DOI: 10.3760/cma.j.issn.1008-1275.2013.02.001 Centre for Joint Surgery, Southwest Hospital, Third Military Medical University, Chongqing 400038, China (Fu DJ, Chen C, Guo L, Yang L)
*Corresponding author: Tel: 23-68765280, Fax: 86-23-65464006, Email: jointsurgery@163.com
Chin J Traumatol 2013;16(2):67-76
Use of intravenous tranexamic acid in total knee
arthroplasty:
a meta-analysis of randomized controlled
trials
FU De-jie, CHEN Cheng, GUO Lin, YANG Liu*
【Abstract】Objective: The effect of tranexamic acid (TA) on patients receiving total knee arthroplasty (TKA) has been reported in many small clinical trials. But single trials are not sufficient enough to clarify the effectiveness
and safety of TA. So, we carried out a meta-analysis of
randomized controlled trials to investigate the efficacy and safety of the intravenous use of TA in TKA.
Methods: Literatures were retrieved in Cochrane Library, OVID, PubMed, EMBASE, CNKI and Wanfang Data. All the related literatures were checked by two independent investigators and only the high quality randomized con-trolled trials were enrolled. Relevant data were analyzed using RevMan 5.1 to compare the difference of blood loss, transfusion and complications between TA group and con-trol group.
Results: There were 353 related literatures and only 22 randomized controlled trials met the inclusion criteria. The
use of TA in TKA significantly reduced total blood loss by
a mean of 435.41 ml (95% CI 300.62-570.21, P<0.01),
post-operative blood loss by a mean of 406.69 ml (95% CI
333.16-480.22, P<0.01). TA also significantly lowered the
transfu-sion rate (risk difference 0.30, 95% CI 0.21-0.39, P<0.01) and
transfusion volume (mean difference 0.95 unit, 95% CI
0.53-1.37, P<0.01). The risks between TA group and control group
in developing deep vein thrombosis and pulmonary embo-lism were not statistically significant.
Conclusion: TA is beneficial for patients undergoing TKA, which can significantly reduce total blood loss, post-operative blood loss, transfusion rate, and transfusion volume. Meanwhile TA is recommended to reduce deep vein thrombosis and pulmonary embolism following TKA.
Key words: Tranexamic acid; Arthroplasty; Knee; Blood loss, surgical; Meta-analysis
I
n recent years there has been an increasing aware-ness of the potential risks in allogenic blood transfusion, such as viral transmission, organic le-sion and ABO incompatibility.1,2 Total knee arthroplasty(TKA) is associated with marked blood loss, so how to avoid blood transfusion has long been a concern among clinicians.3,4 Apparently, minimizing peri- and
post-operative blood loss is the most direct way to avoid allogenic blood transfusion. Several techniques, such as autologous blood transfusion, intraoperative blood saving etc, have been adopted to reduce the need for allogenic blood transfusion.
Nowadays, tourniquet is routinely used in TKA. It not only reduces blood loss during operation but also makes a clear operation area. However, it should be emphasized that the use of tourniquet activates the lo-cal fibrinolytic system and greatly increases blood loss after operation.5 Tranexamic acid (TA) is an analogue
of lysine,6 which has powerful antifibrinolytic potency
through blocking the lysine-binding sites of plasmino-gen molecule and is mainly used in bleeding caused by acute or chronic, localized or systemic accentua-tion of fibrinolysis. Early in 1988, some investigators suggested giving TA before the release of tourniquet for its effectiveness on reducing blood loss after TKA.7 From
then on, a series of clinical trials have been done to verify this assumption. However, the use of TA still re-mains controversial.
In this study, we retrieved the clinical randomized controlled trials (RCTs) concerning the use of TA on patients who undergo a TKA. The purpose of this meta-analysis is to evaluate the effect and safety of TA in patients receiving TKA.
METHODS
Inclusion and exclusion criteria
Articles met the following criteria were included: (1) RCTs in any language; (2) patients who had undergone TKA regardless of age, sex, type or size of prosthesis used and surgical techniques; (3) intravenous use of TA for treatment group and placebo or no treatment for control group; (4) the evaluation of primary outcome by perioperative blood loss including intraoperative, post-operative and total blood loss, while the secondary out-come by the proportion of patients who needed blood transfusion, the amount of blood transfused per patient, and complications such as the incidence of deep vein thrombosis (DVT), pulmonary embolism (PE), etc. Ex-clusion criteria included: (1) RCTs of low quality; (2) simultaneous bilateral primary TKA or revision TKA; (3) original data being not enough for a meta-analysis.
Search strategy
The searching was carried out in the major medical databases including Cochrane Library (CENTRAL, 1948 to December 2012), OVID (1993 to December 2012), PubMed (1966 to December 2012), EMBASE (1966 to December 2012), CNKI (1994 to December 2012), and Wanfang Data (1982 to December 2012). No language restrictions were set. The reference lists were also checked for possible eligible articles. The keywords used for retrieve included Antifibrinolytics, Tranexamic acid, Cyklokapron, Total knee arthroplasty, Total knee replacement, TKA and TKR.
Study selection and data extraction
Two authors independently conducted the search strategy to select qualified references. The titles and abstracts of the references were read. If there was a doubt, the full text would be reviewed for clarification. The two authors respectively decided whether the ar-ticle met the inclusion criteria. Disagreement between the two authors was resolved by a senior author. When necessary, the authors of the eligible trials were con-tacted to obtain missing information.
Evaluation of methodological quality of included studies
Two authors independently assessed the methodo-logical quality of included studies. In this study, we used the tools for assessing quality and risk of bias from Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0.8 Disagreement was resolved
by a senior author.
Statistical analysis
Statistical Software Review Manager (Version 5.1 for Windows, Cochrane Collaboration) was used for meta-analysis. Continuous variables were expressed as mean plus standard deviation (SD), and the outcomes were analyzed by mean difference (MD) with 95% con-fidence interval (CI). Dichotomous variables were ex-pressed as proportions or risks, and the outcomes were analyzed by a risk difference (RD) with 95% CI. Signifi-cance levels for statistical tests were set at P<0.05.
Heterogeneity was tested for all the included articles. Chi-squared test was used to estimate heterogeneity.
P value was set at 0.1, and the quantity of heteroge-neity was measured by I2. If there was significant
he-terogeneity (P<0.1 and I2>50%), a random effect model
was used; if not, a fixed effect model was used. When necessary, subgroup analysis was carried out to ex-plore the source of heterogeneity. Data which could not be incorporated to the meta-analysis were analyzed descriptively.
RESULTS
Literature search and study characteristics
The primary search found 353 potentially relevant articles (Figure 1). After reading the titles and abstracts, 309 articles were excluded. The full texts of the remain-ing 44 articles were read conscientiously, and at last 27 RCTs met the inclusion criteria. Among them, five trials were further excluded because TA was used through knee joint injection in two trials, placebo or blank control group was not set in two trials, and patients accepted concurrent bilateral TKA in the remaining trial. Finally, 22 studies with a total of 1 361 patients were included into this meta-analysis.9-30 Most trials were
small but well designed and had generally high quality (Figure 2). Key information of the 22 publications in-cluded in the meta-analysis was shown in Table 1.
Table 1. Characteristics of included studies Transfusion trigger Hiippala 19959 Benoni 199610 Hiippala 199711 Jansen 199912 Ido 200013 Ellis 200114 Engel 200115 Tanaka 200116 Veien 200217 Good 200318 Zohar 200419 Camaras 200620 Orpen 200621 Molloy 200722 Zhang 200723 Alvarez 200824 Kakar 200925 Charoencholvanich 201126 Gautam 201127 McConnell 201228 Chareancholvanich 201229 Lee 201230 28 (15/13) 86 (43/43) 77 (39/38) 42 (21/21) 43 (21/22) 20 (10/10) 24 (12/12) 99 (73/26) 30 (15/15) 51 (27/24) 40 (20/20) 95 (35/60) 29 (15/14) 100 (50/50) 102 (51/51) 95 (46/49) 24 (12/12) 100 (50/50) 40 (20/20) 44 (22/22) 120 (60/60) 72 (36/36)
15 mg/kg before tourniquet deflation
10 mg/kg before tourniquet deflation then 10 mg/kg after 3 h 15 mg/kg before tourniquet deflation, 2 additional doses of 10 mg/kg after 3-4 h and 6-7 h
15 mg/kg 30 min before tourniquet deflation, then repeated every 8 h for 3 d
1 g just before tourniquet release and then 1 g 3 h after operation
15 mg/kg 30 min before tourniquet deflation, then 10 mg/kg/h infusion for 12 h
15 mg/kg before tourniquet deflation, then 10 mg/kg after 3 h 10 mg/kg before surgery, 10 mg/kg 10 min before tourniquet deflation
10 mg/kg before tourniquet deflation, repeated 3 h later 10 mg/kg before tourniquet deflation, repeated 3 h later 15 mg/kg 15 min before tourniquet deflation, then 10 mg/kg/h infusion for 12 h
10 mg/kg before tourniquet deflation, repeated 3 h later 15 mg/kg at cement mixing commenced
500 mg 5 min before tourniquet deflation, repeated 3 h later 1 g before tourniquet deflation, repeated 3 h later
10 mg/kg 30 min before tourniquet deflation, then 1 mg/kg/h infusion for 6 h
10 mg/kg before tourniquet deflation, then 1 mg/kg/h until wound closure
10 mg/kg 10 min before tourniquet deflation, repeated 3 h later
10 mg/kg 0.5 h before tourniquet deflation, then 2 mg/kg after 3 h
10 mg/kg at induction of anesthesia
10 mg/kg 10 min before tourniquet deflation, then 10 mg/kg 3 h postoperatively
10 mg/kg before tourniquet deflation, repeated 6 h later
LMWH LMWH LMWH LMWH NA LMWH LMWH NA LMWH LMWH LMWH LMWH LMWH Aspirin LMWH LMWH NA Ankle pump exercise NA Aspirin Ankle pump exercise LMWH Saline Saline Saline Saline None Saline None Saline None Saline None Saline Saline None Saline Saline Saline Saline Saline None None None Hb<100 g/L Hb<85 g/L Hb<100 g/L PCV<26% NA Hct<27% Hb<100 g/L NA Hct<28% Hb<90 g/L Hct<28% Hb<80 g/L Hb<90 g/L Hct<25% NA Hb<90 g/L Hb<100 g/L or Hb<80 g/L Hb<100 g/L Hb<80 g/L or Hct<30% NA Hb<100 g/L Hb<80 g/L Author and year Cases
(TA/C) Usage of TA
DVT
prophylaxis Control
Totally there were 693 patients in TA group and 668 patients in control group. The number of patients in these trials ranged from 20 to 120. All the patients received
Figure 1. Flow diagram of study selection.
primary TKA. Osteoarthritis was the main diagnosis, followed by rheumatoid arthritis. Thirteen trials used spinal anesthesia,9-11,15,17,18,20,22,24-27,29 one used a
combi-nation of general and regional anesthesia,28 five used
general anesthesia12,14,19,21,30 and the remaining three did
not mention this information.13,16,23 Saline was used in
sixteen trials as placebo9-12,14,16,18,20,21,23-27,29,30 and control
patients in the other six studies did not receive any treatment.13,15,17,19,22,28 TA was used once in three trials 9,21,28
and used repeatedly in nineteen trials.10-20,22-27,29,30 Eighteen
trials mentioned a transfusion trigger9-12,14,15,17-22,24-27,29,30
ex-cept for four studies.13,16,23,28 To prevent DVT, fourteen
trials used low-molecular-weight heparin,9-12,14,15,17-21,23,24,30
two used aspirin,22,28 two performed a mechanical ankle
pump exercise regimen,26,29 and four did not mention
any preventative measures.13,16,25,27
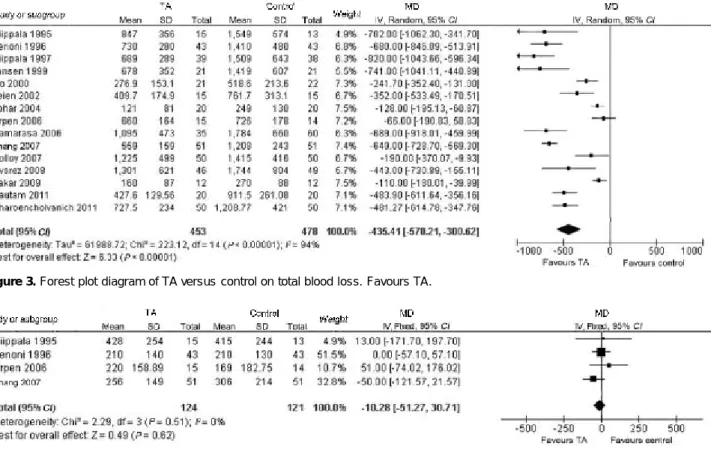
Total blood loss
Fifteen trials described total blood loss of TKA (Figure 3), including 453 patients in TA group and 478 patients in control group. TA significantly reduced total blood loss of TKA (P<0.01, MD=435.41 ml, 95% CI 300.62-570.21). There was significant heterogeneity (P<0.01, I2=94%) in
the trials, so a random effect model was used.
Intraoperative blood loss
Intraoperative blood loss was recorded in 4 trials which included 124 patients in TA group and 121 pa-tients in control group. TA did not significantly reduce intraoperative blood loss of TKA (P=0.62, Figure 4) and there was no heterogeneity between trials (P=0.51, I2=0). Postoperative blood loss
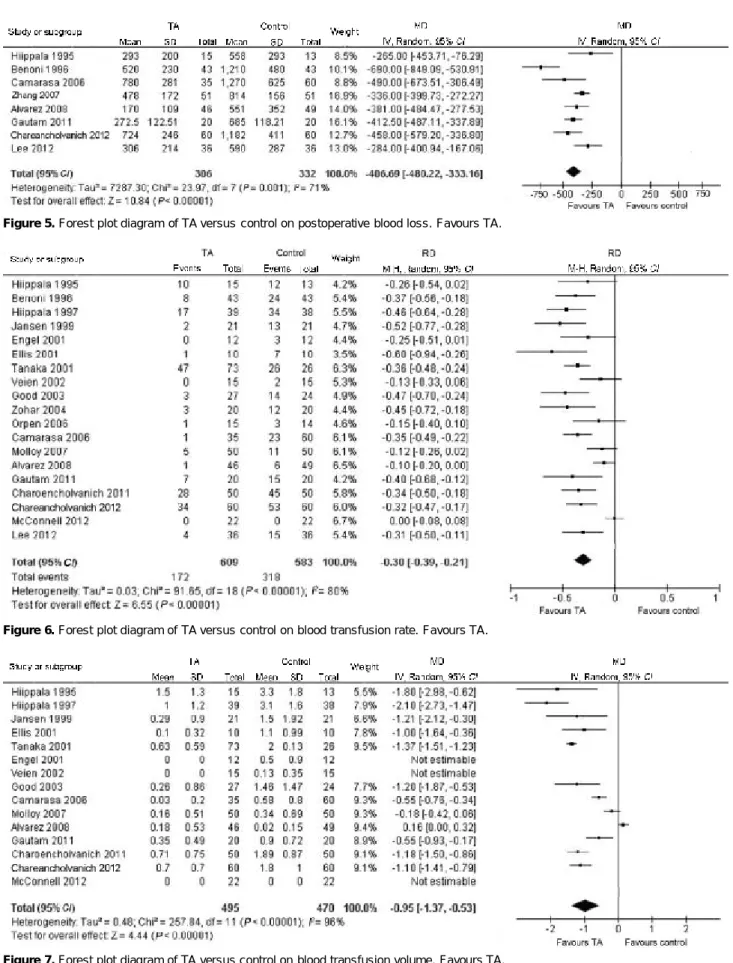
Eight trials involving 638 patients described post-operative blood loss. TA significantly reduced postop-erative blood loss compared with control group (P<0.01, MD=406.69 ml, 95% CI 333.16-480.22). But heteroge-neity was significant between trials (P=0.001, I2=71%,
Figure 5).
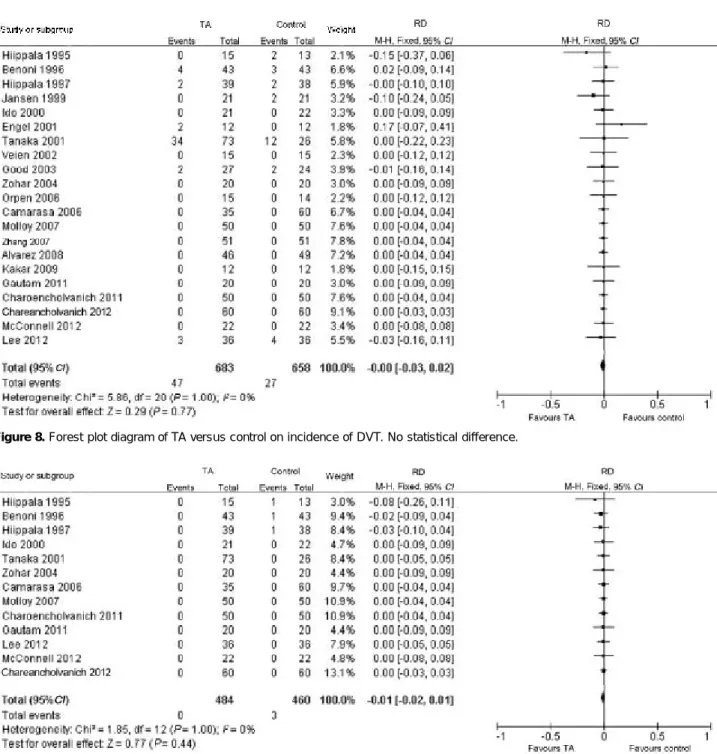
Blood transfusion rate
Blood transfusions were recorded in 19 trials (Figure 6), including 609 patients in TA group and 583 patients in control group. Among them, there were 172 patients in TA group and 318 patients in control group requiring transfusion. TA significantly reduced the proportion of patients who needed transfusion (P<0.01, RD=0.30, 95% CI 0.21-0.39). Heterogeneity still existed between trials (P<0.01, I2=80%).
353 records identified through database searching
309 records excluded on the basis of titles, abstracts or duplicates
44 full-text articles as-sessed for eligibility
22 full-text articles excluded, with reasons Not RCT: 17
Not intravenous TA: 2 No placebo control: 2 Simultaneous bilateral TKA: 1
22 studies included in this meta-analysis
Blood transfusion volume
The units of blood transfused to patients were re-corded in 15 trials (Figure 7), including 495 patients in TA group and 470 patients in control group. The MD cannot be calculated in three trials because the pa-tients in TA group did not receive any blood. TA signifi-cantly reduced blood transfusion volume compared with control group (P<0.01, MD=0.95 unit, 95% CI 0.53-1.37). However, there was significant heterogeneity between these trials (P<0.01, I2=96%).
Incidence of DVT
In 21 trials, data on DVT were available. Studies included 683 and 658 patients in TA group and control group respectively (Figure 8). Among them, 47 patients in TA group and 27 in control group developed DVT. There was no statistical difference between TA group and control group (P=0.77) and no heterogeneity be-tween trials either (P=1.00, I2=0).
Incidence of PE
In 13 trials data were available on patients’ outcome (Figure 9). In TA group, no PE was reported. In control group, 3 patients out of 460 developed PE. There was no statistical difference in the risk of developing PE be-tween TA group and control group (P=0.44). There was
no heterogeneity between trials either (P=1.00, I2=0). Other complications
Besides thromboembolic episodes, some other com-plications were found. Three studies reported 6 cases of infection, 5 in TA group and 1 in control group.10,18,22
He-matoma was reported in three studies, including 9 pa-tients in TA group and 6 in control group.10,26,29
Secre-tions occurred in the wounds of 5 patients, 3 from TA group and 2 from control group. In TA group, one pa-tient with a history of ischemic heart disease suffered from myocardial infarction and recovered after prompt therapy.9 One patient was hospitalized for 11 days
be-cause of heart attack 4 weeks after TKA.11 Three days
after TKA, one patient in TA group suffered from chest pain and showed hypotension.10 In one trial,11 one
pa-tient from TA group who had 30 years history of rheu-matoid arthritis suffered from pneumonia and respira-tory failure 4 days after operation and another developed pneumonia 4 weeks after operation; one patient from control group suddenly died 15 days after operation and the cause of death was certified as PE. Three patients in TA group had mild nausea16 and a few patients complained
of nausea and vomiting.13 In the telephone follow-up, one
patient from TA group died of aggravation of prior pulmo-nary fibrosis 3 months after surgery.20
Figure 3. Forest plot diagram of TA versus control on total blood loss. Favours TA.
Figure 7. Forest plot diagram of TA versus control on blood transfusion volume. Favours TA.
Figure 6. Forest plot diagram of TA versus control on blood transfusion rate. Favours TA.
DISCUSSION
TA could reversibly occupy the lysine-binding sites of the plasminogen molecule and has powerful antifibrinolytic potency. It has been widely used in many kinds of therapies. Besides orthopedic surgery, it has been used in pediatric urinary tract surgery,31 ruptured
intracranial aneurysms,32 oral surgery,33 gynecologic
surgery,34 caesarian section,35 upper gastrointestinal
hemorrhage,36 cardiac surgery,37 and so on. The trial
Clinical Randomization of an Antifibrinolytic in
Signifi-cant Hemorrhage 2 (CRASH-2) showed that TA can sig-nificantly reduce the all-cause mortality as a result of bleeding.38 Cap et al39 suggested that TA should be
in-corporated into trauma clinical practice guidelines and treatment protocols.
In 2008, 615 050 total knee replacements were per-formed in the United States adult population, 134% more than in 1999.40 The number of patients receiving TKA
increases nowadays, which largely raises the risks of allogenic blood transfusion and heavily burdens the
pub-Figure 9. Forest plot diagram of TA versus control on incidence of PE. No statistical difference.
lic health system. TA is cheap and very efficacious in reducing blood loss. Therefore, it is a very promising drug to lower these risks.
According to the results of our meta-analysis, TA can significantly reduce total blood loss andpostoperative blood loss. TA reduced the proportion of patients who needed transfusion by 30% and the decreased average volume of blood transfusion researched 0.95 unit. TA also did not raise the risks of DVT or PE. But signifi-cant heterogeneity existed between trials. Heteroge-neity could result from many factors, such as different types of prosthesis, surgical techniques, TA usage, tourniquet usage and types of anesthesia. We carried out a subgroup analysis on blood transfusion rate ac-cording to the type of anesthesia, use of transfusion protocols, TA dose (high dose >3 000 mg ) and whether the patient received LMWH. Results were accordant to the original analysis. Except for TA dose, other factors could not explain the source of heterogeneity: hetero-geneity did not exist in the high dose subgroup (P=0.79,
I2=0), but existed in the low dose subgroup (P<0.01,
I2=80%).
Complications associated with TA use have been reported. Concerning the occasional thromboembolic events, most of doctors are reluctant to use TA in TKA. However, with many prospective random clinical trials being carried out, the safety of TA has been further confirmed. Intravenous administration of TA was ap-proved by the Food and Drug Administration (FDA) in 1986 for prevention or reduction of bleeding in patients with hemophilia undergoing dental procedures. And in 2009 the FDA approved its oral use to control heavy menstrual cyclic bleeding. In the CRASH-2 study,36 there
was no significant difference between TA group and con-trol group on incidence of DVT and PE. In our study, 47 patients in TA group and 27 patients in control group developed DVT. However, there was no significant dif-ference (P=0.77) between two groups either, which was consistent with the result of CRASH-2. Three patients developed PE events, and they were all from control group unexpectedly. The difference between TA and con-trol group was also not statistically significant (P=0.44). Then, from the result of our meta-analysis, TA did not increase risks of DVT or PE. We presume that use of TA mainly resists the fibrinolytic effect caused by tour-niquet and generally has little influence on the normal fibrinolytic system. Thereby, TA does not obviously
cause thromboembolic events. However, clinical trials are requisite to certify this hypothesis. Additionally, most of the reported thromboembolic events are detected by clinical examinations and Doppler ultrasound. Few studies perform a routine screening for thromboembolic com-plications as the study of Tanaka et al’s16, in which all
the patients receive bilateral radioisotope venography and a standard perfusion lung scan imaging between 7 and 14 days after surgery. We insist on routine screen-ing for thromboembolic complications after surgery in future trials.
There are also many other meta-analysis articles that investigate the effect of TA in TKA. Ho and Ismail41
studied the effect of TA in total hip arthroplasty (THA) and TKA. Data were analyzed without distinguishing TKA and THA. This might be a source of heterogeneity. Cid and Lozano42 carried out a meta-analysis focused
on the effects of TA in TKA. But only 9 trials were in-cluded in the study and the quality of some trials is very low. The result of their meta-analysis also showed that TA is effective to reduce the proportion of patients who need transfusion. However, they did not mention the blood loss as well as the complications. Zufferey et al43 and Kagoma et al44 performed another two
meta-analysis articles. They both assessed the effect of antifibrinolytics on blood loss and blood transfusion rate in orthopedic surgery. But all antifibrinolytics were as-sessed in a single group or one of them was analyzed in many kinds of orthopedic surgeries. Yang et al45 and
Alshryda et al46 also did similar researches, but the
usage of TA included intrav enous and topical administration.
In our study, most of the trials are of high quality, which makes the conclusions drawn from this meta-analysis more reliable. We focused on the efficacy of TA in TKA only to reduce heterogeneity caused by other types of orthopedic surgery. Blood loss was analyzed separately by total, intraoperative and postoperative time period. Besides, we only included trials in which TA is used intravenously, and those that do not set placebo or blank controls are excluded.
There are still many limitations in our analysis. (1) Some of the included studies have an obvious defect, i.e. the collection of autologous blood before surgery. It can be a confusing factor for the determination of blood loss based on decrease of hemoglobin, especially when the
blood collection program is not standard. (2) We mainly analyze the effect and safety of TA used in TKA. However, the recovery of joint function and quality of life after surgery are also very important for patients. Be-cause few trials report these aspects, data are not enough for analysis. Many studies record the number of patients who develop DVT and PE, but the methods and time of follow-up are different, so the results are less comparable. Complications should be examined in larger clinical trials. (3) We searched for trials in the major medical databases and did not search for gray literature, such as special topic report, unpublished documents, government reports and other conventional or unconventional literature. So, publication bias might exist.
In a word, on the basis of these analyzed studies, this meta-analysis concludes that TA effectively reduces blood loss and transfusion rate as well as transfusion volume after TKA and does not apparently increase the risk of DVT or PE. The optimal dose and administra-tion of TA in TKA still need further investigaadministra-tion.
REFERENCES
1. Levy O, Martinowitz U, Oran A, et al. The use of fibrin tissue adhesive to reduce blood loss and the need for blood trans-fusion after total knee arthroplasty. A prospective, randomized, multicenter study. J Bone Joint Surg Am 1999;81(11):1580-8.
2. Lemaire R. Strategies for blood management in orthopaedic and trauma surgery. J Bone Joint Surg Br 2008;90(9):1128-36.
3. Tobias JD. Strategies for minimizing blood loss in orthope-dic surgery. Semin Hematol 2004;41(1 Suppl 1):145-56.
4. Moonen AF, Neal TD, Pilot P. Peri-operative blood ma-nagement in elective orthopaedic surgery. A critical review of the literature. Injury 2006;37 Suppl 5:S11-6.
5. Burkart BC, Bourne RB, Rorabeck CH, et al. The efficacy of tourniquet release in blood conservation after total knee arthroplasty. Clin Orthop Relat Res 1994;(299):147-52.
6. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl 1987;137:22-5.
7. Benoni G, Carlsson A, Petersson C, et al. Does tranexamic acid reduce blood loss in knee arthroplasty? Am J Knee Surg 1995; 8(3):88-92.
8. The Cochrane Collaboration. Cochrane handbook for sys-tematic reviews of interventions. Version 5.1.0 [EB/OL]. [2011-10-30]. www.cochrane-handbook.org/.
9. Hiippala S, Strid L, Wennerstrand M, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with
total knee arthroplasty. Br J Anaesth 1995;74(5):534-7. 10. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss an d blood transfusion after kn ee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br 1996;78(3):434-40.
11. Hiippala ST, Strid LJ, Wennerstrand MI, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg 1997;84(4):839-44.
12. Jansen AJ, Andreica S, Claeys M, et al. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth 1999;83(4):596-601.
13. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg 2000;120(9):518-20.
14. Ellis MH, Fredman B, Zohar E, et al The effect of tourni-quet application, tranexamic acid, and desmopressin on the procoagulan t and fibrin olytic systems during total knee replacement. J Clin Anesth 2001;13(7):509-13.
15. Engel JM, Hohaus T, Ruwoldt R, et al. Regional hemo-static status and blood requirements after total knee arthroplasty with and without tranexamic acid or aprotinin. Anesth Analg 2001; 92(3):775-80.
16. Tanaka N, Sakahashi H, Sato E, et al. Timing of the admin-istration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br 2001;83(5):702-5. 17. Veien M, Sorensen JV, Madsen F, et al. Tranexamic acid given intraoperatively redu ces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand 2002;46(10):1206-11.
18. Good L, Peterson E, Lisander B. Tranexamic acid de-creases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth 2003;90(5):596-9.
19. Zohar E, Ellis M, Ifrach N, et al. The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Analg 2004;99(6):1679-83.
20. Camarasa MA, Olle G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth 2006:96(5):576-82.
21. Orpen NM, Little C, Walker G, et al. Tranexamic acid redu ces early post-operative blood loss after total kn ee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee 2006;13(2):106-10.
22. Molloy DO, Archbold HA, Ogonda L, et al. Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomised controlled trial. J Bone Joint Surg Br 2007;89(3):306-9.
23. Zhang F, Gao Z, Yu J. Clinical comparative studies on effect of tranexamic acid on blood loss associated with total knee
arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2007; 21(12):1302-4.
24. Alvarez JC, Santiveri FX, Ramos I, et al. Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion 2008;48(3): 519-25.
25. Kakar PN, Gupta N, Govil P, et al. Efficacy and safety of tranexamic acid in control of bleeding following TKR: a random-ized clinical trial. Indian J Anaesth 2009;53(6):667-71.
26. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospec-tive randomized controlled trial. Clin Orthop Relat Res 2011;469 (10):2874-80.
27. Gautam PL, Katyal S, Yamin M, et al. Effect of tranexamic acid on blood loss and transfusion requirement in total knee re-placement in the Indian population: a case series. Indian J Anaesth 2011;55(6):590-93.
28. McConnell JS, Shewale S, Munro NA, et al. Reducing blood loss in primary knee arthroplasty: a prospective randomised controlled trial of tranexamic acid and fibrin spray. Knee 2012;19 (4):295-8.
29. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, et al. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord 2012; 13:124.
30. Lee SH, Cho KY, Khurana S, et al. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective ran-domized controlled trial. Knee Surg Sports Traumatol Arthrosc 2012, In press.
31. Ro JS, Knutrud O, Stormorken H. Antifibrinolytic treat-ment with tranexamic acid (AMCA) in pediatric urinary tract surgery. J Pediatr Surg 1970;5(3):315-20.
32. Gibbs JR, Corkill AG. Use of an anti-fibrinolytic agent (tranexamic acid) in the management of ruptured intracranial aneurysms. Postgrad Med J 1971;47(546):199-200.
33. Bedil M. Use of tranexamic acid as hemostatic in oral surgery. Trib Odontol (B Aires) 1971;55(7):175-6.
34. Rybo G, Westerberg H. The effect of tranexamic acid (AMCA) on postoperative bleeding after conization. Acta Obstet Gynecol Scand 1972;51(4):347-50.
35. Gai MY, Wu LF, Su QF, et al. Clinical observation of blood loss reduced by tranexamic acid during and after caesarian
section: a multi-center, randomized trial. Eur J Obstet Gynecol Reprod Biol 2004;112(2):154-7.
36. Cormack F, Chakrabarti RR, Jouhar AJ, et al. Tranexamic acid in upper gastrointestinal haemorrhage. Lancet 1973;1(7814): 1207-8.
3 7 . Ha rdy J F, Desr och e s J . Nat u ral an d syn th et ic antifibrinolytics in cardiac surgery. Can J Anaesth 1992;39(4): 353-65.
38. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376(9734):23-32.
39. Cap AP, Baer DG, Orman JA, et al. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma 2011; 71(1 Suppl):S9-14.
40. Losina E, Thornhill TS, Rome BN, et al. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am 2012;94(3):201-7.
41. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfu sion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care 2003;31 (5):529-37.
42. Cid J, Lozano M. Tranexamic acid reduces allogeneic red cell transfusions in patients undergoing total knee arthroplasty: results of a meta-analysis of randomized controlled trials. Trans-fusion 2005;45(8):1302-7.
4 3 . Zu f fe rey P , M er q u iol F, L ap ort e S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology 2006;105(5):1034-46.
44. Kagoma YK, Crowther MA, Douketis J, et al. Use of antifibrinolytic therapy to reduce transfusion in patients undergo-ing orthopedic surgery: a systematic review of randomized trials. Thromb Res 2009;123(5):687-96.
45. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am 2012;94(13):1153-9.
46. Alshryda S, Sarda P, Sukeik M, et al. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br 2011;93(12):1577-85.
(Received September 25, 2012)