R E S E A R C H A R T I C L E
Open Access
Serum prolactin concentrations as risk factor of
metabolic syndrome or type 2 diabetes?
Lisa Balbach
1†, Henri Wallaschofski
1,2†, Henry Völzke
2,3, Matthias Nauck
1,2, Marcus Dörr
2,4and Robin Haring
1,2*Abstract
Background:To investigate potential associations of serum prolactin concentration (PRL) with metabolic syndrome (MetS) and type 2 diabetes mellitus (T2DM), previously observed in small and selected study samples, in a large population-based cohort.
Methods:Data from 3,993 individuals (2,027 women) aged 20-79 years from the population-based Study of Health of Pomerania (SHIP) were used to analyse cross-sectional and longitudinal associations of PRL with MetS and T2DM risk in age- and multivariable-adjusted Poisson regression models. PRL were log-transformed and modelled as continuous (per standard deviation (SD) increase) and categorical predictor (sex-specific quartiles) variable, separately for men and woman.
Results:Cross-sectional analyses showed an inverse association between low PRL concentrations and prevalent T2DM risk in men and women after multivariable-adjustment (men: Q1 vs. Q4: relative risk (RR), 1.55; 95% confidence interval (CI), 1.13–2.14; women: Q1 vs. Q4: RR, 1.70; 95% CI, 1.10–2.62). Likewise, higher PRL concentrations were associated with significantly lower T2DM risk (RR per SD increase in log-PRL: 0.83; 95% CI, 0.72–0.95 in men, and 0.84; 95% CI, 0.71–0.98 in women, respectively). An inverse association between PRL and MetS risk was not retained after multivariable adjustment. Longitudinal analyses yielded no association of PRL with incident MetS or T2DM.
Conclusion:The present study is the first large population-based study reporting a cross-sectional inverse association between PRL and prevalent T2DM in both genders. But the absent longitudinal associations do not support a causal role of PRL as a risk factor of incident MetS or T2DM.
Background
Prolactin (PRL) is a pituitary hormone essential for vari-ous physiological functions in the human body [1-3]. It is not only important for the initiation and maintenance of lactation, but seems to be also involved in reproduction, growth and development, osmoregulation, immune regu-lation, brain function, behaviour, and metabolism [1-3]. These different functions of PRL can only be fulfilled due to the fact that the PRL receptor is expressed in different tissues and cells such as lymphoid cells, endometrium, prostate, and adipocytes [1-3].
The metabolic syndrome (MetS), a cluster of car-diometabolic risk factors including obesity, hypertrigly-ceridemia, hypertension, and insulin resistance [4,5], is often associated with type 2 diabetes mellitus (T2DM) [1]. Although previous investigations about the potential effects of PRL in T2DM and its complications are scarce, existing experimental studies suggest an influence of PRL on T2DM via its metabolic effects on adipose tissue [2,3], development and growth of pancreatic ß-cells [6,7], insulin resistance [3,8], and lipid metabolism [3,9]. The ability of PRL to stimulate insulin [6] and suppress adiponectin as well as interleukin-6 release further sug-gests a potential role in the manifestation of insulin re-sistance [2]. But although these studies support the view that PRL promotes the growth and survival of pancreatic ß-cells and supports insulin secretion [6,7], other studies were not able to detect any correlation between PRL and metabolic disturbances [10].
* Correspondence:robin.haring@uni-greifswald.de †Equal contributors
1
Institute of Clinical Chemistry and Laboratory Medicine, University Medicine Greifswald, Ferdinand-Sauerbruch-Straße, Greifswald 17475, Germany 2
DZHK (German Centre for Cardiovascular Research), partner site Greifswald, Greifswald, Germany
Full list of author information is available at the end of the article
An observational study among erectile dysfunction patients showed an association between low PRL concen-trations and adverse cardiovascular risk profiles and e-vents [11]. However, in most of these previous studies [10,11] the correlation between PRL and cardiometabolic risk factors including MetS or T2DM were not the main focus. Furthermore, previous studies were conducted in comparably small and selected patient populations with-out consideration of major confounding factors. There-fore, we aimed to investigate potential associations of PRL with MetS and T2DM using a large, representative sample of 3,993 individuals from the population-based longitu-dinal cohort Study of Health in Pomerania (SHIP).
Methods Study population
The SHIP is a population-based cohort study in West Pomerania, a region in north-eastern Germany. Details regarding the design, recruitment, and procedures of the SHIP study have been published previously [12]. In brief, from the total population of West Pomerania comprising 213,057 inhabitants, a two-stage stratified cluster sample of adults aged 20-79 years was drawn in 1996. The net sample (without migrated or deceased persons) comprised 6,265 eligible subjects. Only individuals with German ci-tizenship and main residency in the study area were in-cluded. All subjects received a maximum of three written invitations. In cases of non-response, letters were followed by repeated phone calls or by home visits if contact by phone was not possible [13]. After written informed con-sent was obtained, 4,308 (2,192 women) participants were examined (response 68.8%) between 1997 and 2001. Dur-ing the five-year follow-up, 3,300 (1,711 women) partici-pants were re-examined (response 83.6%) between 2002 and 2006. The study conformed to the principles of the Declaration of Helsinki and the Ethics Committee of the University of Greifswald approved the protocol. Out of 4,308 baseline participants, we excluded individuals with missing PRL data (N = 169), measured PRL >100.0 μg/l (N = 16), pregnancy (N = 8), pituitary disease (N = 1), and missing covariate data (N = 121), yielding a final study sample of 3,993 individuals (2,027 women).
Measures
Information about socio-demographic characteristics and health-related lifestyle including smoking habits (categorized into current, former, and never-smokers), educational level (< 10, = 10, or > 10 years at school), parity (number of children), physical activity (< 1 h/week physical training during summer or winter) and alcohol consumption were collected by personal interviews. Waist circumference was measured to the nearest
0.1 cm using an inelastic tape midway between the lower rib margin and the iliac crest in the horizontal plane, with the subject standing comfortably with weight dis-tributed evenly on both feet. Height and weight were measured for the calculation of body mass index (BMI) (weight [kg]/height [m]2). After a resting period of at least five minutes, systolic and diastolic blood pressure was measured three times on the right arm of seated participants with an oscillometric digital blood pressure monitor (HEM-705CP, Omron Corporation, Tokyo, Japan). The interval between the readings was three mi-nutes. The mean systolic and diastolic blood pressure was calculated from the second and third measurement [14]. Menopausal status (pre- and post-menopausal) was defined according to a previously established definition from our cohort: pre-menopausal: all women < 40 years
of age and between 40 – 60 years who reported
men-strual cycle; post-menopausal: all women ≥ 60 years of
age and all women between 40–60 years who reported
no menstrual cycle [15].
Serum high-density lipoprotein (HDL) cholesterol con-centrations were measured photometrically at baseline (Hitachi 704, Roche, Mannheim, Germany), whereas follow-up HDL concentrations were quantified by lipid electrophoresis (HELENA SAS-3 system, Helena 7 Bio-Sciences Europe, Tyne & Wear, UK). To ensure compara-bility in the longitudinal HDL analyses, we used baseline HDL concentrations as the reference and calculated cor-rected follow-up HDL concentrations based on a previ-ously published conversion formula [16]. Doing so, we found that the average HDL concentrations produced by the two methods were virtually identical, suggesting that the differences in HDL will be small within the range of practical relevance [17]. Serum triglycerides and glucose concentrations were determined enzymatically using reagents from Roche Diagnostics (Hitachi 717, Roche Diagnostics, Mannheim, Germany). Glycated hemoglobin (HbA1c) was determined by high-performance liquid chromatography (Bio-Rad, Munich, Germany). During the course of the study, the inter-assay coefficient of variation for HbA1c was 2.76% in the low pool and 1.38% in the high pool. All assays were performed according to the
manufacturers’ recommendations by skilled technical
personnel and internal quality controls were analyzed daily. In addition, the laboratory participates in official quarterly German external proficiency testing programs.
T2DM was defined based on self-reported physician’s
1. Elevated WC: men≥94 cm; women≥80 cm; 2. Elevated non-fasting glucose:≥8.0 mmol/l or
antidiabetic treatment (ATC codes A10A, A10B); 3. Decreased HDL cholesterol: men < 1.0 mmol/l,
women < 1.3 mmol/l, or lipid-lowering treatment (ATC C10AB, A10AD);
4. Elevated non-fasting triglycerides:≥2.3 mmol/l or lipid-lowering treatment (ATC C10AB, A10AD); 5. Elevated blood pressure:≥130/85 mmHg or
self-reported antihypertensive drug treatment.
Participants fulfilling at least three out of these five components were assigned to MetS.
Serum PRL concentrations were measured from frozen sera of baseline participants. Non-fasting blood samples were drawn from the cubital vein in the supine position between 7.00 a.m. and 7.00 p.m. Samples were stored at -80°C until analysis using a chemiluminescent immuno-metric assay on an Immulite 2500 analyzer (Ref. L5KPR, DPC Biermann GmbH, Bad Nauheim, Germany). An ali-quot of two alternating levels of a third party commercial control material (Bio-Rad Lyphochek Immunoassay Plus Control, lot 40151 and lot 40152; Bio-Rad, Munich, Germany) was included in each series in single determin-ation. During the course of the study the inter-assay coef-ficient of variation was 5.6% with a systematic deviation of -4.3% at the 6.3μg/l level, and 4.3% with a systematic de-viation of -6.4% at the 14.5μg/l level. The analytical sensi-tivity was 0.5μg/l by an effective measurement range of 0.5–150μg/l.
Statistical analysis
Categorical data are expressed as percentage and con-tinuous data as median (p25th, p75th). Sex-related differ-ences were tested using theχ2test (categorical data) and Mann-Whitney-U test (continuous data). We log-transformed the highly skewed PRL variable to achieve normality. To analyse cross-sectional and longitudinal associations of PRL with MetS and T2DM, we imple-mented age- and multivariable-adjusted Poisson regres-sion models with robust standard errors and report the relative risks (RR) and their 95% confidence intervals (95% CI) per SD increase in log-PRL and for sex-specific PRL quartiles (reference: quartile four). Longitudinal in-cidence analyses were performed only in individuals without MetS and T2DM at baseline, respectively. P for trend test was conducted by including PRL quartiles as an ordinal score into the regression models. Covariates adjusted for in multivariable regression models included age, BMI, smoking status, physical activity, educational level, and alcohol consumption. All analyses were performed separately in men and women.
Several sensitivity analyses were performed. In
women, we additionally adjust multivariable regression
models for menopausal status and parity. On the basis of ATC codes for metoclopramide (A03FA01, A03FA03, N02CX59), cimetidine (A02BA01), reserpine (C02LA01), methyldopa (C02AB), and psychoanaleptics (ATC-code N06), we excluded 184 individuals indicative of these PRL influencing medications [24] and repeated the multivari-able regression models accordingly. Furthermore, we used inverse probability weights to adjusted our longitudinal analyses for possible non-response bias from drop-out between the baseline and follow-up examination [25]. Finally, we additionally adjusted the multivariable models for blood sampling time to evaluate the potential impact of the diurnal cycle in PRL secretion on the revealed esti-mates. Two-sided probability values < 0.05 were consid-ered statistically significant. All the statistical analyses were performed using Stata 11.0 (Stata Corporation, College Station, TX, USA).
Results
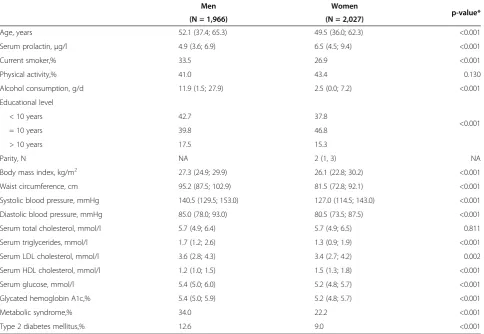
Comparing the baseline characteristics of the study sam-ple by sex, men showed significantly lower PRL concen-trations and a higher cardiometabolic risk factor level (with regard to smoking, alcohol consumption, BMI, blood pressure, lipid levels and parameters of glycemic control) than women (Table 1). Also the prevalence of MetS (34.0% vs. 22.2%) and T2DM (12.6% vs. 9.0%) was higher in men compared to women.
Cross-sectional age-adjusted regression models showed an inverse association between PRL and MetS risk in women (Q1 vs. Q4: RR, 1.32; 95% CI, 1.04–1.66), but not
in men (Q1 vs. Q4: RR, 1.15; 95% CI, 0.97 –1.37). This
sex-specific association was not retained after multiva-riable adjustment (Table 2). Low PRL concentrations were consistently associated with T2DM in age- and multivariable-adjusted regression models (men: Q1 vs. Q4: RR, 1.55; 95% CI, 1.13–2.14; women: Q1 vs. Q4: RR, 1.70; 95% CI, 1.10–2.62). Furthermore, p for trend ana-lyses showed a progressive inverse relationship across PRL quartiles. Likewise, higher PRL concentrations were asso-ciated with a significantly lower T2DM risk (RR per SD increase in log-PRL: 0.83; 95% CI, 0.72–0.95 in men, and 0.84; 95% CI, 0.71–0.98 in women, respectively).
indicative of PRL influencing medications, 3) additional inclusion of ‘drop-out’ weights and 4) additional adjust-ment for blood sampling time showed no impact on the overall estimates (data not shown).
Discussion
To our knowledge, this is the first population-based study to show a cross-sectional inverse association bet-ween PRL and T2DM risk in both men and women. Using a large sample of 3,993 individuals from the gen-eral population, age- and multivariable-adjusted regres-sion models detected an inverse association between low PRL concentrations and increased risk for prevalent T2DM.
These findings are in line with previous observational studies [11,26,27] of the relationship between PRL and T2DM. Among those, the only large-scaled study was conducted in 2,351 male patients with sexual dysfunc-tion, in whom low PRL concentrations were associated with an increased number of MetS factors [26]. Similarly to ours, that study [26] and its subsequent follow-up
[11] showed an inverse association between serum PRL concentrations and prevalent T2DM. However, since both studies based on male patient data, the comparabi-lity with the present population-based findings is quite limited. Furthermore, a cross-sectional study in 345 healthy volunteers aged 30-55 years showed a negative association of PRL with insulin resistance and a doubled risk of prevalent MetS per SD decrease in PRL [27].
Evidence from cross-sectional patient-based studies in drug naive schizophrenia patients suggests that high dopamine and subsequently low PRL concentrations [28,29] are related to an increased T2DM risk, including insulin resistance and impaired fasting glucose [30]. Thus, one may speculate if common anti-psychotics could probably protect from T2DM onset and progres-sion by increasing PRL concentrations through the in-hibition of the D2 receptor [31]. However, since previous studies [28-30] were exclusively cross-sectional, cause and effect in the association of PRL with T2DM are difficult to determine. For example, a small interven-tional study in turn suggests insulin secretion as the major determinant of PRL release [9].
Table 1 Baseline characteristics of the study population stratified by sex
Men Women
p-value*
(N = 1,966) (N = 2,027)
Age, years 52.1 (37.4; 65.3) 49.5 (36.0; 62.3) <0.001
Serum prolactin,μg/l 4.9 (3.6; 6.9) 6.5 (4.5; 9.4) <0.001
Current smoker,% 33.5 26.9 <0.001
Physical activity,% 41.0 43.4 0.130
Alcohol consumption, g/d 11.9 (1.5; 27.9) 2.5 (0.0; 7.2) <0.001
Educational level
<0.001
< 10 years 42.7 37.8
= 10 years 39.8 46.8
> 10 years 17.5 15.3
Parity, N NA 2 (1, 3) NA
Body mass index, kg/m2 27.3 (24.9; 29.9) 26.1 (22.8; 30.2) <0.001
Waist circumference, cm 95.2 (87.5; 102.9) 81.5 (72.8; 92.1) <0.001
Systolic blood pressure, mmHg 140.5 (129.5; 153.0) 127.0 (114.5; 143.0) <0.001
Diastolic blood pressure, mmHg 85.0 (78.0; 93.0) 80.5 (73.5; 87.5) <0.001
Serum total cholesterol, mmol/l 5.7 (4.9; 6.4) 5.7 (4.9; 6.5) 0.811
Serum triglycerides, mmol/l 1.7 (1.2; 2.6) 1.3 (0.9; 1.9) <0.001
Serum LDL cholesterol, mmol/l 3.6 (2.8; 4.3) 3.4 (2.7; 4.2) 0.002
Serum HDL cholesterol, mmol/l 1.2 (1.0; 1.5) 1.5 (1.3; 1.8) <0.001
Serum glucose, mmol/l 5.4 (5.0; 6.0) 5.2 (4.8; 5.7) <0.001
Glycated hemoglobin A1c,% 5.4 (5.0; 5.9) 5.2 (4.8; 5.7) <0.001
Metabolic syndrome,% 34.0 22.2 <0.001
Type 2 diabetes mellitus,% 12.6 9.0 <0.001
Data are given as percentages or median (p25th
; p75th
). NA, not applicable; LDL cholesterol, low-density lipoprotein cholesterol; HDL cholesterol, high-density lipoprotein cholesterol.
* Differences between men and women were tested usingχ2
In the search of potential explanations, two previous studies related alterations of serotonergic pathways caused by low PRL concentrations to the pathogenesis of MetS [27,32]. Furthermore, PRL-receptor deficient mice show an impaired development of pancreatic
ß-cells, finally leading to a blunted insulin response and mild glucose intolerance [7]. In contrast to our find-ings, studies including pathological hyperprolactinemia
caused by PRL-secreting tumors (prolactinomas)
showed that, after treatment with dopamine agonists, Table 2 Cross-sectional and longitudinal associations of serum prolactin with metabolic syndrome and type 2 diabetes mellitus
Metabolic syndrome Type 2 diabetes mellitus
Cross-sectional Longitudinal Cross-sectional Longitudinal
Age-adjusted model
Multivariable model
Age-adjusted model
Multivariable model
Age-adjusted model
Multivariable model
Age-adjusted model
Multivariable model
Prolactin Men
per SD increase
0.95 (0.89; 1.02) 0.95 (0.89; 1.01) 1.05 (0.95; 1.15) 1.04 (0.95; 1.14) 0.84 (0.73; 0.96)* 0.83 (0.72; 0.95)* 1.20 (0.98; 1.47) 1.19 (0.96; 1.47)
Quartiles (Q4 Ref.)
Q1 1.15 (0.97; 1.37) 1.15 (0.98; 1.35) 0.83 (0.64; 1.06) 0.87 (0.68; 1.10) 1.55 (1.12; 2.13)* 1.55 (1.13; 2.14)* 0.71 (0.41; 1.23) 0.78 (0.45; 1.35)
Q2 1.06 (0.89; 1.28) 1.03 (0.87; 1.22) 0.94 (0.74; 1.19) 0.94 (0.75; 1.18) 1.14 (0.81; 1.61) 1.11 (0.79; 1.56) 0.73 (0.42; 1.26) 0.78 (0.45; 1.33)
Q3 1.04 (0.87; 1.25) 1.02 (0.85; 1.21) 0.91 (0.72; 1.16) 0.94 (0.75; 1.18) 0.92 (0.63; 1.33) 0.92 (0.64; 1.33) 0.76 (0.44; 1.32) 0.72 (0.41; 1.27)
P for Trend 0.104 0.076 0.172 0.276 0.002 0.002 0.253 0.472
Women
per SD increase
0.92 (0.85; 1.00) 0.97 (0.90; 1.05) 0.89 (0.79; 1.00) 0.92 (0.83; 1.02) 0.81 (0.68; 0.95)* 0.84 (0.71; 0.98)* 1.09 (0.82; 1.45) 1.13 (0.89; 1.44)
Quartiles (Q4 Ref.)
Q1 1.32 (1.04; 1.66)* 1.11 (0.89; 1.39) 1.26 (0.95; 1.68) 1.15 (0.86; 1.53) 1.89 (1.21; 2.96)* 1.70 (1.10; 2.62)* 0.88 (0.44; 1.78) 0.73 (0.37; 1.43)
Q2 1.13 (0.89; 1.44) 1.04 (0.83; 1.32) 1.22 (0.91; 1.63) 1.16 (0.87; 1.54) 1.82 (1.15; 2.86)* 1.69 (1.09; 2.63)* 0.81 (0.39; 1.68) 0.75 (0.37; 1.50)
Q3 1.11 (0.86; 1.44) 1.07 (0.83; 1.32) 0.73 (0.51; 1.04) 0.78 (0.55; 1.11) 1.29 (0.77; 2.16) 1.26 (0.76; 2.09) 1.00 (0.48; 2.06) 1.08 (0.53; 2.20)
P for Trend 0.016 0.399 0.013 0.106 0.001 0.007 0.633 0.224
* p < 0.05; SD, standard deviation. The multivariable model was adjusted for age, body mass index, smoking status (three categories), physical activity, educational level (three categories), and alcohol consumption.
Figure 1Boxplots for median baseline prolactin concentrations (25thand 75thpercentile) according to the follow-up number of
reduced PRL concentrations led to increased insulin sensitivity [33,34].
These results, however, were collected in small patient-based samples with clinically confirmed prolactinoma and serum PRL concentrations outside the reference range, thus limiting its transferability to the general population. On contrary, HOMA-IR, as a measure of insulin resis-tance, did not change after treatment with dopamine ago-nists [33], and PRL concentrations did not correlate with HOMA-IR change or decrease in glucose, respectively [8,34]. Interestingly, a previous cross-sectional study in obese non-prolactinoma patients showed similar absent correlations of PRL with insulin, HOMA-IR, and glucose levels [10]. Available in-vitro studies rather suggest an influence of prolactin on ß-cell secretion via increased glu-cokinase activity [35], improved ß-cell specific survival [36,37], or inhibition of intrinsic ß-cell apoptosis [38]. However, to provide the necessary level of detail to eluci-date the exact mechanisms of PRL on different tissues and its impact on cardiometabolic risk factors further experi-mental in-vitro research is needed.
We previously published findings from our study sug-gesting a positive association of PRL with biomarkers of inflammation [39] and mortality (cardiovascular and all-cause mortality) [40], as well as an inverse association of PRL with cardiac remodelling [41]. However, these ob-servational findings from our and other studies do not provide consistent risk associations, suggesting the need for further investigations into the potential role of PRL as cardiometabolic risk marker.
For the interpretation of the present study, the follo-wing strengths and limitations need to be considered. Major strengths include the large sample, the longitu-dinal study design, and a broad age range. Furthermore, comprehensive sensitivity analyses were conducted to assess the validity of our findings. An important limita-tion of this study includes the reliance on single serum PRL measurements based on non-fasting blood samples, which is not adapted to the pulsatile release of PRL. But it was shown that the pulsatile secretion occurs mostly during the night and is relatively constant between 9.00 a.m. and 5.00 p.m. [42]. Further limitations arise from the Caucasian study sample, which restricts the generalizability of our results. Finally, we did not exclude other endocrine diseases, which possibly trigger T2DM including hyperthyroidism [43,44], acromegaly and the Cushing Syndrome [45].
However, given the inherent limitations of observational research [46], the definite role of serum PRL concentra-tions as a risk factor or risk marker can not be elucidated based on epidemiological data alone. Previous associations between serum PRL concentrations and disease risk ob-served in clinical study samples, but not in the general population, suggest PRL rather as specific disease marker
than as causal risk factor for MetS and T2DM. Thus, its application and significance in daily clinical practice is very limited to date.
Conclusions
In summary, this is the first population-based study to show low PRL concentrations related to a higher T2DM risk in both genders. But given the variable associations between PRL and T2DM, but not with MetS, together with the absent longitudinal associations with both out-comes, the present study does not support PRL as a causal cardiometabolic risk factor. Therefore, we hypothesize PRL rather as a marker of the complex multi-level alte-rations involved in the onset and progression of T2DM. However, to further elucidate the potential role of PRL in the context of this complex interplay, further observa-tional as well as intervenobserva-tional research is needed.
Competing interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Authors’contributions
RH, LB, and HW contributed to the study design and ideas for the data analysis. HV, MD, MN, and HW organized the sample collection and data preparation. Statistical analyses were performed by RH. LB, RH, and HW contributed to the interpretation of the results and the discussion. LH drafted the manuscript and wrote the final version together with all other co-authors. All authors read, critically revised, and finally gave approval of the version to be published.
Acknowledgements
The contributions to data collection made by field workers, study physicians, ultrasound technicians, interviewers, and computer assistants are gratefully acknowledged.
Funding
SHIP is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grants no. 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania. This work is also part of the research project Greifswald Approach to Individualized Medicine (GANI_MED), funded by the Federal Ministry of Education and Research and the Ministry of Cultural Affairs of the Federal State of Mecklenburg–West Pomerania (03IS2061A). This study was also supported by the DZHK (German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research). The used prolactin reagents were sponsored by Novo Nordisk Pharma GmbH, Mainz, Germany.
Author details
1
Institute of Clinical Chemistry and Laboratory Medicine, University Medicine Greifswald, Ferdinand-Sauerbruch-Straße, Greifswald 17475, Germany.2DZHK (German Centre for Cardiovascular Research), partner site Greifswald, Greifswald, Germany.3Institute for Community Medicine, University Medicine Greifswald, Walther-Rathenau-Straße 48, Greifswald 17475, Germany. 4Department of Cardiology, University Medicine Greifswald, Ferdinand-Sauerbruch-Straße, Greifswald 17475, Germany.
Received: 1 October 2012 Accepted: 15 March 2013 Published: 21 March 2013
References
2. Brandebourg T, Hugo E, Ben-Jonathan N:Adipocyte prolactin: regulation of release and putative functions.Diabetes Obes Metab2007,9(4):464–476. 3. Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR:Focus on
prolactin as a metabolic hormone. Trends in endocrinology and metabolism.TEM2006,17(3):110–116.
4. Prasad H, Ryan DA, Celzo MF, Stapleton D:Metabolic syndrome: definition and therapeutic implications.Postgrad Med2012,124(1):21–30. 5. Rosenzweig JL, Ferrannini E, Grundy SM, Haffner SM, Heine RJ, Horton ES,
Kawamori R:Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline.J Clin Endocrinol Metabol2008,93(10):3671–3689. 6. Brelje TC, Stout LE, Bhagroo NV, Sorenson RL:Distinctive roles for prolactin
and growth hormone in the activation of signal transducer and activator of transcription 5 in pancreatic islets of langerhans.Endocrinology2004, 145(9):4162–4175.
7. Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, Kendall W, Oden J, Bridges S, Binart N,et al:Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance.
Endocrinology2002,143(4):1378–1385.
8. Serri O, Li L, Mamputu JC, Beauchamp MC, Maingrette F, Renier G:The influences of hyperprolactinemia and obesity on cardiovascular risk markers: effects of cabergoline therapy.Clin Endocrinol (Oxf )2006, 64(4):366–370.
9. Mingrone G, Manco M, Iaconelli A, Gniuli D, Bracaglia R, Leccesi L, Calvani M, Nolfe G, Basu S, Berria R:Prolactin and insulin ultradian secretion and adipose tissue lipoprotein lipase expression in severely obese women after bariatric surgery.Obesity (Silver Spring)2008,16(8):1831–1837. 10. Ernst B, Thurnheer M, Schultes B:Basal serum prolactin levels in obesity–
unrelated to parameters of the metabolic syndrome and unchanged after massive weight loss.Obes Surg2009,19(8):1159–1162.
11. Corona G, Rastrelli G, Boddi V, Monami M, Melani C, Balzi D, Sforza A, Forti G, Mannucci E, Maggi M:Prolactin levels independently predict major cardiovascular events in patients with erectile dysfunction.Int J Androl
2011,34(3):217–224.
12. Völzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, Aumann N, Lau K, Piontek M, Born G,et al:Cohort profile: the study of health in pomerania.Int J Epidemiol2011,40(2):294–307.
13. Haring R, Alte D, Volzke H, Sauer S, Wallaschofski H, John U, Schmidt CO: Extended recruitment efforts minimize attrition but not necessarily bias.
J Clin Epidemiol2009,62(3):252–260.
14. Torkler S, Wallaschofski H, Baumeister SE, Volzke H, Dorr M, Felix S, Rettig R, Nauck M, Haring R:Inverse association between total testosterone concentrations, incident hypertension and blood pressure.Aging Male
2011,14(3):176–182.
15. Haring R, Hannemann A, John U, Radke D, Nauck M, Wallaschofski H, Owen L, Adaway J, Keevil BG, Brabant G:Age-specific reference ranges for serum testosterone and androstenedione concentrations in women measured by liquid chromatography-tandem mass spectrometry.J Clin Endocrinol Metabol2012,97(2):408–415.
16. Nauck M, Winkler K, Marz W, Wieland H:Quantitative determination of high-, low-, and very-low-density lipoproteins and lipoprotein(a) by agarose gel electrophoresis and enzymatic cholesterol staining.Clin Chem1995, 41(12 Pt 1):1761–1767.
17. Haring R, Baumeister SE, Völzke H, Dorr M, Felix SB, Kroemer HK, Nauck M, Wallaschofski H:Prospective Association of Low Total Testosterone Concentrations with an Adverse Lipid Profile and Increased Incident Dyslipidemia.Eur J Cardiovasc Prev Rehabil2011,18(1):86–96. 18. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA,
Fruchart JC, James WP, Loria CM, Smith SC Jr:Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity.Circulation2009,120(16):1640–1645. 19. Hannemann A, Meisinger C, Bidlingmaier M, Doring A, Thorand B, Heier M,
Belcredi P, Ladwig KH, Wallaschofski H, Friedrich N,et al:Association of plasma aldosterone with the metabolic syndrome in two German populations.Eur J Endocrinol2011,164(5):751–758.
20. Schipf S, Alte D, Voelzke H, Friedrich N, Haring R, Lohmann T, Nauck M, Felix SB, Hoffmann W, John U,et al:Prävalenz des Metabolischen Syndroms in
Deutschland: Ergebnisse der Study of Health in Pomerania (SHIP).
Diabetologie Stoffwechsel2010,5:161–168.
21. Haring R, Volzke H, Felix SB, Schipf S, Dorr M, Rosskopf D, Nauck M, Schofl C, Wallaschofski H:Prediction of metabolic syndrome by low serum testosterone levels in men: results from the study of health in Pomerania.Diabetes2009,58(9):2027–2031.
22. Haring R, Wallaschofski H, Nauck M, Felix SB, Schmidt CO, Dorr M, Sauer S, Wilmking G, Volzke H:Total and cardiovascular disease mortality predicted by metabolic syndrome is inferior relative to its components.
Exp Clin Endocrinol Diabetes2010,118(10):685–691.
23. Lidfeldt J, Nyberg P, Nerbrand C, Samsioe G, Schersten B, Agardh CD: Socio-demographic and psychosocial factors are associated with features of the metabolic syndrome. The Women's Health in the Lund Area (WHILA) study.Diabetes Obes Metab2003,5(2):106–112.
24. Molitch ME:Drugs and prolactin.Pituitary2008,11(2):209–218. 25. Afifi AA, Kotlerman JB, Ettner SL, Cowan M:Methods for improving
regression analysis for skewed continuous or counted responses.Annu Rev Public Health2007,28:95–111.
26. Corona G, Mannucci E, Jannini EA, Lotti F, Ricca V, Monami M, Boddi V, Bandini E, Balercia G, Forti G,et al:Hypoprolactinemia: a new clinical syndrome in patients with sexual dysfunction.J Sex Med2009,6(5):1457–1466. 27. Muldoon MF, Mackey RH, Korytkowski MT, Flory JD, Pollock BG, Manuck SB:
The metabolic syndrome is associated with reduced central serotonergic responsivity in healthy community volunteers.J Clin Endocrinol Metabol
2006,91(2):718–721.
28. Meaney AM, O'Keane V:Prolactin and schizophrenia: clinical consequences of hyperprolactinaemia.Life Sci2002,71(9):979–992. 29. Rao ML, Gross G, Strebel B, Halaris A, Huber G, Braunig P, Marler M:
Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia.Biol Psychiatry1994,35(3):151–163.
30. Ryan MC, Collins P, Thakore JH:Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia.Am J Psychiatry2003, 160(2):284–289.
31. Oberweis B, Gragnoli C:Potential role of prolactin in antipsychotic-mediated association of schizophrenia and type 2 diabetes.J Cell Physiol
2012,227(8):3001–3006.
32. Muldoon MF, Mackey RH, Williams KV, Korytkowski MT, Flory JD, Manuck SB: Low central nervous system serotonergic responsivity is associated with the metabolic syndrome and physical inactivity.J Clin Endocrinol Metabol
2004,89(1):266–271.
33. Berinder K, Nystrom T, Hoybye C, Hall K, Hulting AL:Insulin sensitivity and lipid profile in prolactinoma patients before and after normalization of prolactin by dopamine agonist therapy.Pituitary2011,14(3):199–207. 34. dos Santos Silva CM, Barbosa FR, Lima GA, Warszawski L, Fontes R,
Domingues RC, Gadelha MR:BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists.
Obesity (Silver Spring)2011,19(4):800–805.
35. Weinhaus AJ, Stout LE, Bhagroo NV, Brelje TC, Sorenson RL:Regulation of glucokinase in pancreatic islets by prolactin: a mechanism for increasing glucose-stimulated insulin secretion during pregnancy.J Endocrinol2007, 193(3):367–381.
36. Yamamoto T, Ricordi C, Mita A, Miki A, Sakuma Y, Molano RD, Fornoni A, Pileggi A, Inverardi L, Ichii H:beta-Cell specific cytoprotection by prolactin on human islets.Transplant Proc2008,40(2):382–383.
37. Yamamoto T, Mita A, Ricordi C, Messinger S, Miki A, Sakuma Y, Timoneri F, Barker S, Fornoni A, Molano RD,et al:Prolactin supplementation to culture medium improves beta-cell survival.Transplantation2010,89(11):1328–1335. 38. Terra LF, Garay-Malpartida MH, Wailemann RA, Sogayar MC, Labriola L:
Recombinant human prolactin promotes human beta cell survival via inhibition of extrinsic and intrinsic apoptosis pathways.Diabetologia
2011,54(6):1388–1397.
39. Friedrich N, Schneider HJ, Spielhagen C, Markus MR, Haring R, Grabe HJ, Buchfelder M, Wallaschofski H, Nauck M:The association of serum prolactin concentration with inflammatory biomarkers - cross-sectional findings from the population-based Study of Health in Pomerania.
Clin Endocrinol (Oxf )2011,75(4):561–566.
41. Haring R, Volzke H, Vasan RS, Felix SB, Nauck M, Dorr M, Wallaschofski H: Sex-specific associations of serum prolactin concentrations with cardiac remodeling: longitudinal results from the Study of Health Pomerania (SHIP).Atherosclerosis2012,221(2):570–576.
42. Katznelson L, Riskind PN, Saxe VC, Klibanski A:Prolactin pulsatile characteristics in postmenopausal women.J Clin Endocrinol Metabol1998, 83(3):761–764.
43. Maratou E, Hadjidakis DJ, Peppa M, Alevizaki M, Tsegka K, Lambadiari V, Mitrou P, Boutati E, Kollias A, Economopoulos T,et al:Studies of insulin resistance in patients with clinical and subclinical hyperthyroidism.
Eur J Endocrinol2010,163(4):625–630.
44. Mitrou P, Raptis SA, Dimitriadis G:Insulin action in hyperthyroidism: a focus on muscle and adipose tissue.Endocr Rev2010,31(5):663–679. 45. Resmini E, Minuto F, Colao A, Ferone D:Secondary diabetes associated
with principal endocrinopathies: the impact of new treatment modalities.Acta Diabetol2009,46(2):85–95.
46. Rothman KJ, Greenland S:Causation and causal inference in epidemiology.Am J Public Health2005,95(Suppl 1):S144–150.
doi:10.1186/1472-6823-13-12
Cite this article as:Balbachet al.:Serum prolactin concentrations as risk factor of metabolic syndrome or type 2 diabetes?.BMC Endocrine Disorders201313:12.
Submit your next manuscript to BioMed Central and take full advantage of:
• Convenient online submission
• Thorough peer review
• No space constraints or color figure charges
• Immediate publication on acceptance
• Inclusion in PubMed, CAS, Scopus and Google Scholar
• Research which is freely available for redistribution