Health Policy Advisory Committee on
Technology
Technology Brief
Exalenz BreathID
®breath test device for the diagnosis of
liver disease
© State of Queensland (Queensland Health) 2012
This work is licensed under a Creative Commons Attribution Non-Commercial No Derivatives 2.5 Australia licence. In essence, you are free to copy and communicate the work in its current form for non-commercial purposes, as long as you attribute the authors and abide by the licence terms. You may not alter or adapt the work in any way.
To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/2.5/au/. For further information, contact the HealthPACT Secretariat at:
HealthPACT Secretariat
c/o Access Improvement Service, Centre for Healthcare Improvement, Queensland Health
Lobby 2, Level 2, Citilink Business Centre 153 Campbell Street, Bowen Hills QLD 4006 Postal Address: GPO Box 48, Brisbane Qld 4001
Email: HealthPACT@health.qld.gov.au Telephone: (07). 3131 6969
For permissions beyond the scope of this licence contact: Intellectual Property Officer,
Queensland Health, GPO Box 48, Brisbane Qld 4001, email ip_officer@health.qld.gov.au, phone (07) 3234 1479.
Electronic copies can be obtained from: http://www.health.qld.gov.au/healthpact
DISCLAIMER: This brief is published with the intention of providing information of interest. It is based on information available at the time of research and cannot be expected to cover any developments arising from subsequent improvements to health technologies. This brief is based on a limited literature search and is not a definitive statement on the safety, effectiveness or cost-effectiveness of the health technology covered.
The State of Queensland acting through Queensland Health (“Queensland Health”) does not guarantee the accuracy, currency or completeness of the information in this brief. Information may contain or summarise the views of others, and not necessarily reflect the views of
Queensland Health.
This brief is not intended to be used as medical advice and it is not intended to be used to diagnose, treat, cure or prevent any disease, nor should it be used for therapeutic purposes or as a substitute for a health professional's advice. It must not be relied upon without verification from authoritative sources. Queensland Health does not accept any liability, including for any injury, loss or damage, incurred by use of or reliance on the information.
This brief was commissioned by Queensland Health, in its role as the Secretariat of the Health Policy Advisory Committee on Technology (HealthPACT). The production of this brief was overseen by HealthPACT. HealthPACT comprises representatives from health departments in all States and Territories, the Australian and New Zealand governments and MSAC. It is a sub-committee of the Australian Health Ministers’ Advisory Council (AHMAC), reporting to AHMAC’s Hospital Principal Committee (HPC). AHMAC supports HealthPACT through funding.
This brief was prepared by Heath White from the Australian Safety and Efficacy Register of New Interventional Procedures – Surgical (ASERNIP-S).
TECHNOLOGY BRIEF
Register ID WP101
Name of technology Exalenz BreathID® platform
Purpose and target group Patients with liver disease, especially those with acute liver failure
Stage of development in Australia
_ Yet to emerge Established
Experimental Established but changed indication
or modification of technique
Investigational Should be taken out of use
Nearly established
Australian Therapeutic Goods Administration approval
Yes ARTG number
_ No
Not applicable
International utilisation
Country Level of use Trials underway or
completed
Limited use Widely diffused
United States 9
Israel 9
Switzerland 9
Impact summary
The term liver disease encompasses a wide range of diseases of various aetiologies. The most common tests employed to diagnose and assess the extent of liver disease are blood tests (primarily to measure alanine aminotransferase [ALT] and aspartate aminotransferase [AST] levels), and liver biopsy. The Exalenz BreathID® platform provides a safe, non-invasive method of assessing liver function by continuously measuring the by-product of 13C-methacetin metabolism in the breath of test subjects. Based on the evidence presented herein, the test provides a relatively accurate measure of fibrosis and inflammation levels; however, several factors can influence the endogenous metabolism of methacetin, and these need to be
been completed or are scheduled for completion in the near future, which have the potential to provide significant data pertaining to the effectiveness of this test for assessing liver function.
Background
Liver disease is a broad term used to cover a range of diseases of various aetiologies, which can either be congenital (e.g. Wilson’s disease, porphyria, pyrosinemia) or acquired (e.g. hepatitis A, B and C, cirrhosis, non-alcoholic fatty liver disease [NAFLD], and hepatocellular carcinoma). Due to the significant variation in liver disease aetiology, no individual test is available through which to diagnose and assess the nature and extent of all liver diseases.1
In the majority of patients, the initial step in identifying liver disease involves a blood test. When the liver is damaged, certain enzymes that are normally contained within liver cells are released into the blood, thereby increasing their plasma
concentrations. For the assessment of liver function, the most frequently measured enzymes are ALT and AST.2
Breath tests have been used to assess liver function for over a decade. These tests are based on measuring exhaled metabolites of labelled substrates that have been ingested and metabolised by the liver; and the concentration of these metabolites in the breath act as a surrogate marker for liver function.3 Specifically, 13C-labelled methacetin is rapidly metabolised by the cytochrome P450 enzyme system, releasing 13
C-labelled carbon dioxide which is subsequently exhaled in the breath and thereby detected using the 13C-methacetin breath test. 13C-labelled methacetin and its associated metabolites are non-toxic in small doses and have not been associated with any adverse reactions. As a result, 13C-methacetin presents a potential diagnostic tool for the assessment of liver damage.4
Exalenz® (Modin, Israel) appropriated the BreathID® platform from then parent company, Oridion (Massachusetts, USA). Now almost fully autonomous, Exalenz® utilises the BreathID® platform to measure gastric emptying rate, detect H. pylori
infection and other gastrointestinal tract conditions, and to assess the severity of liver diseases.5 For the assessment of liver disease, the patient begins by consuming a beverage containing the 13C-methacetin substrate. 13C-labelled carbon dioxide, one of the by-products of 13C-methacetin metabolism, is exhaled into the Exalenz
BreathID® Methacetin Breath Test (MBT) device, which uses molecular correlation spectroscopy to measure the percentage dose recovery (PDR) of 13C and the cumulative PDR (cPDR). Breath samples are automatically collected at the bedside via a nasal cannula.6 The device plots results in real-time, in addition to providing PDR peak value and peak time. The higher the PDR and cPDR, the closer the liver function is to normal.5
Clinical need and burden of disease
In 2007, diseases of the liver accounted for 1,437 deaths in Australia and consisted primarily of alcoholic liver disease (n=676), fibrosis and cirrhosis (n=315) and hepatic failure (n=224).7 In the 2009-2010 financial year, there were 13,555 hospital
separations based on the principal diagnosis of liver disease, with an emphasis on alcoholic liver disease (n=5,854) and fibrosis/cirrhosis (n=2,643).8 Hepatitis C infects an estimated 217,000 Australians, with approximately 46,000 of these currently living with moderate to severe liver disease. Approximately 10,000 new cases are diagnosed each year.9
In the 2010-2011 fiscal year, 3,149 liver biopsy services were claimed under the Medicare Benefits Schedule (MBS) in Australia. In addition, there were almost 13.9 million MBS claims relating to biochemical tests on liver enzymes (including AST and ALT, bilirubin, alkaline phosphatase, gammaglutamyltranspeptidase, lactate
dehydrogenase and total protein), with the bulk of these claims (12.3 million) comprising five or more tests concurrently conducted on a single sample (a total of 64.4 million individual tests).10
Diffusion of technology in Australia
There is no evidence of the 13C-methacetin breath test being employed in an Australian clinical setting.
On 16 September 2011, the United States Food & Drug Administration (FDA) granted humanitarian use device (HUD) designation, which enables Exalenz Biosciences™ to seek Humanitarian Device Exemption (HDE) approval for the Exalenz BreathID®MBT device when used to monitor hepatic metabolism in patients diagnosed with acute liver failure (a rare disease that affects < 4,000 patients in the US each year).11
Comparators
No single test is sufficient to provide a complete estimate of liver injury and
determine aetiology. As such, there are numerous methods employed to assess liver function (Table 1). These tests can be divided into four broad categories:
(1) Common serum liver chemistry tests employed to assess the extent of cholestasis, hepatocellular damage, or synthetic function.
(2) Tests or panels to reveal certain histologic features such as fibrosis, steatosis, and steatohepatitis.
(3) Tests that assist in the accurate diagnosis of liver disease, including specific auto-antibodies, genetic tests, and serologic tests for viral hepatitis.
(4) Tests that assess hepatic metabolic capacity or measure the capacity of the liver to transport organic anions and clear substances from the circulation.12
Table 1 Techniques employed to determine presence and extent of liver disease2
Class Test
Serum biochemical tests Bilirubin
Aminotransferases (ALT and AST) Alkaline phosphatase Gamma glutamyltranspeptidase (GGTP) 5’-Nucleotidase (5NT) Lactate dehydrogenase Proteins Prothrombin time Assessment of hepatic metabolic capacitya Antipyrine clearance
Aminopyrine breath test Caffeine clearance
Galactose elimination capacity Lidocaine metabolite Other tests of liver functiona Serum bile acids
Urea synthesis Bromsulphalein (BSP) Indocyanine green
Non-invasive serum markers of fibrosis
Tissue assessment Liver biopsy
Hepatic imaging Plain abdominal x-ray and barium studies Ultrasonography
Computed tomography (CT) Magnetic resonance imaging (MRI) Radioisotope scanning
Positron emission tomography (PET) Transient elastography
a Tests are rarely used in Australian clinical practice
Liver biopsy remains the gold standard for assessing the severity of liver disease. For the majority of patients the procedure can be performed safely on an outpatient basis with the patient subsequently discharged following several hours of
observation. A local anaesthetic is injected and a biopsy needle is then inserted transthoracically below the diaphragm, often guided by ultrasonography. A sample of 1.5 cm in length, containing at least six portal triads, is adequate for histological interpretation. Although serious complications are uncommon (<3% of patients, primarily consisting of intraperitoneal bleeding), one third of patients experience post-biopsy pain, and vasovagal reactions are also common. Biopsy of malignant neoplasm carries a 1 to 3 per cent risk of seeding of the biopsy tract with tumour. In addition, the fatality rate is between 0.03 per cent and 0.32 per cent.2
The aminotransferases ALT and AST represent the most frequently used biochemical indicators of hepatic injury. Serum ALT and AST levels are elevated to some extent in almost all liver diseases, with the highest levels typically occurring in viral hepatitis, drug- or toxin-induced hepatic necrosis, and circulatory shock (ischemic hepatitis). Determination of ALT and AST levels has proven useful as a screening tool to identify subclinical liver disease in asymptomatic patients. Declining AST and ALT levels may indicate recovery; however, they may also indicate poor prognosis due to paucity of remaining hepatocytes. Precise levels of AST and ALT do not correlate well with the extent of liver disease, nor the prognosis.12
Alternative breath test devices, such as the LiMAx® breath test, are marketed in competition to the Exalenz® BreathID device. The LiMAx® test also involves the administration of 13C-methacetin and the detection of 13CO2 in the breath; however, a laser air analyser (known as the FLIP device), is used for detection.13-15
Furthermore, the LiMAx® breath test device has been demonstrated to predict liver failure pre-operatively.14
Safety and effectiveness Goetze et al (2007)6
Goetze et al (2007) compared the MBT results using the BreathID® system with the gold standard for breath test analysis, isotopic ratio mass spectrometry (IRMS) in patients with chronic hepatitis C (HCV) infection (level III interventional evidence). An additional aim was to assess the diagnostic performance of these tests compared with liver biopsy for the quantification of liver fibrosis; however, no results were presented regarding this comparison. The study enrolled 50 patients with HCV (32 men, 18 women; mean age 46 ± standard deviation (SD) 11 years) at different METAVIR fibrosis stages (F0 to F4; METAVIR is a system used to quantify the degree of inflammation and fibrosis of a liver biopsy in patients with hepatitis C). Sampling for both systems took place over 90 minutes.
Safety
All patients completed the study without complications or adverse events.
Effectiveness
All patients were successfully analysed using the BreathID® system; however, 13 samples could not be analysed with IRMS mainly due to low carbon dioxide content. The 13C/12C isotope ratios of the breath samples were compared between BreathID® and IRMS, with results expressed as delta over baseline (DOB). Mean DOB values were 12.1 ± 7.1 per cent (range -0.7 to 48.6%) for BreathID®, and 11.6 ± 7.1 per cent (range 0.1 to 47.8%) for IRMS. Mean time until peak 13CO2 excretion was 22 ± 13 min for BreathID® and 23 ± 14 min for IRMS. Mean 30-minute cPDR values were 9.3 ± 4.6
per cent (range 0.6 to 22.6%) for BreathID® and 9.7 ± 4.7 per cent (range 0.6 to 23.3%) for IRMS (p=0.3–0.7).
Bland and Altman analysis and linear regression analysis were performed to compare the two tests. A high linear association between the tests was observed for DOB and DOBmax (R
2
=0.95). For DOB and DOBmax, the limits of agreement were within the predefined maximal width of SD <2.5 per cent (mean 0.55 ± 1.69% for DOB; mean -0.57 ± 2.42% for DOBmax).
For both BreathID® and IRMS measurement techniques, cPDR30 min and cPDR60 min were significantly different (p<0.05) in hepatitis C patients with high grades of liver fibrosis compared with patients with low grades of fibrosis.
Lalazar et al (2008)16
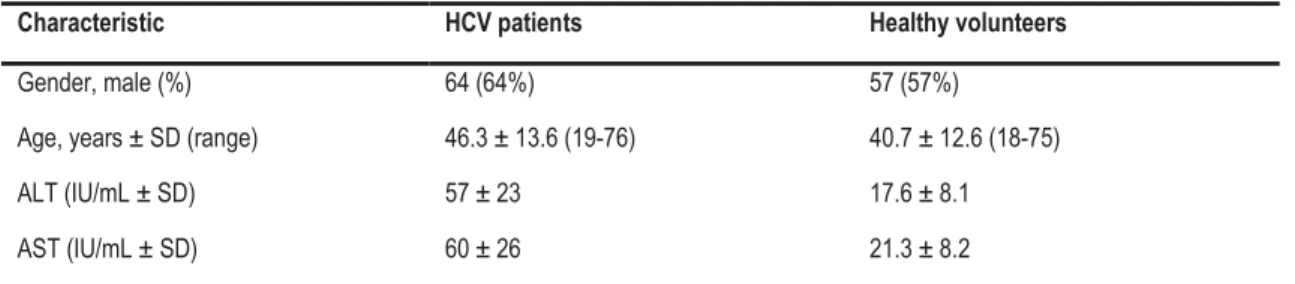
Lalazar et al (2008) assessed the accuracy of BreathID® in assessing the degree of liver fibrosis and inflammation in patients with untreated HCV infection and normal serum ALT levels who had undergone a liver biopsy in the last 12 months. One hundred age- and sex-matched healthy volunteers were enrolled as controls (level III interventional evidence). The proportion of males to females at baseline was similar (Table 2); however, ALT and AST levels were significantly different (p<0.01), as was mean age (p=0.0047).
Table 2 Baseline demographics of chronic HCV and control patients16
Characteristic HCV patients Healthy volunteers
Gender, male (%) 64 (64%) 57 (57%)
Age, years ± SD (range) 46.3 ± 13.6 (19-76) 40.7 ± 12.6 (18-75)
ALT (IU/mL ± SD) 57 ± 23 17.6 ± 8.1
AST (IU/mL ± SD) 60 ± 26 21.3 ± 8.2
ALT: alanine aminotransferase; AST: aspartate aminotransferase; HCV: hepatitis C virus; SD: standard deviation
Based on the results of the pre-test liver biopsy, patients were grouped according to histological activity index (HAI), fibrosis scores of ≤2 (non-significant, n=50) and >2 (significant, n=50), and HAI necroinflammatory scores (HAIa + HAIb + HAIc + HAId) of ≤4 (low, n=32) and >4 (high, n=68). PDR and cPDR were measured at 10, 15, 20, 30 and 60 minutes for all patients. For comparison, values were divided into low- and high-inflammatory groups and significant and non-significant fibrosis groups.
Safety
No diagnosis-related adverse events were identified in either group.
Effectiveness
A significant difference in PDR and cPDR was observed across all time points, with the exception of the PDR of low- and high-inflammation groups at 60 minutes, and
peak time in low and high fibrosis groups (Table 3). Interestingly, patients with low inflammation and low fibrosis scores had both lower peak time values and higher cPDR values at all time points compared to healthy volunteers. PDR values were also lower, with the exception of late PDR readings.
To determine whether use of the MBT test negates the need for a liver biopsy, the authors applied an algorithm that accounted for breath test parameters and baseline patient characteristics. The sensitivity, specificity and positive- and
negative-predictive values of the MBT are presented in Table 4. Based on this algorithm, the authors stated that 67 per cent of liver biopsies performed in the patient group could have been avoided. To assess for reproducibility of results, 42 healthy volunteers and 11 HCV patients each undertook between two and six additional tests. In both groups, inter-test variability of ≤13 per cent was observed for PDR peak height (95% CI, 0.11-0.15).
Table 3 PDR and cPDR values for all groups16
Low inflammation (n=32) High inflammation (n=68)
p value Low fibrosis (n=50) High fibrosis (n=50) p value Healthy volunteers (n=100) PDR peak 38.30 ± 15.73 (2.78) 28.60 ± 11.20 (1.36) 0.0063 36.84 ± 11.37 (1.61) 26.55 ± 13.66 (1.93) <0.0001 35.31 ± 8.94 Peak time 18.86 ± 7.62 (1.35) 22.83 ± 9.47 (1.15) 0.0477 19.86 ± 7.20 (1.02) 23.26 ± 10.42 (1.47) NS 21.04 ± 7.47 PDR10 27.27 ± 16.57 (2.93) 18.98 ± 11.51 (1.40) 0.0148 25.14 ± 12.64 (1.79) 18.14 ± 14.15 (2.0) 0.0030 23.85 ± 10.78 PDR15 33.90 ± 14.90 (2.63) 23.76 ± 12.17 (1.48) 0.0034 31.56 ± 12.38 (1.75) 22.45 ± 13.89 (1.96) 0.0009 30.63 ± 10.67 PDR20 32.59 ± 11.21 (1.98) 24.60 ± 10.65 (1.29) 0.0034 31.88 ± 9.87 (1.4) 22.45 ± 13.89 (1.96) <0.0001 32.18 ± 8.65 PDR30 26.34 ± 7.64 (1.35) 21.71 ± 7.78 (0.94) 0.0170 26.84 ± 6.33 (0.9) 19.55 ± 7.87 (1.11) <0.0001 27.03 ± 5.29 PDR60 14.75 ± 3.82 (0.68) 12.91 ± 4.00 (0.48) NS 15.13 ± 3.28 (0.46) 11.86 ± 4.05 (0.57) 0.0002 15.82 ± 2.6 cPDR10 2.35 ± 1.59 (0.28) 1.51 ± 0.98 (0.12) 0.0072 2.10 ± 1.20 (0.17) 1.46 ± 1.25 (0.18) 0.0017 1.92 ± 0.92 cPDR15 4.83 ± 2.75 (0.49) 3.26 ± 1.91 (0.23) 0.0076 4.40 ± 2.10 (0.3) 3.11 ± 2.37 (0.34) 0.0017 4.12 ± 1.75 cPDR20 7.50 ± 3.63 (0.64) 5.20 ± 2.75 (0.33) 0.0049 6.96 ± 2.84 (0.4) 4.92 ± 3.30 (0.47) 0.0006 6.66 ± 2.44 cPDR30 12.50 ± 4.80 (0.85) 9.14 ± 4.01 (0.49) 0.0028 11.93 ± 3.77 (0.53) 8.50 ± 4.62 (0.65) <0.0001 11.7 ± 3.3 cPDR60 22.26 ± 6.47 (1.14) 17.56 ± 6.03 (0.73) 0.0042 22.01 ± 4.82 (0.68) 16.12 ± 6.71 (0.95) <0.0001 21.9 ± 4.11
Data are reported as mean (%) ± standard deviation. Values in parentheses are standard error of the mean PDR: percentage of administered dose of 13C recovered; cPDR: cumulative PDR
Table 4 Sensitivity and specificity of MBT on inflammation and fibrotic groups16
Value Inflammation group (n=100) Fibrotic group (n=67) Fibrotic + healthy groups (n=98) Inflammation group (n=67)* AUC 0.90 0.92 0.92 0.89 Sensitivity 82%† 91% 91% 83% Specificity 84%† 88% 88% 81% Positive predictive value 92%‡ 88% 79% 91% Negative predictive value 69%‡ 91% 95% 68% Significant fibrosis, n (false positives) NR 34/67 (4/34) 38/98 (8/38) NR Non-significant fibrosis, n (false negatives) NR 33/67 (3/33) 60/98 (3/60) NR
AUC: area under the curve; NR: not reported
*The subset of patients who were analysed in the fibrosis group †based on a set threshold on the point of best agreement of 83% ‡based on the dataset prevalence of 68%
Lalazar et al (2009)3
Lalazar et al (2009) assessed the role of the MBT BreathID® system in 15 patients with severe acute liver disease, and compared these to the results of biochemical tests (level III interventional evidence). All consenting adults with elevated levels of transaminases (≥10 X ULN) or bilirubin (≥10 X ULN) were enrolled between August 2005 and September 2007. In the 15 patients enrolled, aetiologies of liver disease consisted of autoimmune hepatitis (n=5), drug-induced liver injury (n=3), acute hepatitis B virus (HBV; n=2), acute hepatitis A virus (HAV; n=2) and Wilson’s disease (n=1). The remaining two were associated with a neoplastic disease or pregnancy.
Breath samples were analysed at baseline and for 60 minutes, and were compared to normal values determined based on the samples of 100 healthy volunteers (presumably the same 100 monitored for the Lalazar et al (2008) study). Mean patient age was 32.4 ± 15.2 years. Eight of the 15 patients were female. Mean ALT and AST levels were 1,930 ± 2,278 IU and 1,220 ± 1,199 IU, respectively, although these values were higher in male patients compared with female patients.
Safety
Two patients died; one patient with autoimmune hepatitis died of sepsis before a transplant could be provided, and the second patient, with neoplastic disease, died from metastatic cancer. Neither death was related to the use of the BreathID® system.
Effectiveness
The authors defined two clinically-relevant points to assess effectiveness, the point of stable improvement (defined as the point in time after which a continuous improvement was observed in patient MBT scores or blood test results), and
convergence to normality (defined as the point in time at which normal values were achieved, based on the results of healthy volunteers). Both time points were reached in the MBT data before they were achieved in any of the biochemical data (Table 5).
Table 5 Point of stable improvement and convergence to normality, comparison between MBT and biochemical tests3
Test Point of stable improvement (days after treatment)
Convergence to normality (days after treatment)
MBT 2.85 ± 2.23 8.89 ± 11.5
Alanine aminotransferase 7.33 ± 11.06 28.24 ± 21.69 Aspartate aminostransferase 7.62 ± 11.44 12.96 ± 6.96 International normalised ratio 8.62 ± 12.1 16.02 ± 24.71
Bilirubin 7.00 ± 10.61 23.63 ± 22.61
Data are reported as mean (%) ± standard deviation MBT: methacetin breath test
Pearson correlations for breath test values and blood test values ranged between 0.2 and 0.7 (Table 6). Breath test parameters showed better correlation with clinical improvement than all other tests measured.
Table 6 Correlation coefficients for MBT versus blood test parameters3
Test PDR peak Peak time PDR10 PDR30 PDR60 cPDR10 cPDR30 cPDR60
BIL -0.556 0.394 -0.519 -0.588 -0.519 -0.456 -0.580 -0.595 ALT -0.276 0.235 -0.258 -0.318 -0.403 -0.248 -0.306 -0.326 AST -0.338 0.226 -0.306 -0.384 -0.451 -0.279 -0.362 -0.405 AP -0.239 0.219 -0.308 -0.172 -0.110 -0.283 -0.282 -0.198 GGT 0.504 -0.344 0.510 0.418 0.203 0.340 0.497 0.438 LDH -0.313 0.221 -0.313 -0.325 -0.314 -0.264 -0.333 -0.332 INR -0.436 0.441 -0.412 -0.431 -0.386 -0.390 -0.458 -0.562 PTT -0.565 0.239 -0.413 -0.632 -0.471 -0.360 -0.543 -0.598 PT% 0.628 -0.364 0.475 0.673 0.513 0.440 0.609 0.660 F-VII% 0.648 -0.828 0.720 0.613 0.581 0.661 0.725 0.688 F-V% 0.576 -0.495 0.598 0.579 0.503 0.527 0.582 0.591
PDR: percentage of administered dose of 13C recovered; cPDR: cumulative PDR; BIL: bilirubin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; AP: alkaline phosphatase; GGT: gamma glutamyltranspeptidase; LDH: lactate dehydrogenase; INR: international normalised ratio; PTT: partial thromboplastin time; PT%: prothrombin time; F-VII%: coagulation factor VII; F-V: coagulation factor V
Cost impact
No cost data were identified. The manufacturer was contacted in order to acquire price information; however, Exalenz advised that such information could not be included in this report as it is considered confidential.
Ethical, cultural or religious considerations
No ethical, cultural or religious considerations were identified in the literature.
Other issues
In the study by Lalazar et al (2008)16, following the application of fibrosis criteria, there was a mean age difference of approximately nine years between patients with histologically determined non-significant and significant fibrosis (41.3 years versus 50.7 years, respectively). Previously, using the 13C-methacetin breath test,
Ciccocioppo et al (2003)4 identified a significant difference in 13C peak between geriatric (mean age 79.8 years) and adult (mean age 40.6 years) patients (30.66% ± 9.2% versus 38.33% ± 6.05%, respectively; p<0.001). Whilst the age difference is less marked in the present study, if a linear decrease in liver function with age is
assumed, an approximate difference of two per cent in the capacity to metabolise 13
C may exist between the two groups at baseline, irrespective of fibrotic status. No mention was made as to whether the device was calibrated to adjust for these differences, failure of which may bias the results and reduce the significance of group differences.
There are several clinical trials either recruiting or underway involving the use of the Exalenz BreathID® platform to assess liver damage (Table 7).
Table 7 Current and ongoing clinical trials using BreathID® to assess liver function
Location (status) Details Interventions (n) Estimated completion date
NCT01433016 Multiple locations (Not yet open for participant recruitment)
A phase II case-series to detect hepatocellular carcinoma (Sponsor: Exalenz Bioscience) Primary outcome: PDR peak
Secondary outcome: Correlation of 13C-sodium
octanoate breath test to tumour size (based on MRI data)
BreathID® system using
13C-sodium octanoate n=110 (estimated) November 2012 NCT01157845 Multiple locations (Ongoing)
A phase III case-series to predict liver failure in patients with cirrhosis
Primary outcome: Mortality from liver failure Secondary outcome: Liver transplantation
BreathID® system using
13C-methacetin substrate
n=100
July 2012
NCT01281059 Israel (Recruiting)
Case-series study to detect non-alcoholic steatohepatitis and evaluate disease severity Primary outcome: NR
Secondary outcome: NR
BreathID® system using
13C-sodium octanoate
n=NR
NCT00736840 Multiple locations (Completed)
Case-series to determine the incidence of cirrhosis based on ‘hepatic impairment score’ (HIS)
Primary outcome: Number of subjects with likelihood of cirrhosis based on HIS Secondary outcome: AUC of ROC
BreathID® system using
13C-methacetin substrate n=414 September 2009 (no results available) NCT01244503 United States (Recruiting)
A phase II case-series to predict the severity of liver disease in patients with suspected NAFLD. Primary outcome: PDR of 13C-sodium octanoate
breath test
Secondary outcome: Histology-NAS scoring of liver biopsy
BreathID® system using
13C-sodium octanoate n=150 December 2012 NCT01205074 Israel (Recruiting)
A phase II/III trial assessing the effect of various factors on MBT accuracy (Sponsor: Exalenz Bioscience)
Primary outcome: NR Secondary outcome: NR
BreathID® system using
13C-methacetin substrate
n=NR
NR
AUC of ROC: area under receiver operating characteristic curve; HIS: hepatic impairment score; MBT: methacetin breath test; MRI: magnetic resonance imaging; NAFLD: non-alcoholic fatty liver disease; NR: not reported; PDR: percentage of administered dose of 13C recovered
According to the Clinical Trials Manager of Exalenz, the company has been working on several applications of the BreathID® platform in the field of liver disease, including HCV (with and without normal ALT), HBV, NAFLD/non-alcoholic related steatohepatitis (NASH), acute liver failure, advanced (chronic/cirrhotic) liver disease survival prediction, hepatic venous pressure gradient, and liver function after bariatric surgery (personal communication, Mr. Avraham Hershkowitz, 22 May 2012).
Of interest, there is an ongoing clinical trial sponsored by the manufacturer (Exalenz, Clinicaltrials.gov ID: NCT01205074) in which the primary aim is to determine the sensitivity of the MBT to several factors which may affect 13C-methacetin
metabolism in the liver. These factors include: chronic obstructive pulmonary disease (COPD), which is associated with abnormal CO2 production and may affect the MBT given that it is based on CO2 levels; smoking, which is associated with abnormal function of CYP1A2, an enzyme involved in methacetin metabolism; CYP450 1A2 inhibitors, given that several drugs and food items inhibit CYP450 1A2 and may subsequently affect the MBT; alcohol, which may influence enzyme levels and also cause liver damage; beta-blockers, that affect portal hypertension and thus may affect hepatic blood flow; and age. Until the results of this study are known, it would be erroneous to assume a linear relationship between PDR/cPDR values and extent of liver disease across all patient populations undertaking an MBT test.
It is important to note that four of the six authors in the study by Goetze et al (2007)6 have had relationships with the manufacturer of the BreathID® device, whereas there were no conflicts of interest within the studies by Lalazar and colleagues.
Limitations of liver function tests using breath analysis include the effect that anaesthetics and cigarette smoking have on the metabolism of 13C-methacetin, which influence test results and can lead to inaccuracies. As a result, cessation of cigarette smoking must occur for a sufficient duration so as to not affect the breath test results. Furthermore, the breath test does not account for false results arising from patients who have cirrhotic or fibrotic liver disease, as it has not been
confirmed to what extent these patients suffer reduced liver function due to the amount of portosystemic shunting or whether the liver lobules are effectively dead.
The BreathID® device appears to stratify patients to high or low risk of advanced fibrosis, without the provision of data for the range between. This would indicate that the test would confer no additional benefit over similarly non-invasive tests currently used, such as transient elastography that may be easier, quicker and cheaper to use.
Baseline variations in the cytochrome P450 enzyme system function may result due to causes that are unrelated to liver disease, and could potentially confound the results. One potential confounder would be medication use, which can alter P450 function; and patients with liver disease may take more medication than healthy controls, and similarly in patients with advanced disease compared to early stage disease. The studies included have not accounted for this potential confounding effect, and in consequence, the results may be questionable.
Summary of findings
The BreathID® system may be an effective tool to assess liver function and determine the presence and severity of liver disease in patients with chronic HCV and severe acute liver disease. Due to the non-invasive nature of the test, no safety issues were identified that were associated with the use of the device. In addition, the automation of the technology minimises the bias potentially introduced through human error.
Although the technique produced relatively high specificity and sensitivity profiles when comparing low and high inflammation and fibrosis groups, the variability in baseline characteristics (e.g. smoking, alcohol consumption, COPD, beta-blockers, age) were not considered. When such inherent differences in PDR and cPDR among populations are known, the algorithm may be modified to facilitate more accurate calibration of the BreathID® device.
In conclusion, the BreathID® device presents a promising non-invasive alternative to current liver assessment tools; however, further data are required before it becomes a first line investigation method.
HealthPACT assessment:
Based on the scant evidence available at present, and the observation that four registered clinical trials will be completed by the end of 2012, HealthPACT recommended that the technology be monitored for 24 months.
Number of studies included
All evidence included for assessment in this Technology Brief has been assessed according to the revised NHMRC levels of evidence. A document summarising these levels may be accessed via the HealthPACT web site.
Total number of studies: 3
Total number of level III studies: 3
References
1. Obika, M. & Noguchi, H. (2012). 'Diagnosis and evaluation of nonalcoholic fatty liver disease', Exp Diabetes Res, 2012, 145754.
2. Martin, P. & Friedman, L. (2012). ‘Chapter 1 - Assessment of liver function and diagnostic studies’. In: Friedman L. S. & Keefe E. B. (eds), ‘Handbook of Liver Disease (Third Edition)’, Elsevier Saunders, Philadelphia, p. 1-19. 3. Lalazar, G., Adar, T. & Ilan, Y. (2009). 'Point-of-care continuous
(13)C-methacetin breath test improves decision making in acute liver disease: results of a pilot clinical trial', World J Gastroenterol, 15 (8), 966-972. 4. Ciccocioppo, R., Candelli, M. et al (2003). 'Study of liver function in healthy
elderly subjects using the 13C-methacetin breath test', Aliment Pharmacol Ther, 17 (2), 271-277.
5. Exalenz (2012). Innovating Breath Testing Solutions for Liver and GI Disorders
[Internet]. Available from: http://www.exalenz.com/ [Accessed 15 May 2012].
6. Goetze, O., Selzner, N. et al (2007). '13C-methacetin breath test as a
quantitative liver function test in patients with chronic hepatitis C infection: continuous automatic molecular correlation spectroscopy compared to isotopic ratio mass spectrometry', Aliment Pharmacol Ther, 26 (2), 305-311. 7. ABS (2009). 3303.0 - Causes of Death, Australia, 2007 [Internet]. Available
from:
http://www.abs.gov.au/AUSSTATS/subscriber.nsf/log?openagent&3303.0_1 underlying cause of death(australia).xls&3303.0&DataCubes&A934F671CE 9D473FCA25757C001 34947&0&2007&18.03.2009&Latest. [Accessed 18 May 2012].
8. AIHW (2012). Separation statistics by principal diagnosis in ICD-10-AM, Australia, 2008-09 to 2009-10 [Internet]. Available from:
http://www.aihw.gov.au/ hospitals-data-cube/?id=10737419429 [Accessed 22 May 2012].
9. Maher, L. (2012). 'Tackling hepatitis C in Australia: the third national strategy'.
10. MBS (2012). Requested Medicare items processed from July 2010 to June 2011, Liver enzyme tests [Internet]. Available from:
https://www.medicareaustralia.
gov.au/cgibin/broker.exe?_PROGRAM=sas.mbs_item_standard_report.sas&_ SERVICE=default&DRILL=ag&_DEBUG=0&group=66500%2C+66503%2C+6650 6%2C+66509%2C+66512&VAR=services&STAT=count&RPT_FMT=by+state&P TYPE=finyear&START_DT=201007&END_DT=201106 [Accessed 23 May 2012]. 11. Exalenz (2012). Press Release: FDA Grants Humanitarian Use Designation for
BreathID MBT Device for Acute Liver Failure [Internet]. Available from: http://www.exalenz.com/index.aspx?id=3907&itemID=3397 [Accessed 18 May 2012].
12. Poynard, T. & Imbert-Bismut, F. (2012). ‘Laboratory Testing for Liver Disease’. In: Boyer, T., Manns M. & Sanyal, A., (eds), ‘Zakim and Boyer's Hepatology (Sixth Edition)’. Elsevier Saunders, Philadelphia, p. 201-215.
13. Humedics.com (2012). LiMAx liver function test [Internet]. Humedics.com. Available from: http://humedics.de/index.php?article_id=14&clang=1 [Accessed 4 July 2012].
14. Stockmann, M., Lock, J. F. et al (2010). 'The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery', HPB, 12 (2), 139-146.
15. Charite University (2012). The Stockmann liver function breath test [Internet]. Available from: http://www.charite.de/sysbio/research/limax/ [Accessed 4 July 2012].
16. Lalazar, G., Pappo, O. et al (2008). 'A continuous 13C methacetin breath test for noninvasive assessment of intrahepatic inflammation and fibrosis in patients with chronic HCV infection and normal ALT', J Viral Hepat, 15 (10), 716-728.
Search criteria to be used (MeSH terms)