiple sclerosis treatment
(1RS,2RS,3RS,4RS)
-1,2,3,4-Diepoxycyclopentane C5H6O2 98 1.27
Antineoplastic, Gaucher disease treatment,
Antiseborrheic, Antismoking, Alopecia treatment,
Hexadecane, 1-chloro- (CAS) C16H33Cl 260 1.37
Antineoplastic antibiotic Cytoprotectant Antifungal Antihypoxic Antineoplastic
2-Isothiocyanatocyclohex-4-ene-1,1,2,2-tetracarbonitrile C11H5N5S 239 13.49
Alopecia treatment
Antischistosomal Antiviral (HIV) Skin diseases
treatment Immunostimulant
E/Z-4-Bromo-3-methyl-2-buten-1-ol C5H9BrO 164 1.33
Skin diseases treatment Antiviral (Arbovirus) Antiinflammatory,
ophthalmic Gaucher disease treatment Non mutagenic, Salmonella
(meso)-3,4-dihydroxymethyl-3,4-dimethylhexane C10H22O2 174 0.57
Antiseborrheic Gaucher disease treatment Skin diseases treatment Antiviral (Arbovirus) Antitoxic Antineurotoxic Antiepileptic Skeletal muscle relaxant
(3S)-3,6-Dihydro-6-(t-butyl)-
5,8-dimethoxy-1-vinyl-4,7-diaza-spiro[2.5]octane C14H22N2O2 250 1.35
Antineoplastic Anticarcinogenic
Transplant rejection treatment Muscular dystrophy treatment Antineurogenic pain
Antifungal Contraceptive
Hexacosane (CAS) C26H54 366 13.61
treatment Antineurotoxic Antimutagenic Alopecia treatment Multiple sclerosis treatment
1H-1,2,3,4-Triazole-3,5-dicarbaldehyde C4H3N3O2 125 15.64
Antianemic Sickle-cell anemia treatment, Alopecia treatment, Urologic disorders treatment, Antiviral
(Picornavirus), Antineurotoxic Cytoprotectant
3',5-di-o-acetyl-2'-deoxy-2"-
ethoxycarbonyl-8,2'-methylene-cycloadenos ine
C18H21N5O7 419 0.67
[image:8.595.42.558.63.556.2]Metabolic disease treatment Antineoplastic Antiviral (Picornavirus) Antiviral (Herpes) Immunostimulant Antineoplastic (colorectal cancer) Antileukemic
Fig. 1. GC Chromatogram of Albizia lebbeck leaves Petroleum ether extract.
Ethyl acetate extract of A. lebbeck leaves demonstrated the presence of 25 different
compounds. Among them, the major phytoconstituents were
(2R,3S)-2,3-dimethyl-2-vinylcyclobutanones[0.61%]; 3a,5,5,6,7a-Pentachlorotetrahydro
[1,3]dithiolo[4,5-b]dithiin-2-one [0.55%]; 3-t-Butylamino-3-cyclobuten-1,2-di[1,3]dithiolo[4,5-b]dithiin-2-one[1.38%];3-Methylene-5-methylhepten-
3-t-Butylamino-3-cyclobuten-1,2-dione[1.38%];3-Methylene-5-methylhepten-5-ol [1.97%]; Octadecanoic acid, methyl ester (CAS) [1.49%];
3-Neopentoxy-3-cyclobuten-1,2-dione [2.26%]; Heptane [2.07%];
(2S,2'R)-(2'-tert-Butyldimethylsilyloxy-3',3'-dimethyl-1'-pent-4'-enylideneamino)-2-(ethyl-methoxypropyl) pyrrolidine [0.72%];
1-Pentanol, 2-ethyl-4-methyl- (CAS) [9.57%]; (Z)-8-(Trimethylsilyl)-3,3-dimethyl-5-octenol
2-(1',4'-dimethoxy-9',10'-dioxo-5',6',7',8',9',10'-hexahydroanthracen-2'-yl)
methylene]butane-1,4-dioate [0.97%]; 1,2-D2-Imidazole [42%]; (æ,ü5-Pentamethyl-2,3-dihydro-1,3-diborolyl)
(ü5-pentamethylcyclopentadienylruthenium) (ü5-pentamethyl cyclopenta dienylruthenium
hydride) [2.92%]; 2,2-Dimethyl-propyl 2,2-dimethyl-propanesulfinyl sulfone [4.23%];
3,3-dimethyl-bicyclo [2.2.1] hept-2-acetaldehyde [0.77%]; 6,6-bis
(methylthio)-3,4-dimethylhex-5-en-2-one [0.89%]; Carvone Oxide [1.56%];
1-(2-Chloro-1-azaazulene-3-yl)-2,3-cis-diphenylcyclopropane isomer [1.12%]; 1,3-Dimethyl butylbenzene sulfonate [4.00%];
Furan,2,5-dimethyl-(CAS) [13.75%]; (5-Butyl-2,3,7,8,12,13,17,18-octaethylporphyrinato)
nickel (II) [0.47%]. The PASS analysis predicted the biological activities were illustrated in
table 2. Some of the key biological activities are anti-inflammatory, hepatoprotectant,
antipruritic, insecticide, antiviral, ophthalmic drug, cytoprotectant, antibacterial, antifungal,
antiprotozoal, antihelmintic, immunostimulant, anticoagulant, antiseborrheic, antidiabetic,
alopecia treatment, skin diseases treatment, urologic disorders treatment, sickle-cell anemia
treatment, alzheimer's disease treatment, gaucher disease treatment, atherosclerosis treatment
and menopausal disorders treatment (Table 2; Fig. 2).
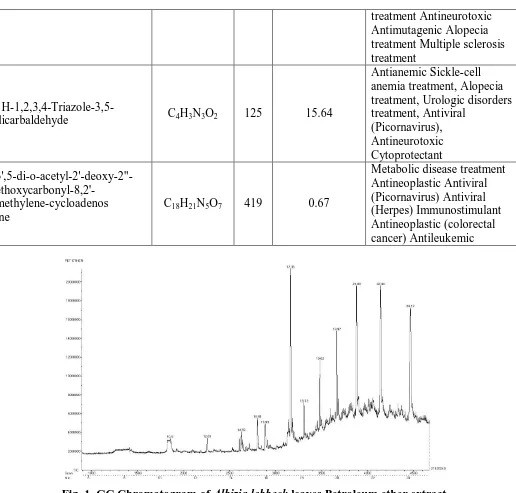
Table 2: Phytocomponents and biological activity of Albizia lebbeck leavesEthyl acetate extract.
Name of the compound Molecular Formula MW Peak Area % Uses
(2R,3S)-2,3-dimethyl-2-vinylcyclobutanones C8H12O 124
0.61
Antiseborrheic Opioid dependency treatment
Anticarcinogenic Antiinflammatory, ophthalmic Ophthalmic drug
3a,5,5,6,7a-Pentachlorotetrahydro[1,3]dithio lo[4,5-b]dithiin-2-one
C5HCl5OS4 380 0.55
Antipsoriatic
Antibacterial activity enhancer Alopecia treatment Antiviral (Arbovirus)
Antineurotoxic
3-t-Butylamino-3-cyclobuten-1,2-dione C8H11NO2 153 1.38
Urologic disorders treatment Urinary incontinence treatment Irritable Bowel
syndrome treatment Antiviral (Influenza) Alopecia treatment
3-Methylene-5-methylhepten-5-ol C9H16O 140 1.97
protector Skin diseases treatment Vascular (periferal) disease treatment Urologic disorders treatment
Octadecanoic acid, methyl ester
(CAS) C19H38O2 298 1.49
Antiseborrheic Gaucher disease treatment Skin diseases treatment Cytoprotectant Antiviral (Arbovirus)
3-Neopentoxy-3-cyclobuten-1,2-dione C9H12O3 168 2.26
Gout treatment Antiseborrheic Antiseborrheic Vascular (periferal) disease treatment Antipruritic, allergic (2S,2'R)-1-(2'-tert- Butyldimethylsilyloxy-3',3'-dimethyl-1'-pent-4'-enyli deneamino)-2-(1-ethyl-1-methoxypropyl)pyrrolidine
C23H46N2O2Si 410 0.72
Multiple sclerosis treatment Antineoplastic Autoimmune disorders treatment 1-Pentanol, 2-ethyl-4-methyl-
(CAS) C8H18O 130 9.57
Antiseborrheic Gaucher disease treatment Multiple sclerosis treatment Autoimmune disorders treatment Non mutagenic, Salmonella
(Z)-8-(Trimethylsilyl)-3,3-dimethyl-5-octenol C13H28OSi 228 0.57
Atherosclerosis
treatment Skin diseases treatment Sleep disorders treatment Cytoprotectant Immunostimulant 1,3-Di(7,7-dimethyl- bicyclo[2.2.1]heptan-1-yl)propan-2-one
C21H34O 302 43.67
Antiseborrheic Alopecia treatment Antiviral (Influenza) Opioid dependency treatment Antipruritic Dimethyl 2-(1',4'-dimethoxy- 9',10'-dioxo-5',6',7',8',9',10'- hexahydroanthracen-2'-yl)methylene]butane-1,4-dioate
C23H26O8 430 0.97
Pulmonary hypertension treatment Antiinflammatory, intestinal Antineoplastic (brain cancer) Antipruritic, allergic
Heptane C7H16 100 2.07
Antiseborrheic Antihypoxic
treatment (æ,ü5-Pentamethyl-2,3-dihydro- 1,3-diborolyl)(ü5-pentamethylcyclopent adienylruthenium)(ü5-pentamethylcyclopentadienylrut heniumhydride)
C28H46B2Ru2 608 2.92
Muscular dystrophy treatment Skeletal muscle relaxant Immunostimulant Sedative CNS active muscle relaxant
Dimethyl-propyl
2,2-dimethyl-propanesulfinyl sulfone C10H22O3S2 254 4.23
Antiseborrheic Antiprotozoal (Amoeba) Alopecia treatment Antiviral (Arbovirus) Cytoprotectant
3,3-dimethyl-bicyclo[2.2.1]hept-2-acetaldehyde C11H18O 166 0.77
Antiprotozoal (Toxoplasma) Opioid dependency treatment Antismoking Muscular dystrophy treatment Antiviral (Influenza)
6,6-bis(methylthio)-3,4-dimethylhex-5-en-2-one C10H18OS2 218 0.89
Mucomembranous protector Antiulcerative Antinephritic
Antiseborrheic Antitoxic
CARVONE OXIDE C10H14O2 166 1.56
Antineoplastic Antipsoriatic Antifungal Antipruritic, allergic 1-(2-Chloro-1-azaazulene-3-yl)-2,3-cis-diphenylcyclopropane isomer
C24H18ClN 355 1.12
Antineoplastic Antineoplastic antibiotic Cytoprotectant Antiseborrheic Menopausal disorders treatment 1,3-Dimethylbutyl
benzenesulfonate C12H18O3S 242 4.00
Gaucher disease treatment< Antiviral (Arbovirus) Antiviral (Picornavirus) Ophthalmic drug Antibacterial activity enhancer
Furan, 2,5-dimethyl- (CAS) C6H8O 96 13.75
Antiseborrheic
(5-Butyl-2,3,7,8,12,13,17,18-octaethylporphyrinato)nickel(II) C40H52N4Ni 646 0.47
Gaucher disease treatment Alopecia treatment
Antiinflammatory, intestinal Antihypoxic Cytoprotectant
1,2-D2-IMIDAZOLE. C3H2D2N2 68 1 0.42
[image:12.595.45.549.68.515.2]Antiseborrheic Cardioprotectant Sickle-cell anemia treatment Restenosis treatment Transplant rejection treatment
Fig. 2. GC Chromatogram of Albizia lebbeck leaves Ethyl acetate extract.
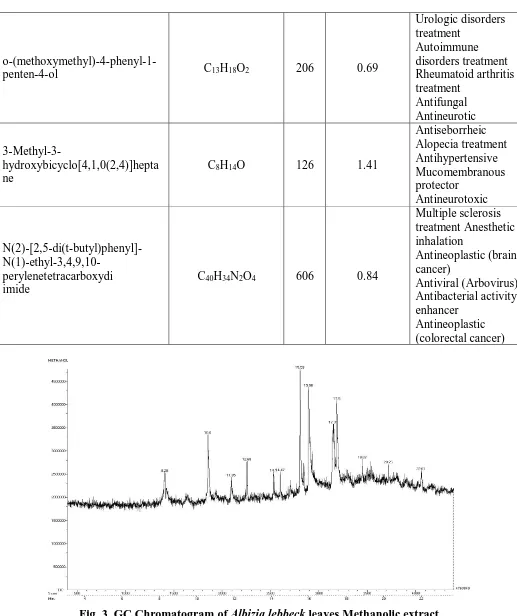
The methanolic extract of A. lebbeck leaves illustrated the presence of 23 different
compounds and the major compounds depicted were (1,1,1-Trifluoro-1,13-octadecyn-2-one
[0.49%]; Nonyl methyl ether [0.90%]; (1S,2R,3S,5R)-2,5-Dimethylbicyclo [3.2.0]
heptan-2,3-diol [1.22%]; 2-Propyldecan-1-ol [0.67%]; Hexacosane (CAS) [57.48%];
1-Hydroxy-1-allyl-3-methoxycyclohexane [0.94%]; 1-(2',3'-Dihydrofuran-2'-yl)nonan-1-one [1.51%];
Eicosane (CAS) [1.78%]; 3-Azido-1-[(tert-butyldiphenylsilyl)oxy]-5-tetradecene [0.51%];
tetracyclo [3.3.0(2,6).0(3,9)] decan-2-ol [0.90%]; RS-2,3-hexanediol [1.80%];
(S)-2-Methyldecan-1-ol [15.16%]; 2-methyl-3-(dimethylpropyl)-1,3-butadiene [0.68%];
2,2-Dimethyl-3-cyanocyclopropane-acetonitrile [0.51%]; Ethyl 2,2,4-Trimethyl-4-pentenoate
[1.65%]; N-(2-Thienyl)-N-methyl-à-(2'-thienethyl) amine [0.59%];
[2.285%];o-(methoxymethyl)-4-phenyl-1-penten-4-ol[0.69%]; 3-Methyl-3-hydroxybicyclo [4,1,0(2,4)%]
heptanes [1.41%]; N(2)-[2,5-di (t-butyl) phenyl]-N (1)-ethyl-3,4:9,10-perylenetetracarboxydi
imide [0.84%]. The PASS analysis predicted the biological activities were depicted in table 3.
Some of the main biological activities are antineoplastic, antiviral (HIV), antibacterial,
antifungal, antianemic, anti-inflammatory, antiseborrheic, antipruritic, antihelmintic,
cardioprotectant, cytoprotectant and are used in multiple sclerosis treatment, pulmonary
hypertension treatment, menopausal disorders treatment, gaucher disease treatment, skin
diseases treatment, sickle-cell anemia treatment, alopecia treatment and gynecological
disorders treatment (Table 3; Fig. 3).
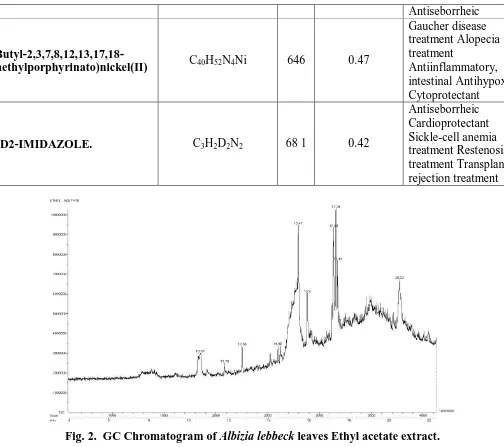
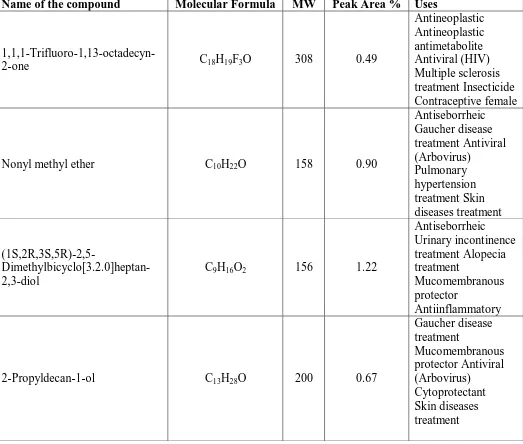
Table 3: Phytoconstituents and biological activity of Albizia lebbeck leavesmethanolic extract.
Name of the compound Molecular Formula MW Peak Area % Uses
1,1,1-Trifluoro-1,13-octadecyn-2-one C18H19F3O 308 0.49
Antineoplastic Antineoplastic antimetabolite Antiviral (HIV) Multiple sclerosis treatment Insecticide Contraceptive female
Nonyl methyl ether C10H22O 158 0.90
Antiseborrheic Gaucher disease treatment Antiviral (Arbovirus)
Pulmonary hypertension treatment Skin diseases treatment
(1S,2R,3S,5R)-2,5- Dimethylbicyclo[3.2.0]heptan-2,3-diol
C9H16O2 156 1.22
Antiseborrheic Urinary incontinence treatment Alopecia treatment
Mucomembranous protector
Antiinflammatory
2-Propyldecan-1-ol C13H28O 200 0.67
Gaucher disease treatment
[image:13.595.38.561.317.761.2]Hexacosane (CAS) C26H54 366 57.48
Gaucher disease treatment Antiseborrheic Pulmonary hypertension treatment Antiviral (Arbovirus) Skin diseases treatment
1-Hydroxy-1-allyl-3-methoxycyclohexane C10H18O2 170 0.94
Antineoplastic Menopausal disorders treatment Antiseborrheic Transplant rejection treatment
Anticarcinogenic
1-(2',3'-Dihydrofuran-2'-yl)nonan-1-one C13H22O2 210 1.51
Sickle-cell anemia treatment Gaucher disease treatment Cytoprotectant Antianemic
Eicosane (CAS) C20H42 282 1.78
Gaucher disease treatment Antiviral (Arbovirus) Skin diseases treatment Acidifying agent non gastric
Psychosexual dysfunction treatment
3-Azido-1-[(tert- butyldiphenylsilyl)oxy]-5-tetradecene
C30H45N3OSi 491 0.51
Multiple sclerosis treatment Gaucher disease treatment Skin diseases treatment Antiviral (Arbovirus) Antiviral
tetracyclo[3.3.0(2,6).0(3,9)]deca
n-2-ol C10H14O 150 0.90
Antiviral, Antihelmintic (Nematodes)
RS-2,3-hexanediol C6H14O2 118 1.80
Pulmonary hypertension treatment Gaucher disease treatment Antiviral (Arbovirus) Cytoprotectant Skin diseases treatment
(S)-2-Methyldecan-1-ol C11H24O 172 15.16
Skin diseases treatment
Sickle-cell anemia treatment
2-methyl-3-(2,2-dimethylpropyl)-1,3-butadiene C10H18 138 0.68
Antiseborrheic Alopecia treatment Anticarcinogenic Mucomembranous protector Antiviral (Arbovirus)
2,2-Dimethyl-3-cyanocyclopropane-acetonitrile C8H10N2 134 0.51
Cytoprotectant Alopecia treatment Menopausal
disorders treatment Opioid dependency treatment
Gynecological disorders treatment Antiviral
(Picornavirus)
Ethyl
2,2,4-Trimethyl-4-pentenoate C10H18O2 170 1.65
Antiseborrheic Skin diseases treatment Cytoprotectant Multiple sclerosis treatment
Metabolic disease treatment
N-(2-Thienyl)-N-methyl-à-(2'-thienethyl)amine C12H15NS2 237 0.59
Antiasthmatic
Antiviral (Arbovirus) Antiallergic
Ophthalmic drug Anesthetic general
2-Thiophenecarboxaldehyde
(CAS) C5H4OS 112 1.71
Amyotrophic lateral sclerosis treatment Antiviral (Arbovirus) Antianemic
Antifungal Antiviral (Picornavirus)
Methyl- 3-Butenyl Ether C5H10O 86 2.28
Prostatic (benign) hyperplasia treatment Antineoplastic (colorectal cancer) Pulmonary
o-(methoxymethyl)-4-phenyl-1-penten-4-ol C13H18O2 206 0.69
Urologic disorders treatment
Autoimmune disorders treatment Rheumatoid arthritis treatment
Antifungal Antineurotic
3-Methyl-3-hydroxybicyclo[4,1,0(2,4)]hepta ne
C8H14O 126 1.41
Antiseborrheic Alopecia treatment Antihypertensive Mucomembranous protector
Antineurotoxic
N(2)-[2,5-di(t-butyl)phenyl]-
N(1)-ethyl-3,4,9,10-perylenetetracarboxydi imide
C40H34N2O4 606 0.84
Multiple sclerosis treatment Anesthetic inhalation
Antineoplastic (brain cancer)
Antiviral (Arbovirus) Antibacterial activity enhancer
[image:16.595.37.554.60.676.2]Antineoplastic (colorectal cancer)
Fig. 3. GC Chromatogram of Albizia lebbeck leaves Methanolic extract.
DISCUSSION
Gas chromatography is a potent separation technique for gas and vapor mixtures. Merging the
including traces of compounds down to parts per trillions in some particular cases. The
significance of gas chromatography in quality control and process control in the chemical and
drug industry, in environmental pollution investigations and in clinical analysis is critical.[26]
In the recent years, Gas Chromatography Mass Spectrometry (GC-MS) is strongly recognized
as a tool to identify the chemical composition of medicinal plants.[27-29] In the present study
also we used the GC-MS analysis to know the chemical constituents of A. lebbeck leaves.
The result of GC-MS analysis revealed the chemcial composition of A. lebbeck leaves and the
results were tabulted in table 1-3. Any biologically active compound has a wide spectrum of
effects. Some of them are useful in the treatment for diseases, but the others cause various
toxic effects. The global research scenario recommends the use of virtual screening
techniques for the discovery of bioactive phytoconstituents. Computer-aided methods could
be extremely useful in pharmacological evaluation of NPs.[30] To analyze the biological and
toxic effects, there are many software programs are available on the internet. PASS is one of
the main programs to analyze the total complex activity of the compound on a biological
entity and biological activity spectrum of the substance.[31] The current version of PASS
predicts around 3750 pharmacological effects, biochemical mechanisms of action, specific
toxicities and metabolic terms on the basis of structural formulae of drug-like substances with
average accuracy of 95%. This can be further validated in in-vitro as well as in-vivo
assays.[32-33] A number of researchers applied the PASS prediction for their biological activity
analysis viz., Anti-tumor, anti-malarial, Anit-HIV and other.[34-42] In the present study also we
applied the PASS prediction for the predicted compounds using GC-MS analysis. Pa
(Probability to be active) and Pi (probability to be inactive) were estimates of probability for
the compound to be active and inactive respecitvely for each type of activity from the
biological activity spectrum. Their values vary from 0.0000 to 1.0000.
CONCLUSION
GC-MS analysis on petroleum ether, metahnolic, ethylacetate extract of A. lebbeck leaves
showed the existence of various compounds with different chemical structures. The
prediction of the biological activities was confirmed with the previous observations which
supplemented the traditional usage of A. lebbeck. Thus, the present study suggests that
methanolic extract is a potent therapeutic agent. It paves the way for the development of
several treatment regimens based on different extracts. Further work is needed to isolate and
CONFLICT OF INTEREST
We declare that we have no conflict of interest.
REFERENCES
1. WHO. General guidelines for methodologies on research and evaluation of traditional
medicine, WHO Consultation on Methodologies for Research and Evaluation of
Traditional Medicine, Hong Kong Special Administrative Region of China, 2000; 1-74.
2. Vasisht K. Quality Control of Medicinal and Aromatic Plants and Their Extracted
Products by HPLC and High Performance Thin Layer Chromatography In: Extraction
Technologies for Medicinal and Aromatic Plants Sukhdev Swami Handa, Suman Preet
Singh Khanuja, Gennaro Longo and Dev Dutt Rakesh (eds). International Centre for
Science and High Technology, Trieste, 2008.
3. Zafar M, Ahmad M, Khan MA, Shazia Sultana, Gul Jan, Ahmad F, Asma Jabeen, Shah
GM, Shabnum Shaheen, Shah A, Nazir A, Marwat SK. Chemotaxonomic clarification of
pharmaceutically important species of Cyperus L. African Journal of Pharmacy and
Pharmacology, 2011; 5(1): 67-75.
4. Gan F, Ye R. New approach on similarity analysis of chromatographic fingerprint of
herbal medicine. J. Chromatogr. A., 2006; 1104: 100-105.
5. Praveen Kumar P, Kumaravel S, Lalitha C. Screening of antioxidant activity, total
phenolics and GC-MS study of Vitex negundo. African Journal of Biochemistry
Research, 2010; 4(7): 191-195.
6. Hema R, Kumaravel S, Alagusundaram K. GC/MS Determination of Bioactive
Components of Murraya koenigii. Journal of American Science, 2011; 7(1): 80-83.
7. Maruthupandian A, Mohan VR. GC-MS analysis of some bioactive constituents of
Pterocarpus marsupium Roxb. International Journal of Chem Tech Research, 2011; 3(3):
1652-1657.
8. Merlin NJ, Parthasarathy V, Manavalan R, Kumaravel S. Chemical Investigation of
Aerial Parts of Gmelina asiatica Linn by GC-MS. Pharmacognosy Res., 2009; 1(3):
152-156.
9. Liebler DC, Burr JA, Philips L, Ham AJL. Gas chromatography-mass spec-trometry
analysis of vitamin E and its oxidation products. Anal. Biochem, 1996; 236: 27-34.
10.Watt JM, Breyer-Brandwijk MG. The Medicinal and Poisonous Plants of South and East
Africa, Second Edition, Livingstone, Edinburgh, 1962.
12.Gupta, Kachhawa JB, Chaudhary R. Anti-fertility effects of methanolic pod extract of
Albizia lebbeck Benth. in male rats. Asian J. Androl, 2004; 6(2): 155-159.
13.Gupta RS, Chaudhary R, Yadav RK, Verma SK, Dobhal MP. Effect of Saponins of
Albizia lebbeck Benth. bark on the reproductive system of male albino rats. J.
Ethnopharmacol, 2005; 96(1-2): 31-36.
14.Chintawar SD, Somani RS, Kasture VS, Kasture SB. Nootropic activity of Albizia
lebbeck in mice. J. Ethnopharmacol, 2002; 81(3): 299-305.
15.Pratibha N, Saxena VS, Amit A, D'Souza P, Bagchi M, Bagchi D. Anti-inflammatory
activities of Aller-7, a novel polyherbal formulation for allergic rhinitis. Int. J.
Tissue. React, 2004; 26(1-2): 43-51.
16.Rahul C, Pankaj P, Sarwan SK, Mahesh JK. Phytochemical screening and antimicrobial
activity of Albizzia lebbeck. J. Chem. Pharm. Res., 2010; 2(5): 476-484.
17.Kapoor BBS, Bhumika, Khatri JS. Antimicrobial activity of some medicinal tree species
of Hanumangarh district of Rajasthan. Journal of Phytological Research, 2007; 20:
325-326.
18.Carpani G, Orsini F, Sisti M, Verotta L. Saponin forms Albizia antheimintica.
Phytochemistry, 1989; 28: 863-866.
19.Orsini F, Pelizzoni F, Verotta L. Saponins from Albizia lucida. Phytochemistry, 1991;
30(12): 4111-4115.
20.Pal BC, Achari B, Yoshikawa K, Arihara S. Saponins from Albizia lebbeck.
Phytochemistry, 1995; 38(5): 1287-1291.
21.El-Mousallamy AMD. Leaf flavonoids of Albizia lebbeck. Phytochemistry, 1998; 48(4):
759-761.
22.Ueda M, Tokunaga T, Okazaki M, Sata NU, Ueda K, Yamamura S. Albiziahexoside: a
potential source of bioactive saponin from the leaves of Albizzia lebbeck. Nat. Prod. Res.,
2003; 17(5): 329-335.
23.Misra LN, Dixit AK, Wagner H. N-demethyl budmunchiamines from Albizia lebbeck
seeds. Phytochemistry, 1995; 39(1): 247-249.
24.Maa YT, Hsiaob SC, Chenb HF, Hsu FL. Tannins from Albizia lebbeck. Phytochemistry,
1997; 46(8): 1451-1452.
25.Nazneen Bobby MD, Wesely EG, Johnson M. High performance thin layer
chromatography profile studies on the alkaloids of Albizia lebbeck. Asian Pacific Journal
26.Guiochon G and Claude L. Guillemin Gas chromatography Rev. Sci. Instrum., 1990;
61(11): 3317 – 3339.
27.Maruthupandian A, Mohan VR. GC-MS analysis of some bioactive constituents of
Pterocarpus marsupium Roxb. International Journal of ChemTech Research, 2011; 3(3):
1652-1657.
28.Hema R, Kumaravel S, Alagusundaram K. GC/MS Determination of Bioactive
Components of Murraya koenigii. Journal of American Science, 2011; 7(1): 80-83.
29.Praveen Kumar P, Kumaravel S, Lalitha C. Screening of antioxidant activity, total
phenolics and GC-MS study of Vitex negundo. African Journal of Biochemistry
Research, 2010; 4(7): 191-195.
30.Rollinger JM, Schuster D, Danzl B, Schwaiger S, Markt P, Schmidtke M, Gertsch J,
Raduner S, Wolber G, Langer T, Stuppner H. In silico target fishing for rationalized
ligand discovery exemplified on constituents of Ruta graveolens. Planta Med, 2009; 75:
195–204.
31.Porovikov V, Filimonov D, Yu B, Lagunin A, Kos A. Robustness of Biological Activity
Spectra Predicting by Computer Program PASS for Non- Congeneric sets of chemical
compounds. J Chem Inform Comput Sci., 2000; 40: 1349-1355.
32.Filimonov DA, Poroikov VV Probabilistic approach in activity prediction. In: Varnek A,
Tropsha A (eds) Chemoinformatics approaches to virtual screening. RSC Publishing,
Cambridge, 2008; 182–216.
33.Poroikov VV, Filimonov DA, Gloriozova TA, Lagunin AA, Druzhilovsky DS,
Stepanchikova AV Computer-aided prediction of biological activity spectra for
substances: virtual chemogenomics. The Herald of Vavilov Society for Geneticists and
Breeding Scientists, 2009; 13: 137–143.
34.Dembitsky VM, Gloriozova TA, Poroikov VV Novel antitumor agents: marine sponge
alkaloids, their synthetic analogs and derivatives. Mini Rev Med Chem, 2005; 5:
319–336.
35.Azhaguraj R, Arockia Lenin E, Viswanathan C, Sangeetha B, Selvanayagam M.
Prediction Biological activity of Algal anti-tumour drugs using PASS.
Pharmacologyonline, 2010; 3: 22-34.
36.John De Britto A. Prediction of Biologicla activity of anti-malarial drugs using PASS.
Indian Journal of Multidisciplinary Research, 2008; 4(2): 271-274.
37.Maridass M, Raju G, Thangavel K, Ghanthikumar S. Prediction of anti-HIV activity of
38.Zotchev SB, Stepanchikova AV, Sergeyko AP, Sobolev BN, Filimonov DA, Poroikov
VV Rational design of macrolides by virtual screening of combinatorial libraries
generated through in silico manipulation of polyketide synthases. J Med Chem, 2006; 49:
2077–2087.
39.Maridass M. PASS: Prediction of activity spectra for biologically active constituents of
Polygodial (A drimane type of Dialdehyde Sesquiterpene) Indian Journal of
Multidisciplinary Research, 2008; 3: 191-197.
40.Abiya Chelliah D. Biological activity proediction of an ethnomedicinal plant
Cinnamomum camphora throough Bioinformatics. Ethnobotanical leaflets, 2008; 12:
181-190.
41.Azhaguraj R, John Milton MC, Ganesh J, Justin Zenith Kumar G, Ramakrishnan M,
Stalin Antony. Prediction of Biological activity Spectra for Secondary metabolites from
marine macroalgae Caulerpa spp (Chlorophyta – Caulerpales) International Research
Journal of Pharmacy, 2012; 3(5): 320-323.
42.John De Britto A, Leon Stephan Raj T, Abiya Chelliah D. Prediction of Biological
Activity Spectra for Few Anticancer Drugs Derived from Plant Sources Ethnobotanical