Potential of New Isolates of Dunaliella salina for Natural β-Carotene Production
Full text
Figure
Related documents
We invite unpublished novel, original, empirical and high quality research work pertaining to recent developments & practices in the areas of Computer Science
Analysis of factors causing payment delays from questionnaires administered to Roads Authority revealed the following that Roads Authority regard lack of adequate funds on the part
Autoregressive Conditional Heterskedastic (GARCH) model and the researcher observed that GARCH (1,1) was identified as the best model for AIICO Insurance Plc and
Treatment of hyperlipidemia with Borassus aethiopum fruit methanol extract at different concentrations (100, 200, 300 and 400 mg/kg) significantly (p≤ 0.05) reduced the
Impact of rabeprazole, a new proton pump inhibitor, in triple therapy for Helicobacter pylori infection- comparison with omeprazole and lansoprazole. Aliment
Relation between thyroid hormone concentration and serum levels of interleukin-6 and interleukin-10 in patients with nonthyroidal illness including chronic kidney disease..
CCL3 and CCL20 -recruited DCs, when modified with tumor antigen gene MAGE-1, could induce not only an effective CTL response against gastric cancer cells ex vivo but also
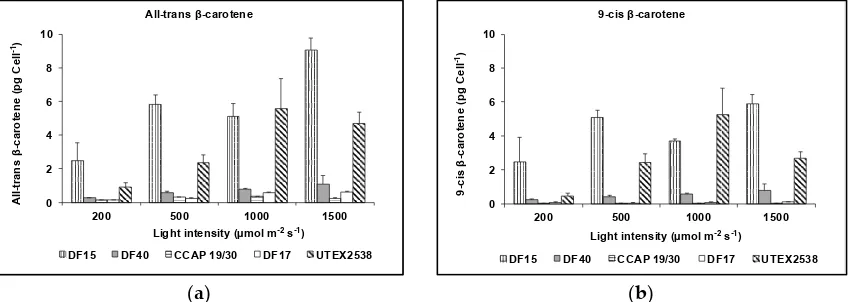
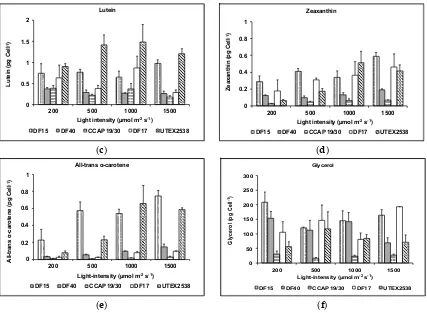
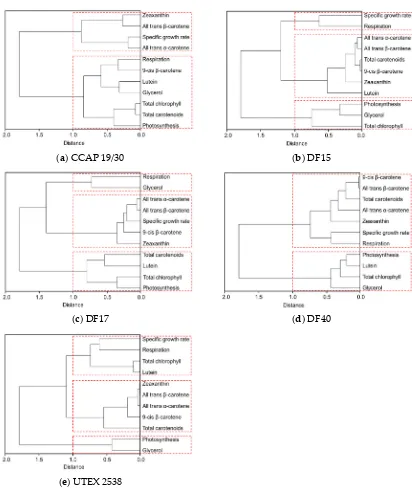
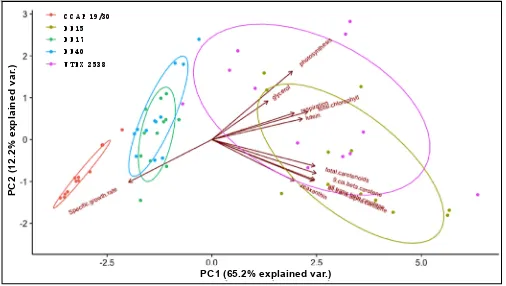
![Figure 10. Phylogenetic tree showing the location of the three newly isolated Dunaliella strains (DF15, DF17 and DF40) compared to CCAP 19/30 and UTEX 2538 used in this study [43]](https://thumb-us.123doks.com/thumbv2/123dok_us/7884707.1308265/13.595.187.432.126.409/figure-phylogenetic-showing-location-isolated-dunaliella-strains-compared.webp)