associated with reversible myositis-myalgia in
statin-treated patients
WAQAS AHMED, NASEER KHAN, CHARLES J. GLUECK, SUMAN PANDEY, PING WANG,
NAILA GOLDENBERG, MUHAMMAD UPPAL, and SURAJ KHANAL
CINCINNATI, OHIO
Our specific aims were to determine whether low serum 25 (OH) vitamin D (D21D3)
(,32 ng/mL) was associated with myalgia in statin-treated patients and whether the
myalgia could be reversed by vitamin D supplementation while continuing statins. After excluding subjects who took corticosteroids or supplemental vitamin D, serum 25 (OH) D was measured in 621 statin-treated patients, which consisted of 128 pa-tients with myalgia at entry and 493 asymptomatic papa-tients. The 128 myalgic papa-tients
had lower mean6standard deviation (SD) serum vitamin D than the 493
asymptom-atic patients (28.6613.2 vs 34.2613.8 ng/mL,P,0.0001), but they did not differ
(p.0.05) by age, body mass index (BMI), type 2 diabetes, or creatine kinase levels.
By analysis of variance, which was adjusted for race, sex, and age, the least square
mean (6standard error [SE]) serum vitamin D was lower in the 128 patients with
my-algia than in the 493 asymptomatic patients (28.7 6 1.2 vs 34.3 6 0.6 ng/mL,
P,0.0001). Serum 25 (OH) D was low in 82 of 128 (64%) patients with myalgia versus
214 of 493 (43%) asymptomatic patients (c2517.4,P,0.0001). Of the 82
vitamin-D–deficient, myalgic patients, while continuing statins, 38 were given vitamin D (50,000 units/week for 12 weeks), with a resultant increase in serum vitamin D from
20.467.3 to 48.2617.9 ng/mL (P,0.0001) and resolution of myalgia in 35 (92%).
We speculate that symptomatic myalgia in statin-treated patients with concurrent vitamin D deficiency may reflect a reversible interaction between vitamin D defi-ciency and statins on skeletal muscle. (Translational Research 2009;153:11–16)
Abbreviations:BMI¼body mass index; CK¼creatine kinase
V
itamin D deficiency or insufficiency1 isbe-coming more widespread in diverse clinical populations in variegated settings and
environ-ments.2-4 Low serum 25 (OH) vitamin D levels have
been associated with myositis.5 Erkal et al5 reported
a strong correlation between low serum 25 (OH) D levels and higher rates and longer duration of general-ized bone pain and/or muscle aches and pains (often
diagnosed as fibromyalgia). Bischoff-Ferrarri al6 have
reported that vitamin D may improve muscle strength through a highly specific nuclear receptor in muscle
tis-sue. Lips7reported that muscle cells contain vitamin D
receptors and noted that serum 25 (OH) D is related to physical performance. Hypovitaminosis D is highly
prevalent in adults with type 2 diabetes.8
Mild clinical muscle problems (myositis-myalgia) are
common in subjects treated with statins.9In a
retrospec-tive analysis of members of a health maintenance orga-nization, which included 10,247 patients with diabetes and 21,978 patients without diabetes, Nichols and
Koro9employed National Heart, Lung, and Blood
Insti-tute categories for clinical muscle problems: myalgia, mild myositis, severe myositis, and rhabdomyolysis. A From the Cholesterol Center, Jewish Hospital of Cincinnati, Cincinnati,
Ohio.
Supported in part by the Lipoprotein Research Fund and the Medical Research Funds of the Jewish Hospital of Cincinnati.
Submitted for publication October 15, 2008; revision submitted November 5, 2008; accepted for publication November 7, 2008. Reprint requests: Charles J. Glueck, Cholesterol Center, ABC Build-ing, 3200 Burnet Ave, Cincinnati, OH 45229; e-mail: glueckch@ healthall.com.
1931-5244/$ – see front matter
Ó2009 Mosby, Inc. All rights reserved. doi:10.1016/j.trsl.2008.11.002
greater proportion of statin initiators versus matched controls experienced ‘‘myopathic events,’’ 7.9% versus 5.5% in diabetics and 9.0% versus 3.7% in the
nondia-betic cohorts.9Nichols and Koro9reported that 95% of
statin-associated events were myalgia-mild myositis. Although uncommon, severe myositis, which is also called myopathy with creatine kinase (CK) more than 10 times the laboratory upper normal limit, is signifi-cantly associated with statin monotherapy (relative
risk 5 2.8; 95% confidence interval,1.3–5.9).10 In
subjects on statin monotherapy, myopathy rates with CK levels more than 10 times the laboratory upper limit have been reported to be 33 per 100,000 person years,
with a mean time to event of 2 years.10
Our specific aims were to determine whether low
serum 25 (OH) vitamin D (D2 1 D3) (,32 ng/mL)
was associated with myalgia in statin-treated patients and whether the myalgia could be reversed by vitamin D supplementation while continuing statins.
MATERIALS AND METHODS
Study design: patients. The study conformed to the ethical guidelines of the Jewish Hospital Institutional Review Board for human research.
From May 2007 to May 2008, in the temporal order of their referral to our outpatient Cholesterol Center for diagnosis and therapy of hyperlipidemia, serum 25
(OH) vitamin D (D2 1D3) was measured in 687
sta-tin-treated patients, 140 of whom had myositis-myalgia at study entry and 547 patients were asymptomatic. After excluding subjects who were taking corticosteroids or supplemental vitamin D, or who had comorbidities that would result in muscle or bone pain (fibromyalgia,
arthritis, peripheral vascular disease, and sensory neu-ropathy), we studied 621 statin-treated patients, of whom 128 had symptomatic myositis-myalgia and 493 were asymptomatic at study entry.
At the initial visit, after an overnight fast, blood was
drawn for a total of 25 (OH) vitamin D levels (D21
D3), quantitated by 2-dimensional liquid chromatogra-phy with tandem mass spectrometry detection after
pro-tein precipitation.11The laboratory lower normal limit
for total 25 (OH) vitamin D was 32 ng/mL.11Additional
measures included plasma cholesterol, triglyceride, and high-density lipoprotein cholesterol, along with CK, glucose, and insulin testing, as well as renal, thyroid, and liver function tests.
At the initial and follow-up visits, a detailed history was obtained for statin, prescription drug, and supple-mental vitamin use. Patients were instructed not to take supplemental vitamins, and, where initial serum vitamin D was low, and when myalgia was present, were given a prescription to take 50,000 units of vitamin D (ergocal-ciferol) once per week for 12 weeks. Most patients were continued during follow-up on the same statins that they had been taking at study entry.
At the initial visit and at every follow-up visit, patients were interviewed by the principal investigators, who em-ployed National Heart, Lung, and Blood Institute cate-gories for clinical muscle problems: myalgia, mild
myositis, severe myositis, and rhabdomyolysis.9The
dis-tinction between myalgia and nonmyalgic groups is
neces-sarily imprecise; it is based entirely on subjective reports.9
In the current study, at entry, we characterized the most severe myositis-myalgias as those that had caused patients to discontinue more than 3 different statins.
In all, 38 statin-treated patients with myalgia and low se-rum vitamin D at study entry had follow-up visits for 3 months on statins plus vitamin D (50,000 units/week for 12 weeks). We prospectively assessed changes in their myositis-myalgia symptoms and serum vitamin D levels. Adherence to the weekly vitamin D (50,000 units/ week) was reviewed by the investigators at each follow-up visit (1 and 3 months after study entry).
Statistical analyses. All statistical analyses were per-formed using SAS (version 9.1; SAS Institute, Inc., Cary, NC). Sample size calculations were based on
population studies of serum vitamin D12and assessments
of optimal13serum vitamin D levels, using an estimate of
mean6standard deviation (SD) serum vitamin D of 28
6 10 ng/mL for statin-using patients with
myositis-myalgia, and 356 10 ng/mL for asymptomatic
statin-using patients. With alpha50.05 and power50.8, 34
statin-using patients with myositis-myalgia and 34 asymptomatic statin-using patients would be required to detect differences in serum 25 (OH) vitamin D levels.
AT A GLANCE COMMENTARY Background
Low serum 25 (OH) vitamin D has been associated with myositis. Myositis is common in statin-treated subjects to promote statin intolerance.
Translational Significance
The current report revealed that patients with statin-induced myalgias had lower serum vitamin D levels than statin-treated patients without myal-gias. Low serum 25 (OH) vitamin D (D2 1D3) (,32 ng/mL) is associated with myalgia in sta-tin-treated patients; while continuing statins, this myalgia can largely be reversed by vitamin D sup-plementation that normalizes serum vitamin D levels. We speculate that vitamin D deficiency reversibly augments statin-induced myalgias.
Comparisons of categorical variables were performed
by X2tests, the Fisher exact tests, or Mantel-Haenszel X2
tests. Comparisons of numerical variables were done using nonparametric Wilcoxon tests. An analysis of variance was used to compare least square mean serum vitamin D in patients with and without entry myositis-myalgia, after covariance adjusting for age, sex, and race. Changes in serum vitamin D after 3 months of supplementation with vitamin D (50,000 units/week) in 38 symptomatic statin users with low serum vitamin D at entry were compared using nonparametric-paired Wilcoxon tests.
RESULTS
We studied 621 statin-treated patients, which
consisted of 128 patients with myalgia at study entry and 493 asymptomatic patients. These patients were categorized by low serum 25 OH D (,32 ng/mL), and subsequent treatment with vitamin D supplementation in 38 myalgic, vitamin-D–deficient patients while
con-tinuing statin therapy (Figs 1 and 2,Tables I–III). All
621 patients had normal thyroid-stimulating hormone
and thyroxine.14
The 128 symptomatic and 493 asymptomatic
statin-taking patients did not differ (P. 0.1) at study entry
by age, body mass index (BMI), type 2 diabetes mellitus,
or high CK, but more nonwhites (12% vs 5%,P5.012)
and women (59% vs 44%,P50.0025) were present in
the symptomatic than in the asymptomatic group (Table I). Of the 128 symptomatic statin-taking patients,
3 had developed myositis-myalgia that caused discontin-uation of more than 3 statins prior to referral to our center. At study entry, the distribution of serum vitamin D was shifted to lower levels in the 128 patients with
myalgia versus the 493 asymptomatic patients (Fig 2).
The mean serum vitamin D was lower in the 128 patients with myalgia than in the 493 asymptomatic patients
(28.66 13.2 vs 34.2613.8 ng/mL, P,0.0001;Fig
1,Table II). By analysis of variance, which was adjusted
for race, sex, and age, the least square mean6standard
error (SE) serum vitamin D was lower in the myalgia
group than in the asymptomatic group (28.7 6 1.2 vs
Fig 1.Characterization of cohort and of vitamin D supplementation.
Fig 2. Distributions of serum 25 OH vitamin D in 128 statin-treated patients with myalgia at study entry and in 493 statin-treated asymp-tomatic patients.
34.3 6 0.6 ng/mL; P , 0.0001; Table II). When the serum vitamin D distribution was examined categori-cally, the myalgia group was overrepresented in the lower end of the distribution, and the asymptomatic group was overrepresented in the upper end of the
distri-bution (Mantel-Haenszelc2516.9,P,0.0001;Table
II). In all, 64% of the 128 patients with myalgia had low
vitamin D versus 43% of the 493 asymptomatic patients
(c2517.4;P,0.0001; Table I).
Vitamin D supplementation was given only to patients who had myalgia symptoms and vitamin D levels less than 32 ng/mL, and it was not given to the 46 symptom-atic patients who had normal entry serum vitamin D
levels (Fig 1). Of the 82 myalgic, vitamin-D–deficient
patients, 38 received 50,000 units vitamin D per week for 12 weeks, 8 had just started vitamin D therapy, 22 had only 1 visit without follow-up, and 14 were currently
untreated (Fig 1). The 38 patients did not differ (P .
0.05) from the 44 patients by entry vitamin D levels
(20.467.3 vs 21.167.0 ng/mL), by age (60612 vs
58 6 12 years), by CK (165 6 153 vs 125 6 98
IU/L), or by BMI (31.165.8 vs 31.16 10.6 kg/m2).
The 38- and 44-patient groups also did not differ by sex (45% male vs 41% male), but they did differ by race, with more nonwhite patients in vitamin D treatment
group (29% vs 5%,P50.0026).
After 3 months follow-up on vitamin D in the 38 myalgic, vitamin-D–deficient, statin-treated patients,
mean 6 SD serum vitamin D increased from 20.4 6
7.3 to 48.2617.9 ng/mL (P,0.0001), and 35 patients
(92%) had become free of myalgia (Table III).
In the 38 symptomatic statin-treated patients with low entry vitamin D who subsequently received vitamin D supplementation, the most frequently used statins were
rosuvastatin at entry and continued (n510), atorvastatin
at entry then switched to rosuvastatin (n57),
atorvasta-tin at entry and then conatorvasta-tinued (n54), and pravastatin at
entry and then continued (n52).
By investigator interview, adherence to the weekly regimen of 50,000 units of vitamin D per week was excel-lent, with no patients recording missed doses. No side ef-fects attributable to the vitamin D therapy were reported.
DISCUSSION
The current report revealed that patients with statin-in-duced myalgias had lower serum vitamin D levels than statin-treated patients without myalgias. Low serum 25
(OH) vitamin D (D21D3) is associated with myalgia
in statin-treated patients and, while continuing statins, this myalgia can largely be reversed by vitamin D sup-plementation which normalizes serum vitamin D levels. We speculate that vitamin D deficiency reversibly augments statin-induced myalgias.
Table I. Characteristics of 621 hypercholesterolemic statin-taking patients with and without myalgia-myositis at study entry Group Serum vitamin D: low ( , 32 mg/mL), normal ( $ 32) Gender Race Age (years) BMI (kg/m 2) Type 2 diabetes CK high ( . 250 and , 2500 U/L) Myalgia (n 5 128) 82 (64% ) low 52 (41%) M 113 (88%) W, 60 6 11 29.6 6 7.6 11 (9%) 15 (12% ) 46 (36% ) norma l 76 (59%) F 12 (9%) B, 3 (2%) O Asymptom atic (n 5 493) 21 4 (43%) low 274 (56% ) M 466 (95%) W, 58 6 12 28.5 6 5.5 44 (9%) 45 (10% ) 27 9 (57%) norma l 219 (44% ) F 22 (4%) B, 5 (1%) O Myalgia vs asy mptom atic group comp arison X 25 17 .38 X 25 9.11 X 25 6.28, df 5 1 P 5 0.16 P 5 0.54 X 25 0.004 X 25 0.57 P , 0.00 01 P 5 0.0025 P 5 0.012 (Wilcoxon) (Wilcoxon) P 5 0.95 P 5 0. 45 ABBREVIATIONS: B , black; F , female; M , male; O , other; W , white.
In the current study, the symptomatic (myositis-myal-gia) and asymptomatic groups did not differ in variables that can affect serum 25 (OH) vitamin D levels, which
include age, BMI,15type 2 diabetes,8and by selection,
exogenous vitamin D, or corticosteroid use.16 More
women (59% vs 44%) and more nonwhites (12% vs 5%) were in the myalgia group than in the asymptomatic group. Statin-treated females seem to have more
myop-athy than males.17This result perhaps is related to lower
female exposure to sun, which results in more common
vitamin D deficiency.18 African Americans are much
more likely than Caucasians to have vitamin D
defi-ciency,19,20 which is related to decreased efficacy of
vitamin D production by darker pigmented skin. Low se-rum vitamin D levels are very common; in this study, lower levels are found in 43% of 493 asymptomatic patients. This result provides a broad base for potential interactions with commonly used statins to facilitate development of myositis-myalgia.
In the current report, vitamin D supplementation that normalized serum vitamin D levels was concurrently asso-ciated with resolution of myalgias in 35 of 38 (92%) statin-treated patients with myalgia and low pretreatment serum vitamin D levels. We speculate that statins and vitamin D deficiency interact additively or synergistically on the muscle to produce myalgia that is reversible by vitamin D supplementation, while continuing statin therapy.
Low 25 (OH) vitamin D levels have been associated
with myositis-myalgia5,6 and reduced muscle
func-tion.21-23 A case report of reversible muscle weakness
in a patient with vitamin D deficiency has been
pub-lished.24The association of vitamin D deficiency, statin
use, and myopathy has been commented on in a case
re-port.25Vitamin D may improve muscle strength through
a highly specific nuclear receptor in muscle tissue.6,7In
healthy elderly subjects, 25 (OH) vitamin D levels are
related to physical performance.26
The pathoetiology of statin-induced myalgia-myopa-thy is not well understood, but common polymorphic
variants within SLCO1B1 on chromosome 12 are
strongly associated with an increased risk of
statin-in-duced myopathy,17 perhaps by increasing statin blood
levels. Although 95% of statin-associated clinical events
involve myalgia or mild myositis,9myalgic symptoms
are often enough to cause the patients to stop statin
ther-apy.27We speculate that symptomatic myositis-myalgia
in statin-treated patients may reflect a potentially revers-ible interaction in muscle between 25 (OH) vitamin D deficiency and statins, which individually are commonly
associated with mild myositis-myalgia.9,10,27
A limitation of our and other studies of myositis-myalgia lies in reliance on patients’ subjective reports of muscle symptoms. Our study was also limited by not having a matched, blinded, vitamin-D–deficient
Table II. Distribution of serum vitamin D levels in 621 hypercholesterolemic patients taking statins: 128 patients with symptomatic myalgias-myositis and 493 asymptomatic patients at study entry Serum vitamin D (ng/mL) Mean 6 SD Adjusted * Mean 6 SE , 10 10– , 20 20– , 32 32– , 40 40– , 60 60– , 80 $ 80 Myal gia (n 5 128) 28.6 6 13.2 28.7 6 1.2 7 (5.4%) 28 (21.9 %) 47 (36.7 %) 19 (1 4.8%) 25 (19.5 %) 1 (0.8 %) 1 (0.8%) Asymp tomati c (n 5 49 3) 34.2 6 13.8 34.3 6 0.6 12 (2.4%) 58 (11.8 %) 144 (29.2 %) 116 (2 3.5%) 145 (29.4 %) 16 (3.3 %) 2 (0.4%) Wil coxon test ANOVA P , 0.0001 P , 0.0001 Mant el-Haenszel X 2 5 16 .9, P , 0.0001 ABBREVIATIONS: ANOVA, analysis of variance. * Adjusted for race, gender, and age.
control group with previous myositis-myalgia on statins, continuing statins, given a vitamin D placebo, and by the absence of a second matched, blinded, vitamin-D– deficient control group with previous myositis-myalgia on statins, given a statin placebo, and vitamin D treat-ment. The lack of blinding in the current study is an important issue, especially in the context of subjective reports of symptomatic myositis-myalgia. Future double
blind, placebo-controlled studies of statin-taking
patients with low serum vitamin D and symptomatic myositis-myalgia will be needed to confirm our current observations, which suggest that in vitamin-D–deficient, statin-taking patients with myalgia, vitamin D supple-mentation that normalizes serum vitamin D is associated with resolution of myalgia in 92% of patients.
Speculations. Symptomatic myositis-myalgia in sta-tin-treated patients may reflect a potentially reversible additive or synergistic interaction in muscle between 25 (OH) vitamin D deficiency and statins, which individ-ually are commonly associated with mild myositis-myal-gia.9,10,27Normalization of low serum 25 (OH) D by oral vitamin D largely reverses myositis-myalgia, which
otherwise might cause statin intolerance.27
REFERENCES
1. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080S–6. 2. Mark S, Gray-Donald K, Delvin EE, et al. Low vitamin D status in
a representative sample of youth from Quebec, Canada. Clin Chem 2008;54:1283–9.
3. Goswami R, Mishra SK, Kochupillai N. Prevalence & potential significance of vitamin D deficiency in Asian Indians. Indian J Med Res 2008;127:229–38.
4. Alsafwah S, Laguardia SP, Nelson MD, et al. Hypovitaminosis D in African Americans residing in Memphis, Tennessee with and without heart failure. Am J Med Sci 2008;335:292–7.
5. Erkal MZ, Wilde J, Bilgin Y, et al. High prevalence of vitamin D deficiency, secondary hyperparathyroidism and generalized bone pain in Turkish immigrants in Germany: identification of risk fac-tors. Osteoporos Int 2006;17:1133–40.
6. Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxy vitamin D concentrations are associated with better lower-extrem-ity function in both active and inactive persons aged.or560 y. Am J Clin Nutr 2004;80:752–8.
7. Lips P. Vitamin D physiology. Prog Biophys Mol Biol 2006;92: 4–8.
8. Targher G, Bertolini L, Padovani R, et al. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 2006;65:593–7.
9. Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther 2007;29:1761–70.
10. McClure DL, Valuck RJ, Glanz M, Murphy JR, Hokanson JE. Sta-tin and staSta-tfibrate use was significantly associated with in-creased myositis risk in a managed care population. J Clin Epidemiol 2007;60:812–8.
11. Tsugawa N, Suhara Y, Kamao M, Okano T. Determination of 25-hydroxyvitamin D in human plasma using high-performance liq-uid chromatography—tandem mass spectrometry. Anal Chem 2005;77:3001–7.
12. Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabe-tes Care 2005;28:1228–30.
13. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28.
14. Thompson PD, Clarkson P, Karas RH. Statin-associated myopa-thy. JAMA 2003;289:1681–90.
15. Ybarra J, Sanchez-Hernandez J, Perez A. Hypovitaminosis D and morbid obesity. Nurs Clin North Am 2007;42:19–27, v. 16. Sentongo TA, Semaeo EJ, Stettler N, Piccoli DA, Stallings VA,
Zemel BS. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am J Clin Nutr 2002;76:1077–81. 17. Link E, Parish S, Armitage J, et al. SLCO1B1 variants and
statin-induced myopathy–a genomewide study. N Engl J Med 2008;359: 789–99.
18. Fuleihan GE, Deeb M. Hypovitaminosis D in a sunny country. N Engl J Med 1999;340:1840–1.
19. Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr 2002;76:187–92. 20. Hannan MT, Litman HJ, Araujo AB, et al. Serum 25-hydroxyvita-min D and bone 25-hydroxyvita-mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab 2008;93:40–6.
21. Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr 2003;89:552–72.
22. Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle func-tion. Osteoporos Int 2002;13:187–94.
23. Shinchuk LM, Holick MF. Vitamin d and rehabilitation: improv-ing functional outcomes. Nutr Clin Pract 2007;22:297–304. 24. Ziambaras K, Dagogo-Jack S. Reversible muscle weakness in
pa-tients with vitamin D deficiency. West J Med 1997;167:435–9. 25. Goldstein MR. Myopathy, statins, and vitamin D deficiency. Am J
Cardiol 2007;100:1328.
26. Bunout D, Barrera G, Leiva L, et al. Effects of vitamin D supplemen-tation and exercise training on physical performance in Chilean vita-min D deficient elderly subjects. Exp Gerontol 2006;41:746–52. 27. Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10
mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006;28: 933–42.
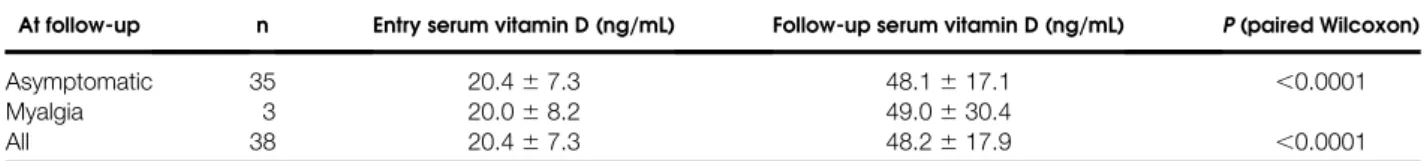
Table III. Thirty-eight statin-taking patients with entry myositis-myalgia and low serum vitamin D (,32 ng/mL)
who were given vitamin D supplementation therapy (50,000 units/week) for 3 months
At follow-up n Entry serum vitamin D (ng/mL) Follow-up serum vitamin D (ng/mL) P(paired Wilcoxon)
Asymptomatic 35 20.467.3 48.1617.1 ,0.0001
Myalgia 3 20.068.2 49.0630.4