LEABHARLANN CHOLAISTE NA TRIONOIDE, BAILE ATHA CLIATH TRINITY COLLEGE LIBRARY DUBLIN
OUscoil Atha Cliath
The University of Dublin
Terms and Conditions of Use of Digitised Theses from Trinity College Library Dublin
Copyright statement
All material supplied by Trinity College Library is protected by copyright (under the Copyright and
Related Rights Act, 2000 as amended) and other relevant Intellectual Property Rights. By accessing
and using a Digitised Thesis from Trinity College Library you acknowledge that all Intellectual Property
Rights in any Works supplied are the sole and exclusive property of the copyright and/or other I PR
holder. Specific copyright holders may not be explicitly identified. Use of materials from other sources
within a thesis should not be construed as a claim over them.
A non-exclusive, non-transferable licence is hereby granted to those using or reproducing, in whole or in
part, the material for valid purposes, providing the copyright owners are acknowledged using the normal
conventions. Where specific permission to use material is required, this is identified and such
permission must be sought from the copyright holder or agency cited.
Liability statement
By using a Digitised Thesis, I accept that Trinity College Dublin bears no legal responsibility for the
accuracy, legality or comprehensiveness of materials contained within the thesis, and that Trinity
College Dublin accepts no liability for indirect, consequential, or incidental, damages or losses arising
from use of the thesis for whatever reason. Information located in a thesis may be subject to specific
use constraints, details of which may not be explicitly described. It is the responsibility of potential and
actual users to be aware of such constraints and to abide by them. By making use of material from a
digitised thesis, you accept these copyright and disclaimer provisions. Where it is brought to the
attention of Trinity College Library that there may be a breach of copyright or other restraint, it is the
policy to withdraw or take down access to a thesis while the issue is being resolved.
Access Agreement
By using a Digitised Thesis from Trinity College Library you are bound by the following Terms &
Conditions. Please read them carefully.
Ultrasonic spray pyrolysis and electroless
deposition for the synthesis of nanostructured
metal/carbon microspheres
A thesis submitted to the University of Dublin for the degree of Doctor of
Philosophy
by
Paul Duffy B. A. (Mod.)
T R !N rrY C O LL E G E
5 JAN 2015
Declaration
I d e c la re th a t this thesis has not been s u b m itte d as an exercise fo r a d e g re e a t this or any other university and it is entirely m y own work. I agree to deposit this thesis in the
U n iv e rs ity ’ s open access institutional repository or a llo w the library to do so on my
behalf, subject to Irish C opyright Legislation and T rin ity College L ib ra ry conditions o f
use and acknowledgement.
I declare that this report details entirely m y own work. Due acknowledgements and
references are given to the w ork o f others where appropriate.
Acknowledgements
I w o u ld like to th a n k D r P au la C o l a v it a fo r h e r su p p o rt an d p atien ce o v e r the c o u rs e o f
m y P h D studies. S h e has b een a c o n s ta n t g u id e in the im p le m e n ta tio n o f this study and
w a s alw ays av a ilab le for d isc u ssio n s a n d insight.
I w o u ld a lso like to tha nk D r L a u r a Soldi an d D r D ilu sh a n Ja y a s a n d ra th e post d o c s in
m y group. Both g av e im m e n s e h elp in the im p l e m e n ta tio n o f m y stu dies, p rac tica lly and
via ideas a n d sug g e stio n s. I w o u ld also like to a c k n o w le d g e all the su p p o rt given to m e
by the o th e r m e m b e rs o f o u r re se arch group. I w o u ld also like to a c k n o w le d g e all the
p e o p le at the c h e m is try d e p a rtm en t, w ith o u t training on in stru m e n ta tio n and b a c k g ro u n d
support and se rvic e, none o f this w o u ld h a v e b ee n possible. In particular, I w o u ld like to
tha nk D r M a n u e l R u e th e r for m u c h help o v e r the years. A lso D r K arsten R o d e fo r help
in data inte rpretatio n.
I w o u ld a lso like to a c k n o w le d g e D r K ev in M e tz , o u r co ffee lovin A m e ric a n
co llaborator. M a n y go o d talks w ere to be h a d on the w ay to S E M sessions.
Lastly, I w o u ld like to thank fam ily a n d frie n d s for su p p o rtin g me th r o u g h o u t m y P h D
studies.
I grate fully a c k n o w le d g e su p p o rt f ro m the E n v iro n m e n t a l P rotection A g e n c y , Ireland
(E P A , Ireland) fo r funding. T rinity C o lle g e D u b lin for its start up fu n d s an d the S chool
Abstract
T h e o b je c tiv e o f this w o rk is to synthesis various n a n o -m e ta llic carbon m icro s p h e re
c o m p o s ite m a te ria ls fo r e n v iro n m e n ta l an d ca ta ly tic ap p licatio n s. U ltraso nic spray
pyrolysis and electro less d ep ositio n te c h n iq u e s w e re used to achieve this. The
co m p o sites m a teria ls w e re ch arac teris ed and th e ir su b se q u en t a c tiv ity w as
d e m o n s tra te d .
C arbon m icro s p h e re p article o f various m o rp h o lo g y and su rface area have been
synthesised using ultraso n ic spray pyrolysis. T hese particles w e re ch arac teris ed using a
c o m b in a tio n o f Ram an spectroscopy, scanning e le c tro n m icroscopy (SEM ), BET
a d s o rp tio n , FTIR and zeta p o te n tia l m e a s u re m e n ts . SEM im ag e ry d e te rm in e d th e
sh ap e an d m o rp h o lo g y o f th e various carb on m a te ria ls . It w as d e m o n s tra te d via
Ram an sp ectro scop y th a t carb on m icro p a rticles w e re g ra p h itic in n a tu re , h o w e v e r,
FTIR and Zeta p o te n tia l m e a s u re m e n ts d e m o n s tra te d th e p resen ce o f c a rb o x ylate
groups on th e ca rb o n m icro s p h e re surface.
It w as d e m o n s tra te d th a t carbon m icrosp heres synthesised using ultraso n ic spray
pyrolysis had c o n tro lla b le size by leveragin g p rec u rso r c o n c e n tra tio n s . This was
c o n firm e d via a c o m b in a tio n o f SEM im ages and d y n a m ic light sc atte rin g e x p e rim e n ts .
FTIR and zeta p o te n tia l e x p e rim e n ts d e m o n s tra te d th a t C M surfaces display ch em istry
w h ich a llo w e d th e g ra ftin g o f d iffe rin g ch em ical m o ie tie s via d ia zo n iu m ch em istry.
Using electro less d ep o s itio n te c h n iq u es , n a n o p a rtic le s o f th r e e m e ta l m a teria ls w e re
n u c le a te d and g ro w n on th e C M surfaces. In p articu lar. P allad iu m and silver m e tal
w e r e n u c le ate d on th e CM surface fro m m e ta l salts using c o ffe e as a g ree n reducing
a g e n t, a t ro o m te m p e ra tu r e . M e ta l co m p o site m a te ria ls w e r e ch arac teris ed using a
c o m b in a tio n o f SEM , x-ray d iffractio n (XRD), e n erg y dispersive x-ray spectroscopy
p a r tic le s o b s e rv e d v ia S E M . U s in g T G A a n d EDS in c o m b in a tio n , m ass lo a d in g s w h e r e
e s tim a te d w it h A g /C a n d P d /C m ass ra tio s o f ( 1 3 .5 ± 1 .5 )% a n d ( 7 .4 ± 1 .0 )% w / w ,
re s p e c tiv e ly .
S u zu k i c o u p lin g r e a c tio n c o n f ir m e d t h e v ia b ility o f t h e P d /C c o m p o s ite m a t e r ia l f o r
c a ta ly tic a p p lic a tio n s . A g /C c o m p o s ite re d u c e d 4 - n it r o p h e n o l, a c h ie v in g re a c tio n ra te s
w h ic h a r e c o m p a r ib le t o o t h e r s u p p o r t s ilv e r n a n o p a r tic le s in t h e lit e r a t u r e .
Iro n a n d iro n o x id e n a n o p a r tic le s w e r e s y n th e s is a t t h e c a rb o n m ic r o s p h e r e s u rfa c e
u s in g e le c tr o le s s d e p o s itio n . T h e r e p o r te d e le c tr o le s s d e p o s itio n a p p ro a c h p r o d u c e d a
c o m p o s ite F e /F e O x /c a r b o n m ic r o s p h e r e w it h n a n o p a rtic le s o f a n a r r o w ly d is p e rs e d
size . A c o m b in a tio n o f X - r a y p o w d e r d if f r a c tio n (X R D ) a n d X -ra y a b s o rp tio n
s p e c tro s c o p ie s (EXAFS a n d X A N E S ) w a s u s e d in o r d e r t o d e t e r m in e t h e s tr u c tu r e a n d
c o m p o s itio n o f t h e F e /F e O x /c a r b o n m ic r o s p h e re s . M ic r o s p h e r e s w e r e f o u n d to d is p la y
( 1 4 ± 1 )% iro n c o n t e n t ( w /w ) , w h e r e b y (1 2 ± 3 )% o f Iro n a to m s w e r e p re s e n t as
m e t a llic iro n a n d t h e r e m a in in g as m a g h e m it e (F e2 0 3>. F in a lly , w e s h o w t h a t t h e r e m o v a l c a p a c ity o f F e /F e O x /c a r b o n m ic r o s p h e re s f o r C r (V I) is ( 2 0 ± 2 ) m g g ' l a n d t h a t
t h e m a x im u m s u r fa c e d e n s ity f o r C r a d s o rb a te s Is ( 6 0 ± 6 ) m g m ‘^, th u s s u g g e s tin g t h a t
t h e s e a r e p ro m is in g m a te r ia ls f o r t h e r e m o v a l o f w a t e r p o llu ta n ts f r o m a q u e o u s
Table of Contents
Declaration... iii
Acknowledgements... iv
Abstract... v
Table of contents... vii
List of figures... xi
List o f tables... xv
1 Introduction... 1
1.1 Background... 2
1.2 Introduction to supported nanoparticle systems... 3
1.2.1 Stabilising agents... 3
1.2.1.1 Polymers... 4
1.2.1.2 Dendrimers... 5
1.2.1.3 Solid support systems... 6
1.3 Applications of nanoparticles... 8
1.3.1 Nanomaterials fo r catalysis... 8
1.3.1.1 Palladium nanomaterials in catalytic application... 9
1.3.1.2 Silver nanomaterials in catalytic application... 9
1.3.2 Nanomaterials fo r environmental applications... 10
1.3.2.1 Review of the issues... 10
1.3.2.2 Metallic iron as remediation m aterial... 11
1.3.2.3 Challenges in the im plementation of iron nanoparticles 14 1.3.2.4 Iron oxide in environmental applications... 16
1.4 Support material fo r Ag, Pd and Fe/FexOy particles... 17
1.4.1 Zeta potential and surface charge... 17
1.4.2 Carbon microsphere with controllable size... 19
1 .5 .1 Basics o f Electroless D e p o s itio n ... 2 0
1 .5 .2 Key requirennents fo r ED... 21
1 .5 .3 Uses o f electro less d ep o s itio n fo r n a n o p a r tic le ... 23
1 .6 Aim s o f this w o r k ... 25
1 .6 .1 C arbon m icro s p h e res... 25
1 .6 .2 Synthesis o f P d /C and A g /C co m p o site m a te ria ls using Electroless d e p o s itio n ... 26
1 .6 .3 Synthesis o f Fe/FexOy/C co m p o site m a teria ls using Electroless d e p o s itio n ... 26
R e fe re n c e s ... 27
2 E xp e rim e n ta l te c h n iq u e s ... 35
2 .1 R eview o f e x p e rim e n ta l m e th o d s ... 36
2 .1 .1 U ltraso nic spray pyrolysis... 36
2 .2 E xp e rim e n ta l T e c h n iq u e s ... 38
2 .2 .1 D ynam ic Light S catterin g (DLS)... 38
2 .2 .2 Z eta P o ten tia l M e a s u re m e n ts ... 42
R e fe re n c e s ... 45
3 Synthesis o f carb on m icrosp heres via u ltraso n ic spray pyrolysis... 4 6 3 .1 In tro d u c tio n ... 47
3 .1 .1 USP m e th o d s fo r carb on m icro s p h e re synthesis... 4 7 3 .1 .2 M o d ific a tio n o f surface ch e m is try and c h a rg e ... 4 8 3 .2 E x p e rim e n ta l... 4 9 3 .2 .1 M a te ria ls ... 4 9 3 .2 .2 Synthesis o f porous ca rb o n m ic ro s p h e re s ... 50
3 .2 .3 F u n ctio n a lisa tio n o f ca rb o n m icro s p h e res... 51
3 .2 .4 C h arac terizatio n te c h n iq u e s ... 52
3 .3 Results and Discussion... 53
3 .3 .1 C h aracterisatio n o f C M s... 53
3.3.3 S u rfac e c h a r g e a n d c h e m i s t r y ... 62
3 .3.4 Discussion a n d s u m m a r y ... 68
3.3.4.1 C arb o n m i c r o s p h e r e c h a r a c t e r i s a t i o n ... 68
3.3.4.2 Size co n tro l o f c a r b o n m i c r o s p h e r e s ... 71
3.3.4.3 S u rfac e c h e m is try a n d f u n c ti o n a lis a ti o n o f c a r b o n m i c r o s p h e r e s ... 78
3.4 C o n c lu sio n s... 79
R e f e r e n c e s ... 81
4 Sy n th esis o f M e t a l / C a r b o n c o m p o s i t e s via g r e e n e l e c t ro l e s s d e p o s i t i o n 83 4 .1 I n t r o d u c t i o n ... 84
4 .1.1 C arb o n s u p p o r t m a t e r i a l ... 84
4.1.2 Reduction of m e ta l ions using c o f f e e ... 85
4.2 E x p e r im e n ta l... 86
4.2.1 M a teria ls a n d r e a g e n t s ... 86
4 .2.2 Synthesis of c a r b o n a n d m e t a l / c a r b o n m i c r o s p h e r e s ... 86
4.2.3 Reactivity s t u d i e s ... 87
4 .2.3 .1 Ag/CM rea ctivity... 87
4 .2.3 .2 Pd/CM rea ctivity... 87
4.2.4. C h a ra c te riz a tio n t e c h n i q u e s ... 88
4.3. R e su lts... 89
4.3.1. S ynthesis a n d c h a r a c t e r is a tio n of m e ta l /C M c o m p o s i t e s ... 8 9 4.3.1.1 S ynthesis a n d c h a r a c t e r i s a t i o n o f a c t i v a t e d C M ... 89
4.3.2 Catalytic activity of m e t a l / c a r b o n c o m p o s i t e m a t e r i a l s ... 101
4.3.2.1. R e d u ctio n o f 4 - n it r o p h e n o l using Ag/CM c o m p o s i t e s 102 4.3.2.2 Suzuki co u p lin g using Pd/C M c o m p o s i t e s ... 104
4.3.3 Discussion a n d s u m m a r y ... 106
4.3.3.1 Effect of S ensitisation s t e p o n c o m p o s i t e s ... 107
4 .4 Conclusions... 113
References... 114
5 Synthesis of Fe/FexOy nanoparticles on porous carbon microspheres: Structure and surface reactivity... 118
5.1 Introduction... 119
5.1.1 Electroless deposition of Iro n ... 119
5.2 Experim ental... 121
5.2.1 M aterials and reagents... 121
5.2.2 Composite m aterial synthesis... 122
5.2.3 Characterization... 123
5.3 Results and discussion... 124
5.3.1 Characterisation of activation steps... 125
5.3.2 Iron deposition utilising m ethod A... 127
5.3.2.1 Synthesis of Fe/FexOy CMs using M etho d A ... 127
5.3.3 Iron deposition utilising m ethod B... 133
5.3.3.1 Synthesis and characterisation of Fe/FexOy CM using method B... 133
5.3.3.2 Removal o f C r(V I)... 140
5.3.4 Discussion and sum m ary... 142
5.3.4.1 M etho d A... 142
5.3.4.2 M etho d B... 145
5.4 Conclusions... 151
References... 153
6 Conclusions and fu tu re w/ork... 157
6.1 Conclusions... 158
List of figures
Figure 1.1: Two polym er families used as metal NP supports fo r catalysis
Figure 1.2: Nanoparticles encapsulated in PAMAM or PPI dendrimers: complexation o f a
metal cation, then reduction to metal(O) by NaBH4, and aggregation giving the NPs inside the
dendrim er. Specifically, the preparation o f dendrimer-encapsulated bim etallic NPs is show/n
Figure 1.3: (a) Schematic illustration o f the steps involved in the functionalization o f carbon
nanofibers and subsequent proceudure fo r electroless deposition, (b) Chemical
transform ations involved in the nanofiber m odification
Figure 1.4: Core shell model o f an iron nanoparticle
Figure 1.5: TEM showing FeNP aggregates; Scale bar 500 nm
Figure 1.6: Model o f Surface charges and potentials. All potentials are defined w ith respect to
the potential in the bulk solution.
Figure 1.7: Electroless deposition processes: (a) Autocatalytic: The reduced noble metal
serves as the catalyst fo r fu rth e r reduction of the metal salt by the external reducing agent,
(b) Substrate catalyzed: The substrate surface catalyzes the reduction o f the metal salt by the
reducing agent (c) Galvanic displacement: The surface serves as the reducing agent and
electron source fo r reduction o f the metal salt
Figure 1.8: Schematic showing electroless deposition utilising catalytic seed particles
Figure 2.1: Schematic o f the USP system used in our studies.
Figure 2.2: Optical setup fo r DLS measurements
Figure 2.3: Example o f an experim ental correlation function
Figure 3.1: Schematic dem onstrating an example of a diazonium grafting reactions.
Figure 3.2: Photo o f the USP setup used fo r the synthesis o f CMs in our laboratory.
Figure 3.3: Raman spectrum o f microspheres (exc. 457 nm); the profile displays the D and G
bands th a t are characteristic o f amorphous carbons.
Figure 3.4: a) Shows SEM image o f a CM synthesized using LiDCA as a precursor solution;
Scale bar = 200 nm, b) Size distribution obtained fro m SEM images from 100+ particles fo r 1.5
Fig u re 3 .5 : a) S h o w s SEM i m a g e o f a CM s y n t h e s i z e d using NaDCA a s a p r e c u r s o r s o l u t io n ; Sc ale b a r = 2 0 0 n m , b) Size d i s t r i b u t i o n o b t a i n e d f r o m SEM i m a g e s f r o m 10 0 + p a r ti c l e s fo r 1.5 M NaDCA p r e c u r s o r so l u t io n .
F ig u re 3 .6 : a) S h o w s SEM i m a g e o f a CM s y n t h e s i z e d u sin g KDCA a s a p r e c u r s o r s o l u t i o n ; Scale b a r = 2 0 0 n m , b) Size d i s t r i b u t i o n o b t a i n e d f r o m SEM i m a g e s f r o m 1 0 0 + p a r ti c l e s f o r 1.5 M KDCA p r e c u r s o r s o l u t io n .
F ig u re 3 .7 : Typical DLS size d i s t r i b u t i o n o f c a r b o n p a r ti c l e s p r o d u c e d by USP o f 1.5 M s o l u t i o n s o f a) LiDCA a n d b) NaDCA.
F ig u re 3 .8 : DLS size d i s t r i b u t i o n s f o r 0 . 1 2 5 , 1 . 0 0 0 a n d 1 . 5 0 0 M c o n c e n t r a t i o n LiDCA p r e c u r s o r s o lu tio n .
F ig u re 3. 9: Size d i s t r i b u t i o n o b t a i n e d f r o m SEM i m a g e s f r o m 1 0 0 + p a r ti c l e s f o r 1.0 M LiDCA p r e c u r s o r s o l u t io n .
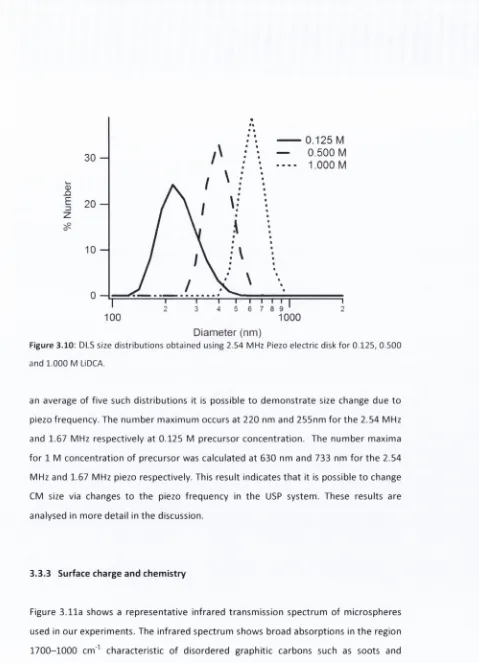
F ig u re 3 .1 0 : DLS size d i s t r i b u t i o n s o b t a i n e d u s in g 2 . 5 4 MHz Piezo e l e c t r i c disk f o r 0 . 1 2 5 , 0 . 5 0 0 a n d 1 . 0 0 0 M LiDCA.
F ig u re 3 . 1 1 : (a) I n f r a r e d t r a n s m i s s i o n s p e c t r u m o f c a r b o n m i c r o s p h e r e s ; (b) 0 - p o t e n t i a l of c a r b o n m i c r o s p h e r e s in a q u e o u s s u s p e n s i o n s a s a f u n c t i o n o f pH.
F ig u r e 3 .1 2 : FTIR s p e c t r u m s o f p r i s t in e c a r b o n (Red) vs CM f u n c t i o n a l i s e d (Blue) w i t h a) p -c a r b o x y b e n z e n e d i a z o n i u m b) p - s u l p h o n a t e d i a z o n i u m a n d -c) n , n - d i e t h y l a n i l i n e d i a z o n i u m . Fig u re 3 .1 3 : z - p o t e n t i a l m e a s u r e m e n t s o f p r i s t in e C M s s h o w n in c o m p a r i s o n t o z - p o t e n t i a l o f CMs f u n c t i o n a l i s e d w i t h a) p - c a r b o x y b e n z e n e d i a z o n i u m b) p - s u l p h o n a t e d i a z o n i u m a n d c) n ,n - d i e t h y l a n i l in e d i a z o n i u m .
Fig u re 3 .1 4 : Plot o f t h e p a r ti c l e d i a m e t e r c u b e d , d e t e r m i n e d via DLS vs c o n c e n t r a t i o n o f LiDCA s o l u t io n
F ig u r e 3 .1 5 : SEM i m a g e s o f C Ms s y n t h e s i s e d f r o m t h e 2 . 5 4 MHz p i e z o u sin g a) 0 . 1 2 5 M a n d b) 0 . 8 5 M LiDCA; Sc a le b a r = 2 0 0 n m
F ig u re 3 .1 6 : Size d i s t r i b u t i o n via SEM o f 6 0 C M s s y n t h e s i s e d u s in g 1.0 M NaDCA
F ig u re 3 .1 7 : a) Typical DLS d i s t r i b u t i o n s o b t a i n e d u sin g NaDCA C M s b) Plot o f t h e p a r ti c l e d i a m e t e r c u b e d vs c o n c e n t r a t i o n o f t h e NaDCA s o l u t io n .
Figure 4.2: Typical SEM images o f th e Ag (a) and Pd (b) carbon m icro sphe res o b ta in e d by using co ffe e as a re d u c ta n t, 3 m m o l so lu tio n A g lN H jjj (a) and (b) 5 m m ol so lu tio n PdClz;
Scalebar = 200 nm.
Figure 4.3; C o n tro l e xp e rim e n ts excluding Sn se n sitisa tio n step fo r A g/C M (a) and Pd/CM (b) co m p o site synthesis; scalebar = 500 nm.
Figure 4.4:(a-c) Surface m o d ific a tio n caused by sen sitisa tion and a c tiv a tio n using 8 m m o l (a), 50 m m o l (b) and lO O m m ol (c) in th e sen sitisa tion step. Figure 4 (d -f) Resulting electroless
de p o sitio n s fro m activ a tio n s observed in fig u re 4.4a-c, resp ective ly. Scalebar= 100 nm , except
b = 50 nm
Figure 4.5: A g/C M synthesized using 0.3 m m o l (a) and 30 m m o l (b) Ag(NH3)2*;scalebar (a) = 100 nm Scalebar (b) = 200nm
Figure 4.6: XRD p a tte rn s o b ta in e d fro m th e A g/C M (a) and Pd/CM (b) samples. Figure 4.7: TGA curves o b ta in e d in air fo r p ris tin e CMs, A g/C M and Pd/CM com posites. Figure 4.8: E volution o f th e UVeVis a b so rp tio n spectra o f 4 -n itro p h e n o l in th e presence o f 1.0 X 10'^ M NaBH4 and A g/C M particles as a fu n c tio n o f re a ctio n tim e ; all spectra w e re corre cte d fo r s c a tte rin g [40]. The firs t sp e ctru m was take n im m e d ia te ly a fte rin je c tio n o f 4 -n itro p h e n o l,
w h ere as th e last sp e ctru m was take n a fte r 27 m in.
Figure 4.9: L o ga rithm ic p lo t o f th e no rm alized absorbance change as a fu n c tio n o f tim e . The lin e a r f it ne ar tim e zero was used to calculate th e ra te c o e ffic ie n t fo r th e re d u c tio n reaction.
Figure 4.10: Suzuki re a ctio n b e tw e e n 4 -b ro m o to lu e n e and p h e n y lb o ro n ic acid, run fo r 18 h at ro o m te m p e ra tu re . Yields o f 40 ± 10% w e re achieved, even up on th e th ir d use o f th e Pd/CM
catalysts.
Figure 4.11: TGA curves fo r washed and unw ashed Ag/C, D iffe re n ce can be a ttrib u te d to u n s u p p o rte d particles.
Figure 4.12: EDX m apping placing highest c o n c e n tra tio n s o f Ag to locatio ns w h e re particles are observed a t th e CM surface.
Figure 5.1: SEM im ages o f th e surface o f carbon m icrospheres as p re pa red (a), a fte r se n sitiza tio n in a Sn^* s o lu tio n (b), and a fte r a ctiv a tio n in a Pd^* s o lu tio n (c); scale bar =200
Figure 5.2: XP sp e ctru m o f carbon m icro sphe res a fte r un d e rg o in g th e tw o step s e n s itiz a tio n /a c tiv a tio n process th a t nucleates Pd° n a n o p a rtic le s at th e carbon surface (a SEM
Figure 5.3: SEM image showing CM samples after deposition in hypophosphite and Fe^* showing a) the heterogeneous reaction occurring on the CM surface and b) colloidal particles
present which represent the bulk o f the sample; Scale bar = 200 nm.
Figure 5.4: SEM images showing CM surface a fte r utilising m ethod A w ith 0.0125 M Fe^* in solution. Scale bar = 200 nm.
Figure 5.5: SEM images showing CM surface after utilising m ethod A w ith 0.025 M sodium hypophosphite concentration in solution. Scale bar = 200 nm
Figure 5.6: SEM images showing CM surface a fte r utilising m ethod A at room tem perature. Scale bar = 200 nm
Figure 5.7: SEM images showing CM surface a fte r utilising m ethod A at pH value 6. Scale bar = 300 nm
Figure 5.8: Typical SEM images o f CMs obtained after deposition in DMAB/Fe^* solution after 0.5 h (a) and a fte r 1.5 h (b); scale bar = 200 nm. The size of primary particles increases w ith
deposition tim e, (c) Size distrib u tio n o f iron clusters obtained a fte r 1.5 h o f deposition
Figure 5.9: Size d istribution o f iron particles obtained after 1.5 hr deposition.
Figure 5.10: XRD pattern obtained a fte r sensitization/activation and deposition in DMAB/Fe^* solutions fo r 1.5 h on activated CM powders
Figure 5.11: Fe K-edge absorption threshold obtained from (a) a-Fe, (b) hem atite, (c)magnetite and (d) maghem ite standards compared to th a t o f (e) Fe/CM. The linear com bination (LC) fit
w ith the parameters reported in Table 1 and the residual are drawn in the b o tto m traces.
Figure 5.12: k3-weighted EXAFS data o f Fe/CM compared to the fittin g curve in k-space (a) and R-space (b). The model did not include distances larger than 0.35 nm.
Figure 5.13: Cr(VI) removal fro m solution as a function o f tim e fo r pristine CM and Fe/CM particles
Figure 5.14: Shows the resulting decomposition o f Bath A absent glycine; Scale bar = 4 nm Figure 5.15: TGA showing th e residual masses le ft for CMs a fte r undergoing deposition fo r various ED times
Figure 5.16: SEM showing NPs at the CM surface a fte r 2.5 hr deposition utilising m ethod B Figure 5.17: XRD o f m ethod B perform ed on a flat carbon surface
List of Tables
Table 3.1: Surface area and pore size results from BET analys
Table 3.2: Comparison of 2.54 and 1.67 M HZ using 1.000 and 0.125 M LiDCA
Table 3.3: Average Zeta potential difference after surface functionalisation of CMs
Table 4.1: Summary of the effect of metal ion concentration on particle growth at the CM
surface
Table 5.1: Demonstrating the components of Methods A and B
Table 5.2: Results of a linear combination fit on XANES data of Fe/CM sample (see Figure 6).
Error bar is reported in brackets
Table 5.3: Summary of scattering paths obtained from a best fit of the EXAFS spectrum of
Fe/CM powders
Chapter 1
1.1 Background
C o n trary to p o p u la r b elie f, n a n o te ch n o lo g y is n o t exclusive to m o d e rn science.
C olloidal gold n a n o p a rtic le s (NPs) w as disco vered o v e r 2 5 0 0 years ago [1]. Colloidal
gold w/as used to m a k e ruby glass and to im b u e ceram ics w ith co lou r, and th ese
ap p licatio n s a re still co n tin u in g n ow . This w as d u e to th e in ten se colours th a t colloidal
n o b le m e ta l NPs m a ke . Also, o f in te re s t w as th e use o f co llo idal gold NPs in th e
diagnosis o f syphilis in th e m id d le age. A m e th o d w h ich re m a in e d ac tiv e and in use
u n til th e 20'*'’ c e n tu ry , a m e th o d w hich also h appens to be c o m p le te ly u n re lia b le [2].
H o w e v e r, ev en th o u g h NPs had b een aro u n d f o r this long, it w a s n 't until F araday
sh o w ed th a t th e in te n s e co lo u r is d ue to m e ta llic gold in co llo idal fo rm th a t
u n d erstan d in g o f th e p o te n tia l ap p licatio n s o f NPs w as slo w ly realised [3 ,4 ]. In 19 0 8 ,
M ie e x p la in ed th e p h e n o m e n a o f co lo u re d co llo idal m e ta l particles by solving
M a x w e ll's eq u a tio n s fo r th e ab so rp tio n and sc atte rin g o f e le c tro m a g n e tic ra d ia tio n by
spherical m e ta llic particles [5]. In te res tin g ly , th e s e tw o results in d icate d th a t m e ta l
nan o m a te ria ls had d iffe re n t o ptical p ro p e rtie s th a n th e ir b ulk c o u n te rp a rts , k n o w as
q u a n tu m size e ffe c ts [6 ,7 ]. These effects a re n ot a resu lt o f a scaling fa c to r b u t are
d ire c tly d ue to th e size and shape o f th e NPs. In fa c t, m a n y o f th e p ro p e rtie s o f bulk
m a te ria ls change w h e n th e m a te ria l is p ut in to th e n an o scale. O p tical, physical,
ch em ical and e le ctrica l p ro p e rties have all been o b s erv ed to be size d e p e n d e n t fo r
various m a te ria ls [8 ,9 ]. Effects such as this d ue to sm all p a rtic le size led to g re a t
curiosity a b o u t th e p ro p e rtie s o f nano-sized m a te ria ls in th e scientific c o m m u n ity
w h ich has c o n tin u e d to this day. In fa c t, in th e last te n years alo n e , th e n u m b e r o f
research papers on n a n o m a te ria ls has g ro w n e x p o n e n tia lly in n u m b e rs, ind icatin g th a t
research into n a n o m a te ria ls is still a re la tiv e ly n e w and u n e x p lo re d field [1 0 ]. This
research is d riven by a desire to d iscover m a teria ls w ith n e w p ro p e rtie s an d to
u n d e rs ta n d th e science b eh in d q u a n tu m size effe cts fo r d iffe rin g m a teria ls in th e hop e
All o f this research has led to m a n y p o te n tia l ap p licatio n s o f n a n o m a te ria ls fro m biological, optics, e n v iro n m e n ta l an d catalysis. H o w e v e r, in this re p o rt w e w ill be focusing on th e n a n o m a te ria ls w hich have p o te n tia l c a talytic and e n v iro n m e n ta l applications.
Synthesis o f n a n o m a te ria ls and p article is g e n e ra lly achieved in tw o w ays, to p d o w n ap p ro ach and b o tto m up ap p ro ac h . T op d o w n ap p ro ac h g en era l involved th e use o f p h o to c h e m is try o r e le c tro n b eam lith o g ra p h y fo r etchin g surfaces to c re a te n a n o m e tre sized structures and fe a tu re s [1 1 ,1 2 ], This ro u te fo r synthesis o f n a n o m a te ria ls w ill n ot be discussed o r re p o rte d on h ere in .
T he b o tto m up ap p ro ac h g e n e ra lly involves th e n u c le a tio n o f particles fo llo w e d by su b se q u en t g ro w th o f th e p article. T he easiest m e th o d fo r o b ta in in g NPs using this ap p ro ach is utilising w e t ch em istry. This ty p e o f synthesis starts o ff w ith m o le c u la r p recurso r solutions. Typically, th e y include a m e ta l salt, a reducing ag en t and a s u p p o rt/s ta b ilis in g a g e n t. G e n e ra lly th e m e ta l ion in so lutio n is re du ced to its m e ta llic s ta te , fo rm in g a p article. This process Is g o v ern e d by th e th e o ry o f n u c le atio n and g ro w th [6 ]. O th e r m e th o d s fo r b o tto m up synthesis h ave b een ac h ie ved . Specifically, m e th o d s involving sp ray pyrolysis [1 3 ,1 4 ], e le c tro -d e p o s itio n [1 5 ,1 6 ], and sol gel. Synthesis o f n an o s tru ctu res using e le c tro -d e p o s itio n is discussed In m o re d e ta il In section 1 .5 .3 .
1.2 Introduction to supported nanoparticle systems
1.2.1 Stabilising agents
crucial fo r m a n y p o te n tia l ap p lications. In s tab ility in so lutio n com es fro m a ttra c tiv e
forces b e tw e e n NPs in so lutions such as van d e r vaals fo rce s and m a g n e tic in te rac tio n s
[1 8 ]. If b o tto m up m e th o d s a re being used th e re m u st be a m ech an ism fo r restricting
g ro w th o f th e NP to keep it in th e desired size range. This is g e n e ra lly achieved using
stabilising ag en ts and s u p p o rt m aterials.
By in tro d u cin g stabilising s u p p o rt m a teria ls , it is possible to m a n ip u la te th e size o f NPs
d u rin g synthesis and NP s ta b ility post synthesis. The sta b ilis atio n o f NPs is g e n e ra lly o f
th e fo rm e le c tro s ta tic , steric, ele c tro s te ric and use o f ligands. Stabilisers w o rk by
crea tin g an e n e rg e tic b a rrie r to c o u n te ra c t th e van d e r W a a ls an d m a g n e tic (m a g n etic
m a te ria ls ) a ttra c tio n s b e tw e e n n an op articles. This can be used to in h ib it g ro w th in th e
n u c le atio n processes o f NP fo rm a tio n or to stabilise dispersions o f NPs in so lutio n [6].
1 .2 .1 .1 Polym ers
An e x a m p le o f a steric stabilising ag en t is polym ers. Figure 1 .1 shows p o ly (N -v in y l-2 -
p yrro lid o n e) (PVP) and p o ly (2 ,5 -d im e th y lp h e n y le n e o xid e ) (PPO ) w h ich are used in th e
synthesis o f NPs b ecause th e y b ehaves as steric and ligand stabiliser.
P V P
poly( vi ny IpyiTol idone)
PPO
poly(2.3-cliniethylphenylene oxide)
[image:20.521.46.510.23.821.2]Using th is p o ly m e r, Pt, Pd and Rh NPs have been synthesised w hich catalyse o le fin and
b e n ze n e h y d ro g e n a tio n reactions [1 9 ]. H o w e v e r th ro u g h o u t th e lite ra tu re a vast
n u m b e r o f p o lym ers have b een used in NP synthesis, including, polyacrylic acid [20 ],
oligosaccharides, p o ly e th y le n e glycol [2 1 ], chitosan [22] and p o ly e le c tro ly te film s [23 ].
1 .2 .1 .2 D e n d rim e rs
D e n d rim e rs h ave also seen use fo r s u p p o rtin g and stabilising m e ta l NPs. M e ta ls NPs
such as Cu, Au, Pt, Pd, Fe, Ag and Ru have all b een synthesis using d e n d rim e rs [2 4 -2 6 ].
D e n d rim e rs b e h a v e sim ilarly to p o lym ers in th a t th e y en cap su late th e NP, p articu larly
if h e te ro ato m s o f t h e m e ta l a re lo c a te d in th e d e n d rim e r in te rio r. T he branches o f th e
d e n d rim e r th a n c o n tro l access to th e n u c le atin g NP, w hich lim its g ro w th and stabilise
th e NP in so lutio n. Figure 1.2 show s th e stra te g y p io n e e re d by croo k e t al fo r th e
synthesis o f NPs via en ca p su la tio n in a d e n d rim e r [24].
P A M A M -O H
Complexatlon
Product Reactant
Pd/Rh bim etallic cat. Reduction
[image:21.521.10.510.41.789.2]dendrim er. Specifically, the preparation of dendrim er-encapsulated bimetallic NPs is shown
[27],
O th e r fo rm s o f stabilisers used include Ligands [1 ], m icro -e m u lsio n s, re ve rse m icelles
[9] an d su rfa cta n ts [10 ]. These m e th o d s ach ieve sta b ilis atio n and c o n tro lle d p article
g ro w th by using th e sam e principles and co n cepts as ex am p les previously o u tlin e d
ab o v e . H e n c e, th e y w ill n ot be discussed fu r th e r in this re p o rt. In g en era l, use o f som e
fo rm o f stabilising agents is essential in o rd e r to o b ta in nan o-sized m a te ria ls using a
b o tto m up s o lu tio n based ap p ro ach.
1 .2 .1 .3 Solid su p p o rt system s
In re c e n t lite ra tu re , th e re is an Increasing n u m b e r o f re p o rts on NPs a re being placed
on va rio u s solid su p po rts a re being published. T h e b e n e fit o f an ch o ring NPs a t a solid
su rfa ce is in c rea tin g synergies b e tw e e n th e d e s ira b le p ro p e rties o f m e ta l
n a n o p a rtic le s and th o s e o f th e solid m a trix. For e x a m p le , ease o f re m o va l and h an dling
o f NP system fro m a re ac tio n m e d iu m and m in im is a tio n o f p article a g g reg a tio n are
sig nifican t b en efits. A n o th e r ap p lica tio n fo r solid su p po rts is in use as a te m p la te
m a te ria l in o rd e r to achieve stru c tu red n a n o m a te ria ls . For e x a m p le , Kim e t a l[2 8 ],
n u c le a te d a Pd film by h e a t tre a tin g silica spheres w hich had p a lla d iu m
a c e ty la c e to n a te (P d (aca c)2 ) adsorbed a t th e silica su rface. S u bseq uent etchin g o f th e
silica s p h e re w ith HF le ft a h o llo w Pd m e ta l s p h e re n a n o s tru c tu re . M e n o n e t al u tilised
a p o ly c a rb o n a te m e m b ra n e as a te m p la te fo r synthesis Au n a n o e le c tro d e s in th e 10
nm size ra n g e [2 9 ]. In th e lite ra tu re , m o st solid su p po rts ta k e th e fo rm o f m e ta l oxides.
For e x a m p le . Si [3 0 ], Al [3 1 ], and Tl [32 ] oxides have all b een utilised as solid s u p p o rt
m a te ria ls fo r NPs. O xide su pports are n o t exclusive to th e s e m etals and w ill n o t be
discussed fu r th e r in this re p o rt.
M o re re c e n tly , carb o n m a teria ls have seen use as solid s u p p o rt m a te ria ls fo r NPs. T h e
properties fo r a variety o f applications. Carbon provides m any advantages over
polym eric o r inorganic supports due to its resistance to corrosion and its relatively
good b iocom patibility. F urtherm ore, th e surface chem istry o f carbon can be fin e tuned
to display d iffe re n t chemical groups th a t can fo r instance im p art charge, regulate
basicity/acidity, co ntrol w e ttin g behaviour o r p ro m p t biological recognition. Finally,
carbon supports can be designed to display a large specific surface area w hich can be
leveraged fo r the delivery o f large loads o f nanoparticles o r small molecules. For this
reason, th ere have recently been increased e ffo rts aimed at developing new
m ethodologies fo r th e c o ntrolle d d ep osition /e m b e dd in g o f m etal nanoparticles at
carbon scaffolds [33-36].
Examples o f carbon m aterials fo r nanom aterials support Include: M etz et al. have
synthesized nano-structured com posite Au/C and Pt/C electrodes fo r energy storage
and conversion applications, by using vertically aligned carbon nano-fibres (VACNF) as
a scaffold [36-38].
Photochemical Electroless
VACNF functionallzation
b)
CH3 D
'C=0
1) hv, 254nm
2) deprotection 1) 2)
OH OH C=0 CO
H H H
±
Au* Au
Au Au N ^ Sn^* ✓ V
o
o
'CO po
VACNF VACNF VACNF VACNF
Figure 1.3: (a) Schematic illustration of the steps involved in the functionalization of carbon
nanofibers and subsequent proceudure for electroless deposition, (b) Chemical
Figure 1.3 shows h o w IVletz and co w o rk e rs used solid ca rb o n fibres as a s u p p o rt fo r g ro w in g gold nano - ele ctro d es. T he ca rb o n fibres act as a te m p la te in o rd e r to shape th e g ro w th o f th e m e ta l n an o m a te ria l. Lipshuts e t al [40 ] use o f Nickel on Charcoal N i/C as an Inexp en sive H e te ro g e n e o u s C atalyst fo r Cross-Couplings b e tw e e n Aryl C hlorides and O rg an o m eta llics is a n o th e r e x a m p le o f a carb on su p p o rte d n a n o m a te ria l.
1.3 Applications of nanoparticles
In this section th e use o f n an o m a te ria ls w ith reg ard to specific areas w ill be discussed. T h e firs t p art w ill consist o f n a n o m a te ria ls fo r catalysis. A sh o rt re v ie w w ill be given, fo llo w e d by ap p lica tio n in catalysis fo r th e tw o m e tals utilised in th is re p o rt fo r catalysis. Part tw o w ill consist o f n a n o m a te ria ls fo r e n v iro n m e n ta l ap p licatio n s. H e re in , a re v ie w w ill be given o f c u rre n t te c h n o lo g ie s in e n v iro n m e n ta l re m e d ia tio n , th e p o te n tia l o f n a n o m a te ria ls and th e p ro b lem s w ith im p le m e n ta tio n o f n a n o m a te ria ls in this field .
1.3.1 Nanomaterials for catalysis
[image:24.521.15.511.52.597.2]b o n d s , m ak in g it po ssib le t o f u n c tio n alis e olefins a n d a r o m a t i c s in a facile m a n n e r . M o r e o v e r, significant n u m b e r s o f d iv e rse r e a c tio n s c a n b e c a ta ly s e d by v ario u s n oble m e ta l NPs, w h ic h a r e b e y o n d t h e s c o p e o f th is r e p o r t [10]. For th is r e p o r t , w e will utilise Pd a n d Ag m e ta l s in o r d e r t o achieving t h e aim s s t a t e d p reviously (s ee se c tio n 1.6.2).
1.3.1.1 Palladium n a n o m a t e r i a l s in cata lytic ap p lic a tio n
Pd n a n o p a r t i c l e s fin d i m p o r t a n t a p p lic a tio n s as c a ta ly s ts in h y d r o g e n a t i o n a n d C-C b o n d f o rm i n g r e a c tio n s like Suzuki, Heck a n d S o n o g a s h ir a co u p lin g [45,46]. H o w ev er, p r o b l e m s w ith ca ta ly s t re c o v e ry m e a n t t h e p r e s e n c e of u n d e s i r e d m e t a l c o n t a m i n a n t s in t h e e n d p r o d u c t s in r e a c tio n s f o r w hich it w a s utilised. Im m o b ilizatio n of catalytically ac tiv e Pd n a n o p a r t i c l e s a t solid s u p p o r t s fac ilita tes c a ta ly s t r e m o v a l an d rea c tio n w o rk up w h e n c o m p a r e d t o h o m o g e n e o u s cata ly sts. T h e r e f o r e , m u c h e ffo rt has r e c e n tly b e e n d e v o t e d t o d e v e lo p in g a n c h o r i n g p r o to c o l s f o r Pd n a n o p a r tic l e s , t h u s f o rm i n g a c o m p o s i t e m a te r ia l t h a t p r e s e r v e s t h e original ca taly tic p r o p e r t i e s w h ile im p roving h a n d lin g a n d r e a c tio n w o rk up. Mei e t al u s e d p o ly e le c tr o ly te b r u s h e s a n d c o r e shell m icro gels in o r d e r t o e n c a p s u l a t e t h e PdNP [26]. O t h e r g r o u p s h a v e u s e d c a r b o n n a n o t u b e s [47,48], silica [49,50], p o ly e le c tr o ly te films [51], a n d g r a p h i t e [52]. Im p o rt a n tl y t h e resu lts o b t a i n e d by m a n y of t h e s e r e s e a r c h e r s in d ic a t e t h a t t h e n a t u r e o f t h e s u p p o r t u s e d c an h a v e an i m p a c t o n t h e r a t e o f catalysis. This is n o t u n e x p e c t e d as catalysis is a h e t e r o g e n e o u s p ro c e s s . T h e r e f o r e , a n y m a te r ia l t h a t blocks a c c e s s t o t h e NP s u r f a c e h a s t h e p o te n t i a l t o r e d u c e c a ta ly tic p e r f o r m a n c e .
1.3.1.2 Silver n a n o m a t e r i a l s in ca taly tic ap p lic a tio n
[2 5 ,5 9 ], In o rd e r to achieve this goal, several research groups h ave fo cu sed on
d e v e lo p in g s u p p o rte d Ag n an o p artic le s, e.g. A g /p o lysa cc h a rid e [2 2 ,5 8 ], A g /g ra p h e n e
[6 0 ] o r A g / p o ly m e r [61 ] n a n o c a rrie r com p o sites, im p ro vin g tra n s p o rt and m o d u la tin g
ag g reg a tio n o f Ag n a n o p a rtic le active cen tres. Sim ilar to Pd p articles, th e su p p o rt
system chosen can in flu en ce th e ra te o f catalysis fo r AgNPs. This m eans selectio n o f an
a p p ro p ria te s u p p o rt m a te ria l can be essential fo r utilising NPs in c a talytic ap p lications
in an e ffic ie n t m a n n e r (See section 1.4).
1.3.2 Nanomaterials for environmental applications
1 .3 .2 .1 R eview o f th e issues
D u e to g ro w in g aw a ren es s a b o u t th e effe cts o f subsurface co n ta m in a n ts in soil and
g ro u n d w a te r in th e e n v iro n m e n t, increased research and fu n d in g is being allo c a te d to
th e a re a o f re m e d ia tio n tec h n o lo g ie s and c o n ta m in a n t m a n a g e m e n t. R e m e d ia tio n o f
th e s e c o n ta m in a te d sites is a vast and c o m p lex a re a . T he vast scale o f th e p ro b le m is
e x a s p e ra te d by th e s h e e r n u m b e r o f c o n ta m in a te d sites. In th e US th e re a re o v e r 1 5 0 0
o f w h a t are kn o w n as su p erfu n d sites and on av erag e a su p erfu n d site costs
a p p ro x im a te ly $ 2 5 m illio n to re m e d ia te . R ecently, it has been e s tim a te d th a t Irela n d
has close to 2 0 0 0 c o n ta m in a te d sites d u e old ind u stries, u n m a n a g e d spills and o th e r
in a d e q u a te w as te m a n a g e m e n t p ro ced u res [6 2 -6 4 ]. T h e c o m p le x ity o f th e p ro b le m is
in th e v a rie ty o f c o n ta m in a n ts a t th e sites, v a rie ty o f c o n ta m in a n t source ty p e and
lo catio n s and also th e v a rie ty o f soils and porous m e d ia w h ich co n tain th e
c o n ta m in a n ts . This m eans d iffe re n t m e th o d s fo r clean up h ave to be id e n tifie d in a
case by case basis. T ra d itio n a l ap p ro aches to tre a tin g soil an d g ro u n d w a te r
c o n ta m in a n ts have used p u m p an d t r e a t o r c o n ta in m e n t m e th o d s via p e rm e a b le
re a c tiv e b arriers (PRB). These m e th o d s h ave serious disad vantages. T he biggest
d is a d v a n ta g e is th e cost associated w ith th e m . Installing, m anagin g and re m o vin g
c o n ta m in a n t sources in d e e p aq u ife rs and u n d e r buildings m ay n ot be accessible to
th ese tec h n o lo g ie s [6 5 -6 7 ].
In re c e n t tim e s th e use o f n a n o te c h n o lo g y fo r e n v iro n m e n ta l re m e d ia tio n has been investig ated [1 8 ]. N a n o p article s have a h ig h er p ro p o rtio n o f a to m s n e a r o r a t th e
surface. This leads to a h ig h er p ro p o rtio n o f a to m s having d angling bonds and h ig h er surface en erg y . This m eans th a t th e s e a to m s h ave a m u ch g re a te r cap acity to
p ro m o te a d s o rp tio n and to in te ra c t w ith o th e r m o lecules in o rd e r to c o m p en s ate fo r
th e excess surface en ergy. A lre a d y , n a n o m a te ria ls ex h ib itin g prom ising re a c tiv e and
ad so rp tive p ro p e rtie s have b een successfully used in w a te r p u rific a tio n and
e n v iro n m e n ta l re m e d ia tio n . N a n o p article s also have th e a d v a n ta g e o f being ab le to p e n e tra te in tra p a rtic le pores o f soils. This en ab les th e ir use in slurry reactors fo r th e
re m e d ia tio n o f c o n ta m in a te d soils and s e d im en ts. C u rre n tly , th e m o st prom ising
n a n o m a te ria ls fo r e n v iro n m e n ta l a p p lica tio n s, co n sidering re m e d ia tio n p o te n tia l and cost a re m e ta llic iron an d iron oxides.
1 .3 .2 .2 M e ta llic iron as re m e d ia tio n m a te ria l
In re c e n t lite ra tu re , m e ta llic iron Fe° has b een re p o rte d as a successful re m e d ia tin g ag e n t fo r e n v iro n m e n ta l co n ta m in a n ts . Fe° is an e x c e lle n t e le c tro n d o n o r in w a te r ,
resulting in its use as a re d u ctiv e ag e n t th a t can e ffe c tiv e ly re m e d ia te c o n ta m in a n ts
such as ino rg an ic anions [6 8 -7 3 ], heavy m e tals [7 4 -7 6 ], and o rg an o h alid e s [7 7 -7 9 ]. In
fa c t, m e ta llic iron has b een so ve rs a tile in re m e d ia tin g m a n y d iffe re n t v a rie tie s o f
co n ta m in a n ts th a t an explosion o f w o rk has o ccu rred in this research fie ld in th e last fifte e n years.
Fe° particles can be d escribed using a c o re shell m o d e l as show n in Figure 1.4 . T he
co re m ain ly consists o f Fe w h e re a s th e o u te r shell consists o f a m ix tu re o f Fe^'^ and
H,0
Figure 1.4: Core shell model of an iron nanoparticle[18]
Figure 1 .4 also shows tw o o f th e m ain pro po sed m ech an ism s by w hich re m e d ia tio n
occurs on p a rtic le surfaces in th e case o f a c h lo rin a te d o rg an ic c o m p o u n d . Several
c o n ta m in a n ts ad so rb on th e oxide phase layer, o x id a tio n o f th e Fe° in th e p article
pro vides e le ctro n s fo r th e re d u ctio n o f tric h lo ro e th y le n e . T he Fe° core shrinks and is
used up in th e re ac tio n . T he particles a re no lon g er ac tiv e as a redu cin g a g e n t once all
th e Fe° is oxidised.
T h e re d u ctio n re a c tio n ind u ced by Fe° is a h e te ro g e n e o u s process. This m eans th a t
th e to ta l n u m b e r o f ac tiv e surface sites w hich can in te ra c t w ith co n ta m in a n ts
d e p e n d s only on th e to ta l surface area . H ence, by increasing th e surface to v o lu m e
ra tio {i.e d ecrea se d p article size), th e efficie n c y an d p e rfo rm a n c e o f th e re d u ctiv e
processes can be im p ro v e d . This led to th e in te re s t in n ano-sized Fe° across research
g roups fo r e n v iro n m e n ta l re m e d ia tio n . In te re s tin g ly , Fe°NPs have displayed
p ro p e rtie s u n iq u e to nan o-sized p article ind icatin g th e r e a re so m e q u a n tu m size
effe c ts . For e x a m p le , nano iron can d e c h lo rin a te p o ly c h lo rin a te d bip h en yl com p o un d s
(PCBs) a t ro o m te m p e ra tu r e and pressure w h e re a s g ra n u la r iron does n ot [8 0 -8 2 ].
H o w e v e r, m a n y re d u ctio n d u e to iron d o n 't have an y q u a n tu m e ffe cts . In fa c t w h e n
n o rm a lis e d fo r su rface a re a , re actio n ra te co nstants a re s im ilar fo r m a n y re d u ctiv e
re ac tio n s [6 5 ,8 0 ,8 3 ].
For successful use o f n an o m a te ria ls a t a large scale, cost e ffe c tiv e and robust m eth o d s
[image:28.521.13.509.33.720.2]q u a n titie s o f re m e d ia tio n agents a t lo w costs. Fe°NPs have b een synth esised using a
w id e ra n g e o f m e th o d s: d e c o m p o s itio n o f iron p e n ta c a rb o n y l in o rg an ic solvents
[8 4 ,8 5 ], e le c tro d e p o s itio n o f fe rro u s salts , vacu u m s p u tte rin g [8 6], re d u c tio n o f
g o e th ite an d h e m a tite p articles w ith h yd ro g e n gas a t high te m p e r a tu r e [8 7 ] and
fin a lly , re d u ctio n o f F e[lll] and F e[ll] salts using sodium b o ro h y d rid e o r o th e r redu cin g
agents. [8 8]. This last m e th o d uses aq u eo u s based c h e m is try an d is fa c ile to
im p le m e n t. Fe° is p re p a re d according to th e fo llo w in g re ac tio n :
4Fe^^ + 3BH4 + 9H2O ^ 4 F e ° 4 . + 3H 2 B 03 ' + 12H^ +6H2 t (e q n 1 .1 )
W h e n th e re a c tio n is ca rrie d o u t u n d e r co n s ta n t stirrin g an d u n d e r in e rt a tm o s p h e re
it leads to th e fo rm a tio n o f p olyd isp erse p olycrystallin e o r a m o rp h o u s n a n o p a rtic le s ,
w ith d ia m e te rs ty p ic a lly in th e rang e 2 0 -1 0 0 nm . This e q u a tio n show s th e basic
re ac tio n th a t occurs. It is im p o rta n t to n o te th a t m a n y side re ac tio n s can o ccu r h ere .
For e x a m p le , th e re a c tio n sh o w n in eq n 1.2 b e lo w occurs
B H 4 + 2 H 2 O - > B O 2 ■ + 4 H 2 (eq n 1.2)
This re a c tio n causes th e fo rm a tio n o f th e basic M e ta b o r a te ion. This can th e n lead to
th e fo rm a tio n o f m e ta llic Boron in o u r m ix tu re via th e h a lf re a c tio n
BO2 +2H2 0 + 3 e ^ B + 4 0 H ' (eq n 1 .3 )
These reactio n s re su lt in a F e/B alloy. T h e Boron c o n c e n tra tio n o f th is allo y is sensitive
to p a ra m e te rs such as pH, te m p e r a tu r e , c o n c e n tra tio n o f salts an d t h e ra tio o f th e
fe rro u s salt to th e B o ro h yd rid e salt. T h e se have b een in v e s tig a te d in d e ta il in th e
p revious lite ra tu re [8 9 -9 1 ].
T h e increased p e rfo rm a n c e o f Fe° o ffers m any ad va n ta g es; (a) im p ro v e d p e rfo rm a n c e
h a r m f u l b y - p r o d u c t in t e r m e d ia t e s . F in a lly , n a n o - m a t e r ia ls can b e d e liv e r e d d ir e c tly
in to t h e c o n t a m in a n t s o u rc e v ia in je c tio n s o f a q u e o u s s lu rrie s c o n ta in in g
n a n o p a r tic le s . T h is in je c tio n c a p a b ility e n a b le s s ite s s u c h as c o n t a m in a t e d d e e p
a q u ife r s t o b e s e le c tiv e ly t a r g e t e d [ 9 2 - 9 6 ] , th u s re p la c in g c o s tly e x c a v a tio n s .
H o w e v e r , a n u m b e r o f c h a lle n g e s m u s t b e o v e r c o m e in o r d e r t o im p le m e n t n a n o F e°
in t h e fie ld ; k e y issues t h a t r e p r e s e n t a n o b s ta c le a r e o u t lin e d in t h e f o llo w in g s e c tio n .
1 .3 .2 .3 C h a lle n g e s in t h e im p le m e n t a t io n o f iro n n a n o p a r tic le s
T h e f ir s t p r o b le m w ith t h e im p le m e n t a t io n o f F e ° is t h e p re s e n c e o f c o m p e t in g
o x id a n ts . In a c o n t a m in a n t p lu m e F e° n o t o n ly re a c ts w it h c o n t a m in a n ts b u t a ls o w ith
d is s o lv e d o x y g e n a n d w a t e r a c c o rd in g to t h e f o llo w in g r e a c tio n s :
2Fe°(s) + 02(g) + 4H ^ 2Fe^^(aq| + 2 H 2 0 (i) (e q n 1 .4 )
2Fe°,s) + 2 H 2 0 (|) 2Fe'",aq) + H2,g) + 20H -,aq ) (e q n 1 .5 )
Fe^'^ c a n b e f u r t h e r o x id is e d t o Fe^'^ a c c o rd in g to :
4 F e % ) + 02(g) + 4 H " 4Fe'"(aq> + 2 H 2 0 (,) (e q n 1 .6 )
T h e s e s id e re a c tio n s can a f f e c t t h e s e rv ic e lif e t im e o f F e ° NPs b a s e d PRB's, s in c e
c o n t a m in a n t p lu m e s c a n t a k e s e v e ra l y e a rs to c o m p le t e ly pass a p o in t in t h e
s u b s u r fa c e . This p r o b le m has t h e r e f o r e r e s tric te d t h e u s e o f F e° as a r e m e d ia t io n
a g e n t t o g r o u n d w a t e r , w h ic h is a r e la t iv e ly a n o x ic m e d iu m . H o w e v e r , t h e b ig g e s t
p r o b le m w it h f u r t h e r a p p lic a tio n o f F eN P s is in its m o b ilit y in p o ro u s m e d ia [9 7 ].
R e c e n t s tu d ie s o n b a r e u n s u p p o r te d F e °N P s h a v e r e p o r t e d lim it e d m o b ilit y in
s a tu r a t e d p o ro u s m e d ia [9 7 ] . T r a n s p o r t d is ta n c e s o f a f e w c e n t im e t r e s o r in c h e s h a v e
b e e n r e p o r t e d . T h is p o o r m o b ilit y is c a u s e d v ia t w o m e c h a n is m s , s u c c e s s fu l f ilt r a t io n
an o p tim u m size range fo r particles in o rd er to minimise th e ir filtra tio n by porous media. In general, sm aller particles below 100-200nm are susceptible to adsorptive processes on soil grains. Electrostatic interactions, London van der vaals forces and Brownian m otio n all influence these absorptive processes; consequently, soil pH, ionic strength and w a te r com position can all affect adsorption. Larger particles, above 1-2 nm in size, are susceptible to filtra tio n via sedim entation instead. Therefore, depending on soil typ e and w a te r conditions, the optim um range fo r particles to m inim ise filtra tio n is in th e o rd er o f 100 nm to 2000 nm. This means th a t Fe°NPs are vulnerable to filtra tio n by adsorptive process due to th e ir small size [98,99].
The colloidal chem istry and fe rro m a gn etic behaviour o f Fe particles results in severe aggregation. Aggregation prevents particle flo w through porous media such as soils a fte r aggregates reach a fe w microns in diam eter. Lowry et al have rep orte d DLS m easurem ents taken at d iffe re n t tim es a fte r sonication o f Fe° NPs dispersions, and observed th a t average p article size increased w ith in 15 min fro m lOOnm to SOOOnm in a concentrated solution. Even fo llo w in g attem pts to prepare extrem ely dilute dispersions it was not possible to reduce aggregation.
500nm
[image:31.521.11.510.51.797.2]Even w ith an im p ro v e m e n t in th e result and m e a s u re m e n t q u a lity , th e ag g reg atio n
ap p ea rs to be still occurring. Figure 1.5 shows a T E M o f FeNPs synthesised in o ur
la b o ra to ry using th e b o ro h y d rid e m e th o d o u tlin e d ab o ve. Large m ic ro m e te r sizes
chain like ag g reg ates can be o bserved . A g g regation such as this, also have th e e ffe c t
o f lo w e rin g re a c tiv ity d u e to su b se q u en t lo w e rin g o f specific surface area av a ila b le fo r
ad s o rp tio n an d re a c tio n [1 0 0 ,1 0 1 ].
In re c e n t tim e s , m a n y groups h ave trie d to o ve rc o m e th e s e d ifficu ltie s by use o f
su rfa ce m o d ific a tio n and su pports fo r th e NPs. S urface m o d ifica tio n s h ave involved
th e use o f su rfa cta n ts [9 9 ,1 0 2 ], ca rb o h y d ra te s [1 0 3 ,1 0 4 ], p o lye le ctro lyte s [1 0 5 ] and
tri-b lo c k co p o lym ers [1 0 6 ], These app ro aches aim at p re v e n tin g ag g reg a tio n via
e le c tro s ta tic repulsion a n d /o r steric stabilisatio n. In lite ra tu re th e s e m eth o d s have
b e e n sh o w n to im p ro v e tra n s p o rt in m o d el soils and to m in im is e ag g lo m e ra tio n ,
increasing sta b ility . H o w e v e r, this o fte n occurs at a loss in re a c tiv ity since th ese
su rfa c e m o d ifica tio n s have th e e ffe c t o f sim u ltan eo u s ly blocking and inh ib itin g
re a c tiv e sites on th e particle.
T h e re fo re , th e re is a need fo r n e w m e th o d s and te c h n o lo g ie s th a t can im p ro v e
tra n s p o rt an d m o b ility in soils fo r th e n an o p articles. These n e w te c h n o lo g ie s should
a im to im p ro v e tra n s p o rt and m o b ility w h ils t m a in ta in in g th e ad va n ta g e o f increased
re a c tiv ity g ained fro m using FeNP.
1 .3 .2 .4 Iro n o xid e in e n v iro n m e n ta l ap p lications
H ig h e r su rfa ce en ergies o f m e ta l o xide n an o p artic le s d u e to surface to v o lu m e ratio's
m a k e iron oxide n a n o p a rtic le m o re p ro n e to ad so rp tio n effects. A large body o f w o rk
has been d o n e on ad so rp tio n m echanism s o f ions o n to m e ta l o xid e surfaces [1 0 7 ,1 0 8 ].
S u rfac e h yd roxides in m e ta l oxides have a d o u b le p air o f ele ctro n s w ith a dissociable H
[1 8 ,1 0 9 ]. Iron o xide NPs have also been used in e n v iro n m e n ta l ap p licatio n s. Iro n oxide NPs have been sh o w n to be e ffe c tiv e in th e ad so rp tio n o f C r(V I) [1 1 0 ], a rs e n ite and
ars e n a te [1 1 1 ].
1.4 Support material for Ag, Pd and Fe/FCxOy particles
Porous carb on m icro s p h e res (C M s) a re p a rtic u la rly a d va n ta g eo u s fo r n a n o p a rtic le s u p p o rt since th e y can lev era g e all o f th e advan tag es o f ca rb o n m a te ria ls w h ile displaying a high specific surface and good tra n s p o rt/d e liv e r y p ro p e rtie s , as re c e n tly sh o w n by w o rk in o u r g ro u p [1 1 2 ]; h o w e v e r, t h e ir ap p licatio n s as n a n o p a rtic le s u p p o rt have re m a in e d re la tiv e ly u n e xp lo re d .
C arb on m a te ria ls displayed c h em istry w h ich fa c ilita te s th e fu n c tio n a lis a tio n o f th e su rfa ce [1 1 3 -1 1 5 ]. This fa c ilita te s a m ech an ism fo r changing su rfa ce ch arg e and ch e m is try in C M s. Surface ch arge m o d ific a tio n can be m e a s u re d using th e zeta p o te n tia l fo r susp end ed particles.
1.4.1 Zeta potential and surface charge
M o s t p articles in a colloidal suspension have a surface charge associated w ith th e m . This surface ch arge a ttra c ts an excess o f a co u n te rio n species in so lutio ns. This leads to th e fo rm a tio n o f tw o layers o f o p p o site charge. These a re th e fix e d surface ch arg e and th e d iffu se charge fro m ions in so lu tio n . This is kn o w n as th e ele c tric a l d o u b le la y e r (E D L )[1 1 6 ].
th e o u te r H e lm h o ltz lay er (OHL) to distinguish b e tw e e n m o ieties th a t have a chem ical a ffin ity fo r th e surface as w e ll as co u lo m b ic in te rac tio n s. T an g e n tial flu id flo w along a ch arged su rface can be caused by ap p lyin g an ele c tric fie ld . It has b een sh o w n th a t a th in la y e r o f flu id sticks to th e surface. This is kn o w n as th e h yd ro d y n a m ica ily stag n an t layer. This la y e r goes fro m th e surface to so m e d istance d®'' w h e re a slip plane is assum ed to exist and flu id flo w begins again. This assu m p tion m eans th a t th e v o lu m e c o n ta in e d w ith in th e slip p la n e Is c o n stan t and h en ce th e space charge fo r e v e ry th in g w ith in th e slip plane is co n stan t as e v e ry th in g is assum ed to be static w ith in th e slip p lane. T he p o te n tia l a t this p o in t is d e fin e d as th e zeta p o te n tia l. T h e slip p lane w ill be a g re a te r th a n o r eq u al to d is tan ce fro m th e su rfa ce as th e OHL.
a° a' 0^ a®*'
IH P
O H P
Slip p la n e
d d®'^
Distance
Figure 1.6: M odel o f Surface charges and potentials. All potentials are defined with respect to th e potential in the bulk solution.
The zeta potential is not directly measurable but its value can be obtained via electrokinetic m easurem ents coupled to modelling of the electrophoretic mobility. In our studies w e have used this approach to investigate the effects of surface functionalisation on CM surface charge using methods outlined in section 2.2.2.
1.4.2 Carbon microsphere w ith controllable size
Also, CMs w/ere synthesised utilising ultrasonic spray pyrolysis. Using this m ethod CMs with varying porosity have been synthesised[117]. This m ethod also has potential to control CM size (See section 2.1.1). Tufenkji-Elim elech model predicts th a t filtratio n effects are minimized fo r particles b etw een 0.1 nm and 1 nm in d iam eter [118]; th erefore, CM size is in th e optim al range fo r applications requiring delivery of nanoparticles through porous matrices. These CM o ffe r a vast range of selectivity and tuneable param eters fo r potential applications as a nanomaterials support. If control of CMs used fo r support can be gained, th ey would provide an excellent support fo r Ag, Pd and Fe/FexOy NP's in all th ere potential applications.

![Figure 1.1: Two polymer families used as metal NP supports for catalysis [10].](https://thumb-us.123doks.com/thumbv2/123dok_us/8811651.918735/20.521.46.510.23.821/figure-polymer-families-used-metal-np-supports-catalysis.webp)


![Figure 1.4: Core shell model of an iron nanoparticle[18]](https://thumb-us.123doks.com/thumbv2/123dok_us/8811651.918735/28.521.13.509.33.720/figure-core-shell-model-iron-nanoparticle.webp)