The temperature dependence of the F band in magnesium oxide
Full text
Figure
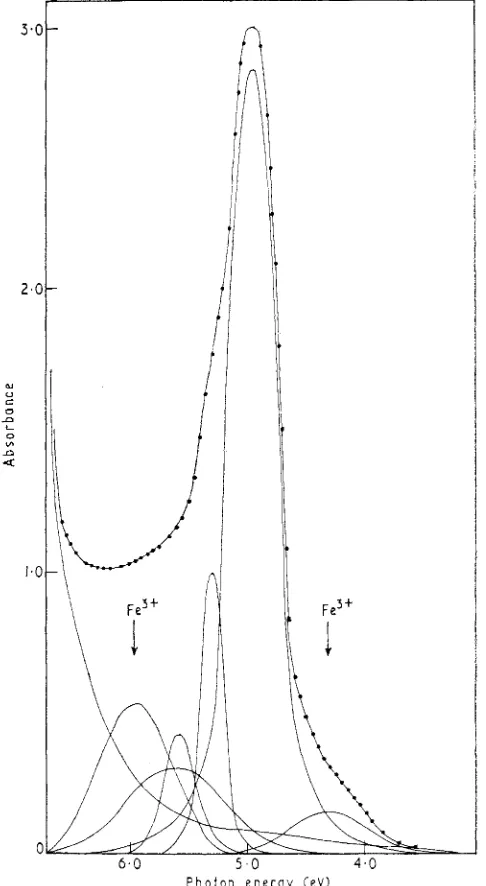
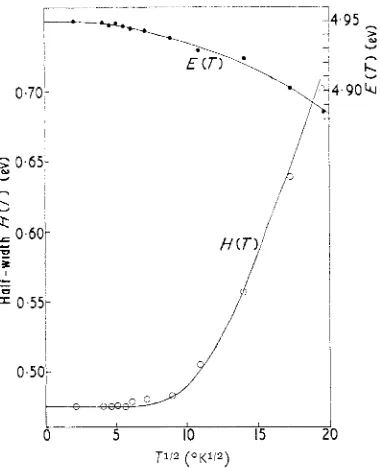
Related documents
• Follow up with your employer each reporting period to ensure your hours are reported on a regular basis?. • Discuss your progress with
4.1 The Select Committee is asked to consider the proposed development of the Customer Service Function, the recommended service delivery option and the investment required8. It
The corona radiata consists of one or more layers of follicular cells that surround the zona pellucida, the polar body, and the secondary oocyte.. The corona radiata is dispersed
Board for Master Plumbers Board for Master Plumbers. APPROVED: APPROVED:
Field experiments were conducted at Ebonyi State University Research Farm during 2009 and 2010 farming seasons to evaluate the effect of intercropping maize with
Results suggest that the probability of under-educated employment is higher among low skilled recent migrants and that the over-education risk is higher among high skilled
Using text mining of first-opinion electronic medical records from seven veterinary practices around the UK, Kaplan-Meier and Cox proportional hazard modelling, we were able to
National Conference on Technical Vocational Education, Training and Skills Development: A Roadmap for Empowerment (Dec. 2008): Ministry of Human Resource Development, Department