metal-organic papers
Acta Cryst.(2006). E62, m511–m512 doi:10.1107/S1600536806004740 Yinet al. [Fe(C
5H5)(C16H20N)]
m511
Acta Crystallographica Section E
Structure Reports Online
ISSN 1600-5368
N
-(1-Ferrocenylethyl)-
N
-(1-phenylethyl)-methylamine
Zhi-Gang Yin,* Heng-Yu Qian, Jia Jia and Chun-Xia Zhang
School of Materials & Chemical Engineering, Zhengzhou University of Light Industry, Zhengzhou 450002, People’s Republic of China
Correspondence e-mail: hengyuqian@yahoo.com
Key indicators
Single-crystal X-ray study
T= 291 K
Mean(C–C) = 0.008 A˚
Rfactor = 0.056
wRfactor = 0.130
Data-to-parameter ratio = 15.6
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
Received 4 January 2006 Accepted 8 February 2006
#2006 International Union of Crystallography All rights reserved
The title compound, [Fe(C5H5)(C16H20N)], was synthesized by
reductive methylation of the corresponding secondary amine. In the crystal structure, C—H interactions are observed between neighboring molecules.
Comment
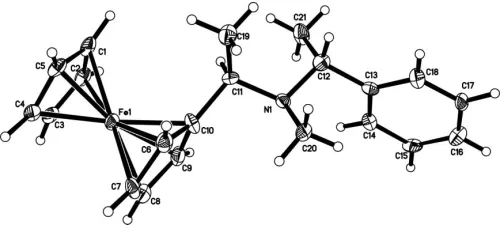
Ferrocenylalkylamine derivatives have been used in asym-metric catalytic hydrogenation (Hayashi et al., 1986), allylic substitution (Sawamura & Ito, 1992) and aldol reactions (Togni et al., 1996). The structure of 1-ferrocenyl-N -(1-phenylethyl)ethylamine (Qianet al., 2005) has been reported by our group. As part of our study of the cyclopalladation of tertiary amines, the crystal structure of the title compound, (I), is presented here.
The molecular structure of (I) is shown in Fig.1, and selected bond lengths and angles are given in Table 1. The cyclopentadienyl rings of the ferrocene moiety are parallel to each other, with a dihedral angle of 2.7 (3). Atom N1 is
coplanar with the C11/C12/C13 plane, the deviation being 0.022 (4) A˚ . Atoms C11 and N1 are displaced from the benzene plane by 1.308 (5) and 1.261 (4) A˚ , respectively.
In the crystal structure of (I), molecules are linked by C— H interactions [H7A Cg1i = 3.10 A˚ and C7— H7A Cg1i = 153, and H8A Cg2ii
= 3.14 A˚ and C8— H8A Cg2ii= 126, whereCg1 andCg2 are the centroids of
the C1-containing and C13-containing rings, respectively; symmetry codes: (i) 1x,1
2+y, 1z; (ii) 1x, 1
2+y,z].
Experimental
2983, 1615, 1484, 1446, 1120, 1000, 822;1H NMR (CDCl3):7.25–7.39
(m, 5H), 4.10–4.11 (m, 4H), 4.02 (s, 5H), 3.79 (dd, 1H,J= 6.8 Hz andJ= 12.9 Hz), 3.49 (dd, 1H,J= 6.4 Hz andJ= 12.6 Hz), 1.92 (s, 3H) 1.37 (d, 3H,J= 6.8 Hz), 1.24 (d, 3H, J= 6.4 Hz); Elemental analysis calculated for C21H25FeN: C 72.62, H 7.20, N 4.03%; found: C
72.38, H 7.49, N 4.13%.
Crystal data
[Fe(C5H5)(C16H20N)]
Mr= 347.27 Monoclinic,P21
a= 5.8833 (12) A˚
b= 10.748 (2) A˚
c= 14.191 (3) A˚ = 97.82 (3)
V= 889.0 (3) A˚3
Z= 2
Dx= 1.297 Mg m
3
MoKradiation Cell parameters from 714
reflections = 2.1–12.4
= 0.85 mm1
T= 291 (2) K Block, orange 0.200.180.16 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer ’and!scans
Absorption correction: multi-scan (SADABS; Bruker, 2000)
Tmin= 0.849,Tmax= 0.876
5026 measured reflections
3239 independent reflections 2928 reflections withI> 2(I)
Rint= 0.060 max= 26.0
h=7!7
k=13!13
l=17!17
Refinement
Refinement onF2 R[F2> 2(F2)] = 0.056
wR(F2) = 0.130
S= 1.07 3239 reflections 208 parameters
H-atom parameters constrained
w= 1/[2
(Fo2) + (0.0644P)2
+ 0.55P]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001
max= 0.33 e A˚
3 min=0.45 e A˚
3
Absolute structure: Flack (1983), 1394 Friedel Pairs
Flack parameter: 0.06 (3)
Table 1
Selected geometric parameters (A˚ ,).
N1—C12 1.489 (6)
N1—C11 1.497 (6)
C11—C19 1.522 (7)
C12—C13 1.517 (7)
C12—C21 1.532 (7)
C17—C18 1.390 (8)
C20—N1—C12 110.3 (4) C20—N1—C11 111.6 (4) C12—N1—C11 113.3 (4) N1—C11—C19 115.2 (4)
N1—C11—C10 107.4 (4) N1—C12—C13 110.1 (4) N1—C12—C21 111.3 (4)
Methyl H atoms were placed in calculated positions, with C—H = 0.96 A˚ , and refined withUiso(H)= 1.5Ueq(C). Other H atoms were
placed in calculated positions, with C—H = 0.93–0.98 A˚ , and refined as riding, withUiso(H)=1.2Ueq(C).
Data collection:SMART(Bruker, 2000); cell refinement:SAINT (Bruker, 2000); data reduction:SAINT; program(s) used to solve structure: SHELXTL (Bruker, 2000); program(s) used to refine structure:SHELXTL; molecular graphics:SHELXTL; software used to prepare material for publication:SHELXTL.
The work was supported by the Startup Foundations for Natural Science of Zhengzhou University of Light Industry (Nos. 2005001 and 000455).
References
Bruker (2000).SMART,SAINT,SADABSandSHELXTL. Bruker AXS Inc., Madison, Wisconsin, USA.
Flack, H. D. (1983).Acta Cryst.A39, 876–881.
Hayashi, T., Konishi, M., Okamoto, K., Katsumura, K. & Kumada. M. (1986).
J. Org. Chem.51, 3772–3781.
Qian, H.-Y., Cui, X.-L. & Yin, Z.-G. (2005).Acta Cryst.E61, m2641–2642. Sawamura, M. & Ito, Y. (1992).Chem. Rev.92, 857–871.
Togni, A., Burckhardt, U., Gramlich, V., Pergosin, P. S. & Salzmann, R. (1996).
[image:2.610.315.565.71.189.2]J. Am. Chem. Soc.118, 1031–1037. Figure 1
supporting information
sup-1
Acta Cryst. (2006). E62, m511–m512
supporting information
Acta Cryst. (2006). E62, m511–m512 [https://doi.org/10.1107/S1600536806004740]
N
-(1-Ferrocenylethyl)-
N
-(1-phenylethyl)methylamine
Zhi-Gang Yin, Heng-Yu Qian, Jia Jia and Chun-Xia Zhang
N-(1-Ferrocenylethyl)-N-(1-phenylethyl)methylamine
Crystal data
[Fe(C5H5)(C16H20N)]
Mr = 347.27
Monoclinic, P21 Hall symbol: P 2yb
a = 5.8833 (12) Å
b = 10.748 (2) Å
c = 14.191 (3) Å
β = 97.82 (3)°
V = 889.0 (3) Å3
Z = 2
F(000) = 368
Dx = 1.297 Mg m−3 Melting point: 198 K
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 714 reflections
θ = 2.1–12.4°
µ = 0.85 mm−1
T = 291 K Block, orange
0.20 × 0.18 × 0.16 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer
Radiation source: sealed tube Graphite monochromator
φ and ω scans
Absorption correction: multi-scan (SADABS; Bruker, 2000)
Tmin = 0.849, Tmax = 0.876
5026 measured reflections 3239 independent reflections 2928 reflections with I > 2σ(I)
Rint = 0.060
θmax = 26.0°, θmin = 1.4°
h = −7→7
k = −13→13
l = −17→17
Refinement
Refinement on F2 Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.056
wR(F2) = 0.130
S = 1.07 3239 reflections 208 parameters 1 restraint
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained
w = 1/[σ2(F
o2) + (0.0644P)2 + 0.55P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001 Δρmax = 0.33 e Å−3 Δρmin = −0.45 e Å−3
Absolute structure: Flack (1983), 1394 Friedel Pairs
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Fe1 0.51940 (10) 0.85961 (7) 0.36379 (4) 0.02886 (18)
N1 0.2664 (7) 0.8054 (4) 0.0590 (3) 0.0275 (8)
C1 0.5643 (8) 0.6780 (5) 0.4086 (4) 0.0347 (11)
H1A 0.5067 0.6045 0.3720 0.042*
C2 0.7782 (8) 0.7338 (5) 0.4062 (4) 0.0346 (11)
H2A 0.8963 0.7060 0.3684 0.041*
C3 0.7931 (8) 0.8328 (4) 0.4657 (3) 0.0353 (13)
H3A 0.9256 0.8885 0.4786 0.042*
C4 0.5879 (8) 0.8430 (5) 0.5065 (3) 0.0339 (12)
H4A 0.5544 0.9044 0.5537 0.041*
C5 0.4451 (8) 0.7451 (5) 0.4694 (4) 0.0324 (11)
H5A 0.2919 0.7259 0.4853 0.039*
C6 0.2194 (9) 0.9202 (5) 0.2888 (3) 0.0367 (11)
H6A 0.0651 0.8931 0.2984 0.044*
C7 0.3422 (9) 1.0221 (5) 0.3355 (4) 0.0377 (12)
H7A 0.2880 1.0781 0.3822 0.045*
C8 0.5540 (8) 1.0272 (5) 0.3020 (4) 0.0363 (12)
H8A 0.6756 1.0878 0.3215 0.044*
C9 0.5625 (11) 0.9327 (6) 0.2342 (4) 0.0436 (13)
H9A 0.6925 0.9159 0.1995 0.052*
C10 0.3585 (7) 0.8649 (7) 0.2256 (3) 0.0358 (10)
C11 0.3029 (9) 0.7548 (4) 0.1581 (4) 0.0332 (11)
H11A 0.4410 0.7029 0.1633 0.040*
C12 0.2412 (10) 0.7068 (5) −0.0155 (4) 0.0393 (12)
H12A 0.1013 0.6593 −0.0101 0.047*
C13 0.2191 (10) 0.7653 (4) −0.1136 (4) 0.0377 (12)
C14 0.3994 (10) 0.8343 (5) −0.1411 (4) 0.0423 (14)
H14A 0.5363 0.8423 −0.1003 0.051*
C15 0.3729 (9) 0.8921 (4) −0.2313 (4) 0.0359 (12)
H15A 0.4892 0.9429 −0.2479 0.043*
C16 0.1785 (9) 0.8744 (7) −0.2943 (4) 0.0438 (13)
H16A 0.1635 0.9111 −0.3542 0.053*
C17 0.0063 (9) 0.8021 (5) −0.2684 (4) 0.0378 (12)
H17A −0.1245 0.7875 −0.3117 0.045*
supporting information
sup-3
Acta Cryst. (2006). E62, m511–m512
H18A −0.0968 0.7040 −0.1608 0.053*
C19 0.1104 (9) 0.6728 (6) 0.1846 (4) 0.0401 (12)
H19A 0.0832 0.6058 0.1397 0.060*
H19B −0.0269 0.7214 0.1834 0.060*
H19C 0.1533 0.6395 0.2472 0.060*
C20 0.0696 (8) 0.8911 (4) 0.0445 (4) 0.0340 (12)
H20A 0.0516 0.9223 −0.0194 0.051*
H20B 0.0959 0.9593 0.0883 0.051*
H20C −0.0671 0.8476 0.0553 0.051*
C21 0.4457 (10) 0.6173 (6) −0.0029 (5) 0.0480 (16)
H21A 0.4610 0.5814 0.0596 0.072*
H21B 0.5832 0.6620 −0.0107 0.072*
H21C 0.4211 0.5525 −0.0497 0.072*
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
Fe1 0.0291 (3) 0.0301 (3) 0.0258 (3) 0.0066 (4) −0.0020 (2) 0.0019 (3)
N1 0.027 (2) 0.0238 (18) 0.030 (2) 0.0010 (15) −0.0032 (16) −0.0030 (16)
C1 0.032 (3) 0.040 (3) 0.030 (2) 0.016 (2) −0.0055 (19) 0.012 (2)
C2 0.028 (2) 0.041 (3) 0.034 (3) 0.012 (2) −0.0011 (19) 0.012 (2)
C3 0.030 (2) 0.033 (3) 0.037 (2) 0.0006 (19) −0.0142 (18) 0.013 (2)
C4 0.041 (2) 0.027 (3) 0.032 (2) 0.019 (2) −0.0020 (17) 0.003 (2)
C5 0.032 (3) 0.033 (3) 0.035 (3) 0.016 (2) 0.015 (2) 0.014 (2)
C6 0.039 (3) 0.041 (3) 0.029 (2) 0.013 (2) 0.001 (2) 0.000 (2)
C7 0.034 (3) 0.045 (3) 0.029 (3) 0.016 (2) −0.012 (2) −0.011 (2)
C8 0.027 (2) 0.045 (3) 0.035 (3) −0.012 (2) −0.004 (2) 0.006 (2)
C9 0.056 (3) 0.049 (3) 0.024 (2) −0.010 (3) −0.001 (2) 0.007 (2)
C10 0.034 (2) 0.044 (3) 0.027 (2) −0.003 (3) −0.0022 (16) −0.012 (3)
C11 0.044 (3) 0.020 (2) 0.035 (3) −0.0041 (19) 0.000 (2) 0.0043 (19)
C12 0.048 (3) 0.034 (3) 0.035 (3) 0.002 (2) 0.004 (2) 0.002 (2)
C13 0.054 (3) 0.019 (2) 0.038 (3) 0.003 (2) −0.001 (2) −0.0070 (19)
C14 0.054 (3) 0.039 (4) 0.031 (2) −0.007 (2) −0.004 (2) −0.003 (2)
C15 0.045 (3) 0.026 (3) 0.042 (3) −0.0106 (19) 0.024 (2) 0.0038 (19)
C16 0.043 (3) 0.045 (4) 0.043 (3) 0.009 (3) 0.004 (2) 0.012 (3)
C17 0.036 (3) 0.034 (3) 0.037 (3) 0.011 (2) −0.017 (2) −0.003 (2)
C18 0.045 (3) 0.049 (3) 0.036 (3) −0.005 (3) −0.008 (2) −0.002 (2)
C19 0.035 (3) 0.049 (3) 0.036 (3) −0.008 (2) 0.003 (2) 0.007 (2)
C20 0.031 (2) 0.032 (3) 0.035 (2) 0.0161 (18) −0.0075 (18) 0.0015 (18)
C21 0.055 (5) 0.047 (3) 0.038 (3) 0.024 (3) −0.007 (3) −0.017 (2)
Geometric parameters (Å, º)
Fe1—C4 2.018 (5) C8—H8A 0.9800
Fe1—C8 2.026 (5) C9—C10 1.396 (8)
Fe1—C5 2.032 (5) C9—H9A 0.9800
Fe1—C3 2.033 (4) C10—C11 1.529 (8)
Fe1—C7 2.046 (5) C11—H11A 0.9800
Fe1—C9 2.048 (5) C12—C13 1.517 (7)
Fe1—C1 2.058 (5) C12—C21 1.532 (7)
Fe1—C10 2.059 (4) C12—H12A 0.9800
Fe1—C2 2.064 (5) C13—C18 1.372 (8)
N1—C20 1.472 (6) C13—C14 1.393 (8)
N1—C12 1.489 (6) C14—C15 1.412 (7)
N1—C11 1.497 (6) C14—H14A 0.9300
C1—C5 1.386 (7) C15—C16 1.367 (8)
C1—C2 1.398 (8) C15—H15A 0.9300
C1—H1A 0.9800 C16—C17 1.366 (8)
C2—C3 1.355 (8) C16—H16A 0.9300
C2—H2A 0.9800 C17—C18 1.390 (8)
C3—C4 1.412 (7) C17—H17A 0.9300
C3—H3A 0.9800 C18—H18A 0.9300
C4—C5 1.404 (8) C19—H19A 0.9600
C4—H4A 0.9800 C19—H19B 0.9600
C5—H5A 0.9800 C19—H19C 0.9600
C6—C10 1.424 (7) C20—H20A 0.9600
C6—C7 1.425 (8) C20—H20B 0.9600
C6—H6A 0.9800 C20—H20C 0.9600
C7—C8 1.394 (8) C21—H21A 0.9600
C7—H7A 0.9800 C21—H21B 0.9600
C8—C9 1.404 (8) C21—H21C 0.9600
C4—Fe1—C8 119.7 (2) C10—C6—C7 108.4 (5)
C4—Fe1—C5 40.6 (2) C10—C6—Fe1 70.4 (3)
C8—Fe1—C5 154.5 (2) C7—C6—Fe1 69.8 (3)
C4—Fe1—C3 40.8 (2) C10—C6—H6A 125.8
C8—Fe1—C3 108.51 (19) C7—C6—H6A 125.8
C5—Fe1—C3 67.6 (2) Fe1—C6—H6A 125.8
C4—Fe1—C6 126.3 (2) C8—C7—C6 107.0 (5)
C8—Fe1—C6 67.7 (2) C8—C7—Fe1 69.2 (3)
C5—Fe1—C6 108.7 (2) C6—C7—Fe1 69.3 (3)
C3—Fe1—C6 164.1 (2) C8—C7—H7A 126.5
C4—Fe1—C7 107.4 (2) C6—C7—H7A 126.5
C8—Fe1—C7 40.0 (2) Fe1—C7—H7A 126.5
C5—Fe1—C7 120.5 (2) C7—C8—C9 108.8 (5)
C3—Fe1—C7 126.4 (2) C7—C8—Fe1 70.8 (3)
C6—Fe1—C7 40.8 (2) C9—C8—Fe1 70.7 (3)
C4—Fe1—C9 154.6 (2) C7—C8—H8A 125.6
C8—Fe1—C9 40.3 (2) C9—C8—H8A 125.6
C5—Fe1—C9 163.8 (2) Fe1—C8—H8A 125.6
C3—Fe1—C9 120.7 (2) C10—C9—C8 109.1 (5)
C6—Fe1—C9 67.3 (2) C10—C9—Fe1 70.6 (3)
C7—Fe1—C9 67.5 (2) C8—C9—Fe1 69.0 (3)
C4—Fe1—C1 66.8 (2) C10—C9—H9A 125.5
supporting information
sup-5
Acta Cryst. (2006). E62, m511–m512
C5—Fe1—C1 39.6 (2) Fe1—C9—H9A 125.5
C3—Fe1—C1 65.8 (2) C9—C10—C6 106.8 (6)
C6—Fe1—C1 122.0 (2) C9—C10—C11 124.6 (5)
C7—Fe1—C1 155.6 (2) C6—C10—C11 128.7 (5)
C9—Fe1—C1 127.9 (2) C9—C10—Fe1 69.7 (3)
C4—Fe1—C10 164.00 (19) C6—C10—Fe1 68.9 (3)
C8—Fe1—C10 67.9 (3) C11—C10—Fe1 127.3 (5)
C5—Fe1—C10 127.0 (3) N1—C11—C19 115.2 (4)
C3—Fe1—C10 153.9 (2) N1—C11—C10 107.4 (4)
C6—Fe1—C10 40.7 (2) C19—C11—C10 113.4 (4)
C7—Fe1—C10 68.5 (2) N1—C11—H11A 106.8
C9—Fe1—C10 39.7 (2) C19—C11—H11A 106.8
C1—Fe1—C10 110.1 (3) C10—C11—H11A 106.8
C4—Fe1—C2 67.1 (2) N1—C12—C13 110.1 (4)
C8—Fe1—C2 126.5 (2) N1—C12—C21 111.3 (4)
C5—Fe1—C2 67.19 (19) C13—C12—C21 109.6 (5)
C3—Fe1—C2 38.6 (2) N1—C12—H12A 108.6
C6—Fe1—C2 155.9 (2) C13—C12—H12A 108.6
C7—Fe1—C2 162.3 (2) C21—C12—H12A 108.6
C9—Fe1—C2 109.7 (2) C18—C13—C14 118.3 (5)
C1—Fe1—C2 39.6 (2) C18—C13—C12 121.4 (5)
C10—Fe1—C2 121.4 (2) C14—C13—C12 120.3 (5)
C20—N1—C12 110.3 (4) C13—C14—C15 119.6 (5)
C20—N1—C11 111.6 (4) C13—C14—H14A 120.2
C12—N1—C11 113.3 (4) C15—C14—H14A 120.2
C5—C1—C2 109.0 (5) C16—C15—C14 120.7 (5)
C5—C1—Fe1 69.2 (3) C16—C15—H15A 119.6
C2—C1—Fe1 70.4 (3) C14—C15—H15A 119.6
C5—C1—H1A 125.5 C17—C16—C15 119.2 (5)
C2—C1—H1A 125.5 C17—C16—H16A 120.4
Fe1—C1—H1A 125.5 C15—C16—H16A 120.4
C3—C2—C1 107.7 (5) C16—C17—C18 120.6 (5)
C3—C2—Fe1 69.5 (3) C16—C17—H17A 119.7
C1—C2—Fe1 70.0 (3) C18—C17—H17A 119.7
C3—C2—H2A 126.2 C13—C18—C17 121.4 (6)
C1—C2—H2A 126.2 C13—C18—H18A 119.3
Fe1—C2—H2A 126.2 C17—C18—H18A 119.3
C2—C3—C4 109.2 (5) C11—C19—H19A 109.5
C2—C3—Fe1 71.9 (3) C11—C19—H19B 109.5
C4—C3—Fe1 69.0 (3) H19A—C19—H19B 109.5
C2—C3—H3A 125.4 C11—C19—H19C 109.5
C4—C3—H3A 125.4 H19A—C19—H19C 109.5
Fe1—C3—H3A 125.4 H19B—C19—H19C 109.5
C5—C4—C3 106.9 (4) N1—C20—H20A 109.5
C5—C4—Fe1 70.3 (3) N1—C20—H20B 109.5
C3—C4—Fe1 70.2 (3) H20A—C20—H20B 109.5
C5—C4—H4A 126.5 N1—C20—H20C 109.5
Fe1—C4—H4A 126.5 H20B—C20—H20C 109.5
C1—C5—C4 107.2 (4) C12—C21—H21A 109.5
C1—C5—Fe1 71.2 (3) C12—C21—H21B 109.5
C4—C5—Fe1 69.2 (3) H21A—C21—H21B 109.5
C1—C5—H5A 126.4 C12—C21—H21C 109.5
C4—C5—H5A 126.4 H21A—C21—H21C 109.5
Fe1—C5—H5A 126.4 H21B—C21—H21C 109.5
C4—Fe1—C1—C5 38.8 (3) C5—Fe1—C7—C8 158.0 (3)
C8—Fe1—C1—C5 156.1 (7) C3—Fe1—C7—C8 74.7 (4)
C3—Fe1—C1—C5 83.5 (3) C6—Fe1—C7—C8 −118.5 (4)
C6—Fe1—C1—C5 −80.7 (4) C9—Fe1—C7—C8 −37.8 (3)
C7—Fe1—C1—C5 −42.3 (6) C1—Fe1—C7—C8 −172.1 (5)
C9—Fe1—C1—C5 −165.4 (3) C10—Fe1—C7—C8 −80.7 (3)
C10—Fe1—C1—C5 −124.3 (3) C2—Fe1—C7—C8 46.7 (8)
C2—Fe1—C1—C5 120.4 (5) C4—Fe1—C7—C6 −125.9 (3)
C4—Fe1—C1—C2 −81.5 (3) C8—Fe1—C7—C6 118.5 (4)
C8—Fe1—C1—C2 35.7 (9) C5—Fe1—C7—C6 −83.5 (3)
C5—Fe1—C1—C2 −120.4 (5) C3—Fe1—C7—C6 −166.9 (3)
C3—Fe1—C1—C2 −36.8 (3) C9—Fe1—C7—C6 80.7 (3)
C6—Fe1—C1—C2 159.0 (3) C1—Fe1—C7—C6 −53.7 (6)
C7—Fe1—C1—C2 −162.6 (4) C10—Fe1—C7—C6 37.7 (3)
C9—Fe1—C1—C2 74.2 (4) C2—Fe1—C7—C6 165.1 (6)
C10—Fe1—C1—C2 115.4 (3) C6—C7—C8—C9 1.4 (6)
C5—C1—C2—C3 0.9 (5) Fe1—C7—C8—C9 60.7 (4)
Fe1—C1—C2—C3 59.5 (3) C6—C7—C8—Fe1 −59.3 (4)
C5—C1—C2—Fe1 −58.6 (3) C4—Fe1—C8—C7 −81.8 (3)
C4—Fe1—C2—C3 −38.0 (3) C5—Fe1—C8—C7 −48.5 (6)
C8—Fe1—C2—C3 72.9 (4) C3—Fe1—C8—C7 −125.1 (3)
C5—Fe1—C2—C3 −82.2 (4) C6—Fe1—C8—C7 38.4 (3)
C6—Fe1—C2—C3 −167.0 (5) C9—Fe1—C8—C7 119.0 (4)
C7—Fe1—C2—C3 37.2 (8) C1—Fe1—C8—C7 168.3 (7)
C9—Fe1—C2—C3 114.9 (3) C10—Fe1—C8—C7 82.5 (3)
C1—Fe1—C2—C3 −118.8 (5) C2—Fe1—C8—C7 −164.0 (3)
C10—Fe1—C2—C3 157.3 (3) C4—Fe1—C8—C9 159.2 (3)
C4—Fe1—C2—C1 80.8 (3) C5—Fe1—C8—C9 −167.5 (5)
C8—Fe1—C2—C1 −168.3 (3) C3—Fe1—C8—C9 115.9 (4)
C5—Fe1—C2—C1 36.6 (3) C6—Fe1—C8—C9 −80.6 (4)
C3—Fe1—C2—C1 118.8 (5) C7—Fe1—C8—C9 −119.0 (4)
C6—Fe1—C2—C1 −48.2 (6) C1—Fe1—C8—C9 49.3 (9)
C7—Fe1—C2—C1 156.1 (6) C10—Fe1—C8—C9 −36.5 (3)
C9—Fe1—C2—C1 −126.3 (3) C2—Fe1—C8—C9 77.0 (4)
C10—Fe1—C2—C1 −83.9 (4) C7—C8—C9—C10 −1.4 (6)
C1—C2—C3—C4 −0.6 (5) Fe1—C8—C9—C10 59.4 (4)
Fe1—C2—C3—C4 59.2 (3) C7—C8—C9—Fe1 −60.8 (4)
C1—C2—C3—Fe1 −59.8 (3) C4—Fe1—C9—C10 −166.4 (5)
C4—Fe1—C3—C2 119.8 (5) C8—Fe1—C9—C10 −120.4 (5)
supporting information
sup-7
Acta Cryst. (2006). E62, m511–m512
C5—Fe1—C3—C2 81.0 (3) C3—Fe1—C9—C10 157.0 (4)
C6—Fe1—C3—C2 160.5 (7) C6—Fe1—C9—C10 −38.5 (4)
C7—Fe1—C3—C2 −166.8 (3) C7—Fe1—C9—C10 −82.9 (4)
C9—Fe1—C3—C2 −83.3 (4) C1—Fe1—C9—C10 75.1 (5)
C1—Fe1—C3—C2 37.8 (3) C2—Fe1—C9—C10 115.8 (4)
C10—Fe1—C3—C2 −48.6 (7) C4—Fe1—C9—C8 −45.9 (7)
C8—Fe1—C3—C4 114.3 (3) C5—Fe1—C9—C8 160.4 (7)
C5—Fe1—C3—C4 −38.7 (3) C3—Fe1—C9—C8 −82.6 (4)
C6—Fe1—C3—C4 40.7 (9) C6—Fe1—C9—C8 81.9 (4)
C7—Fe1—C3—C4 73.4 (4) C7—Fe1—C9—C8 37.5 (3)
C9—Fe1—C3—C4 156.9 (3) C1—Fe1—C9—C8 −164.5 (3)
C1—Fe1—C3—C4 −82.0 (3) C10—Fe1—C9—C8 120.4 (5)
C10—Fe1—C3—C4 −168.4 (6) C2—Fe1—C9—C8 −123.8 (3)
C2—Fe1—C3—C4 −119.8 (5) C8—C9—C10—C6 0.8 (7)
C2—C3—C4—C5 0.1 (5) Fe1—C9—C10—C6 59.2 (4)
Fe1—C3—C4—C5 61.0 (3) C8—C9—C10—C11 179.6 (5)
C2—C3—C4—Fe1 −60.9 (3) Fe1—C9—C10—C11 −122.0 (6)
C8—Fe1—C4—C5 158.6 (3) C8—C9—C10—Fe1 −58.4 (4)
C3—Fe1—C4—C5 −117.2 (4) C7—C6—C10—C9 0.0 (6)
C6—Fe1—C4—C5 75.7 (4) Fe1—C6—C10—C9 −59.7 (4)
C7—Fe1—C4—C5 116.8 (3) C7—C6—C10—C11 −178.7 (5)
C9—Fe1—C4—C5 −169.0 (5) Fe1—C6—C10—C11 121.6 (7)
C1—Fe1—C4—C5 −37.9 (3) C7—C6—C10—Fe1 59.7 (4)
C10—Fe1—C4—C5 44.1 (12) C4—Fe1—C10—C9 158.5 (9)
C2—Fe1—C4—C5 −81.2 (3) C8—Fe1—C10—C9 37.0 (4)
C8—Fe1—C4—C3 −84.2 (3) C5—Fe1—C10—C9 −167.0 (4)
C5—Fe1—C4—C3 117.2 (4) C3—Fe1—C10—C9 −50.0 (8)
C6—Fe1—C4—C3 −167.2 (3) C6—Fe1—C10—C9 118.2 (6)
C7—Fe1—C4—C3 −126.1 (3) C7—Fe1—C10—C9 80.3 (4)
C9—Fe1—C4—C3 −51.8 (7) C1—Fe1—C10—C9 −125.8 (4)
C1—Fe1—C4—C3 79.3 (3) C2—Fe1—C10—C9 −83.3 (5)
C10—Fe1—C4—C3 161.3 (10) C4—Fe1—C10—C6 40.3 (13)
C2—Fe1—C4—C3 36.0 (3) C8—Fe1—C10—C6 −81.1 (4)
C2—C1—C5—C4 −0.8 (5) C5—Fe1—C10—C6 74.8 (5)
Fe1—C1—C5—C4 −60.1 (3) C3—Fe1—C10—C6 −168.2 (4)
C2—C1—C5—Fe1 59.3 (3) C7—Fe1—C10—C6 −37.9 (4)
C3—C4—C5—C1 0.5 (5) C9—Fe1—C10—C6 −118.2 (6)
Fe1—C4—C5—C1 61.4 (3) C1—Fe1—C10—C6 116.1 (4)
C3—C4—C5—Fe1 −61.0 (3) C2—Fe1—C10—C6 158.6 (3)
C4—Fe1—C5—C1 −117.6 (4) C4—Fe1—C10—C11 −83.0 (11)
C8—Fe1—C5—C1 −164.8 (4) C8—Fe1—C10—C11 155.6 (5)
C3—Fe1—C5—C1 −78.6 (3) C5—Fe1—C10—C11 −48.4 (5)
C6—Fe1—C5—C1 117.9 (3) C3—Fe1—C10—C11 68.6 (7)
C7—Fe1—C5—C1 161.2 (3) C6—Fe1—C10—C11 −123.3 (6)
C9—Fe1—C5—C1 45.4 (9) C7—Fe1—C10—C11 −161.1 (5)
C10—Fe1—C5—C1 76.3 (4) C9—Fe1—C10—C11 118.6 (6)
C2—Fe1—C5—C1 −36.7 (3) C1—Fe1—C10—C11 −7.2 (4)
C3—Fe1—C5—C4 39.0 (3) C20—N1—C11—C19 64.9 (5)
C6—Fe1—C5—C4 −124.5 (3) C12—N1—C11—C19 −60.3 (6)
C7—Fe1—C5—C4 −81.2 (3) C20—N1—C11—C10 −62.5 (5)
C9—Fe1—C5—C4 162.9 (8) C12—N1—C11—C10 172.3 (4)
C1—Fe1—C5—C4 117.6 (4) C9—C10—C11—N1 −70.3 (7)
C10—Fe1—C5—C4 −166.1 (3) C6—C10—C11—N1 108.2 (7)
C2—Fe1—C5—C4 80.9 (3) Fe1—C10—C11—N1 −160.0 (3)
C4—Fe1—C6—C10 −167.2 (4) C9—C10—C11—C19 161.3 (6)
C8—Fe1—C6—C10 81.5 (4) C6—C10—C11—C19 −20.2 (8)
C5—Fe1—C6—C10 −125.6 (4) Fe1—C10—C11—C19 71.6 (5)
C3—Fe1—C6—C10 160.8 (7) C20—N1—C12—C13 57.5 (5)
C7—Fe1—C6—C10 119.1 (5) C11—N1—C12—C13 −176.6 (4)
C9—Fe1—C6—C10 37.7 (4) C20—N1—C12—C21 179.2 (5)
C1—Fe1—C6—C10 −83.9 (4) C11—N1—C12—C21 −54.9 (6)
C2—Fe1—C6—C10 −49.9 (7) N1—C12—C13—C18 −117.6 (6)
C4—Fe1—C6—C7 73.6 (4) C21—C12—C13—C18 119.6 (6)
C8—Fe1—C6—C7 −37.7 (3) N1—C12—C13—C14 63.7 (6)
C5—Fe1—C6—C7 115.3 (3) C21—C12—C13—C14 −59.1 (6)
C3—Fe1—C6—C7 41.7 (9) C18—C13—C14—C15 3.6 (8)
C9—Fe1—C6—C7 −81.5 (4) C12—C13—C14—C15 −177.6 (5)
C1—Fe1—C6—C7 156.9 (3) C13—C14—C15—C16 −4.4 (8)
C10—Fe1—C6—C7 −119.1 (5) C14—C15—C16—C17 1.5 (9)
C2—Fe1—C6—C7 −169.0 (5) C15—C16—C17—C18 2.0 (9)
C10—C6—C7—C8 −0.9 (6) C14—C13—C18—C17 −0.2 (8)
Fe1—C6—C7—C8 59.2 (4) C12—C13—C18—C17 −178.9 (5)
C10—C6—C7—Fe1 −60.1 (4) C16—C17—C18—C13 −2.7 (9)