*Corresponding author: Murali Markandan ISSN: 0976-3031
Research Article
BACTERIAL (BACILLUS LICHENIFORMIS) BIODEGRADATION OF UV EXPOSED
PET (POLY ETHYLENE TEREPHTHALATE)
Murali Markandan
1*, Umamaheswari. S
2and Anuradha. S
31
Department of Zoology, St Joseph University Dimapur, Nagaland, India
2
Department of Zoology, Periyar EVR College, Tiruchirapalli, Tamil Nadu, India -620 023
3
Department of Botany, Adiyaman Arts and Science College for Women’s, Uthangarai Tk, Krishnagiri- Dt
DOI: http://dx.doi.org/10.24327/ijrsr.2019.1008.3866
ARTICLE INFO ABSTRACT
PET consist several enviable characteristics and thus are mostly used in daily life. On the other hand, non-biodegradability, once thought to be an advantage offered by plastics, is causing major environmental problem. UVlight may be accelerator of bacterial biodegradation of PET plastics, In this study soil bacteria were selected, specifically Bacillus licheniformis. It was interesting to observe that UV treated PET on inoculation with bacteria lead to biofilm formation, surface corrosion and rod shape bacterial colonization as evinced , further the PET degradation was confirmed by FTIR studies, which indicated the formation of ester group, methylene group as evinced in UV exposed PET. Thus bacteria (Bacillus licheniformis) could be used as biological agents to degrade of PET.
INTRODUCTION
Since the discovery, it is first synthesis and patenting in 1941, polyethylene terephthalate (PET) became a widely used material in several industrial branchesal.,1949 [1]. The world wide PET resin production amounted to 27.8 million tons in 2015 [2]. One of the current mass-produced synthetic polymers that is highly resistant to biological and hydrolytic degradation. Poly(ethylene terephthalate) (PET), a thermoplastic aromatic polyester that has excellent material properties. PET is commercially used in various applications, such as textile fibers, soft-drink bottles, and packaging films. Owing to the increased awareness of environmental issues, recycling of PET has been practiced. However, this is difficult or inexpedient due to technical and economic considerations [3,4].
The ability of UV light to drive radical processes to transform surface chemistry. There are few reported studies of the effect of deep ( < 250 nm ) UV on PET [5]. The phenomenon of ‘weathering’ of polymeric materials is usually caused by a complex series of chemical reactions initiated by the absorption of ultra-violet light which ultimately result in the deterioration of the physical properties of the polymer. One of the most likely initiating sites for ultra-violet absorption in polymer is the ketone carbonyl group which is often introduced
into the polymer by oxidation during its processing or preparation. UV light may be change hydrophobic of on plastics surface into hydrophilic, Natural polymers, on the other hand, although they also degrade by photochemical mechanisms usually are more rapidly degraded biologically, that is by the attack of micro-organisms. The microorganisms participating in the degrading process attack the surface and settle in a biofilm-like colony, which produce alterations as it comes into contact with the polymer[6]. Biofilms are embedded in the polymer at source, consisting basically of extracellular polysaccharides, proteins and microorganisms [5,6]. The formation of biofilms is a prerequisite to substance corrosion and / or material deterioration. Some polymers are susceptible to direct biodegradation via enzymes and /or microorganisms, while others may allow degrading only after a hydrolytic stage or scission of the oxidant chain[7]. The current study aims to investigate the capability of bacterial from the soil environment to associate with and adhere to the surface a common plastic packaging material namely PET understanding the factors that influence bacteria attachment on plastic surface will be useful for promoting bacterial growth on such substrate in order to better facilitate their biodegradation ultimately it is hope that a methodology for biodegradation of plastic in the environment can be developed.
International Journal of
Recent Scientific
Research
International Journal of Recent Scientific Research
Vol. 10, Issue, 08(E), pp. 34310-34314, August, 2019
Copyright © Murali Markandan et al, 2019, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.
DOI: 10.24327/IJRSR CODEN: IJRSFP (USA)
Article History: Received 4th May, 2019
Received in revised form 25th June, 2019 Accepted 23rd July, 2019
Published online 28th August, 2019
Key Words:
MATERIAL AND METHODS
Isolation of bacteria from PET waste
Poly (ethylene terephthalate) waste (PET bottles) were collected from garbage dumped site. The soil particles on the surface of the PET waste was removed and washed with sterile distilled water and inoculated in the nutrient broth. After 24 hours of incubation, 100 µl of broth culture was inoculated into nutrient agar plates. After 24 hours of incubation the bacterial isolates were identified by the methods described in Bergeys Manual of Determinative Bacteriology [8] and Finally conformation of bacterial sample using 16s rRNA methods was done by Microbial type culture collection and Gene Bank, Institute of Microbial Technology, Chandigarh, India.
Incubation of PET flakes in MSM inoculated with bacteria
Purchased PET bottles were cut into small flakes (0.2mm ) and exposed to UV radiation at period of 30 days by UV light (500W) with an optical filter (250–380 nm) at room temperature. The distance between PET flakes and lamp was 3 feet. Then the PET flakes were washed with 70 % ethanol and again washed with distilled water and finally the samples were kept in at 45 oC for drying. Then the samples were directly inoculated into Minimal Salt Medium containing Bacillus licheniformis. They were kept in orbital shaker for a period of one month at 37 o C temperature with 120 rpm.
Scanning Electron Microscopy
Scanning electron microscope (SEM) (VEGA3 TESCAN) was used to determine the changes on the surface of PET flakes and colonisation of bacteria. Control and bacterial treated samples are generally sputter-coated with gold or some metal ions before SEM examination. Analysis was carried out using low vacuum 0.68 Torr mode, 10 to 30 kv at different magnification 6.13 kx to 500 kx and LFD (large field Detector)[9].
Strums test
Carbon dioxide (CO2) evolution as a result of PET (UV treated
and Untreated) biodegradation was determined by Sturm Test [10]. UV exposed and unexposed PET flakes were added to the conical flask containing 150 ml of mineral salts medium without any other carbon source. Selected bacteria was used for PET biodegradation Bacillus licheniformis(109 ×109 cfu / ml), .The control conical flasks were without any PET flakes. Sterilized air was supplied to keep conditions aerobic. The test and control conical flasks were stirred continuously by placing them on the Orbital shaker at 120 rpm and test was performed at room temperature (300 C) for four weeks. Every week the amount of carbon dioxide produced was calculated in the test and control bottles gravimetrically. Evolution of CO2, as a
result of degradation of polymeric chain was trapped in the absorption conical flakes containing KOH (1M).Barium chloride solution (0.1M ) was added to the CO2 containing
KOH bottles and as a result precipitates of Barium Carbonate (using CO2 released from breakdown of polymer) were
formed. CO2 Produced can be calculated gravimetrically by
measuring amount (weight) of CO2 precipitates evolved by
addition of Bacl2.
FTIR Spectroscopic analysis of bacterial degradation of PET flakes
Fourier transform infrared (FT-IR) measurements were carried out with a Perkin Elmer Spectrum two (Version 10.03.09) in the range of 4000-400 cm-1. FT-IR spectra were recorded at a resolution of 2 cm-1 and at an accumulation of 32 scans. FTIR analysis was done to detect the chemical changes of PET inoculated in MSM containing Bacillus licheniformis.
RESULT AND DISCUSSION
The. surface topography of untreated, UV-irradiated PET and Bacterial inoculated with UV exposed PET in Minimal salt medium period of one month are investigated by SEM. From the plate -1, it is apparent that the untreated PET sample surface was smooth without any cracks or fracture and were free from defects. There was no visible structural changes on the surface of the UV treated PET. Rough surface of UV untreated and UV exposed PET flake inoculated with Bacillus licheniformis was observed in the SEM image when compared to the control. Inoculation of 30 days UV exposed PET with Bacillus licheniformis resulted in roughness and bacterial propagate very rapidly on the PET surface. Bacterial Rod shapes colonization can be seen on surface of PET flakes. The biofilme formation evinced indicates that Bacillus licheniformis, could serve as an agent for biodegradation. (Plate-1).
Smooth surface of PET evinced in the SEM image is in good accord with the observations of Umamaheswari et al.,(2013), who have also visualised smooth surface of PET in the SEM image. They also noticed no visible structural changes on the surface of UV treated PET. In addition, they also noticed Pseudomonas sp., colonies on the surface as well as inside PET on inoculation of UV treated PET with Pseudomonas sp.,. These observation are in line with the present findings[11]. In addition, they have detected surface changes in the SEM images of short UV treated polypropylene films after 12 months of incubation with Bacillus flexu[ 11]. Bacterial biofilm formation on UV treated PET flakes is in good coincide that they also detected in the SEM image of polyethylene terephthalate sheet kept in a closed drawer untouched for nearly a year, crystals of degraded products as well as microbial colony growing inside as well as on the surface of the crystals [12].
The data displayed in Table-3 reveal significant (115.06, P < 0.001) increase in CO2 evolved in MSM containing PET
inoculated with Bacillus licheniformis and MSM without PET (0.19 g/ L). Among the treatments, PET powder containing MSM inoculated with Bacillus licheniformis elicited significantly higher CO2 production ( 0.70 g/ L) when
compared to UV exposed PET flakes (0.60 g/ L) and PET flakes (0.46 g/ L) containing MSM inoculated with Bacillus licheniformis . Significant increase ( F = 107.458, P< 0 .001) in CO2 evolution was evident during the period of experiment
( I week: 0.06 g L-1 ; II week: 0.54 g L-1; III week: 0.57 g L-1 ; IV week : 0.59 g L-1 ;V week: 0.67 g L-1 ( Table-1).
Shakina et al., (2012) have observed that test sample ( with polyester sheets ), the amount of CO2 produced were greater
consortia grow more in the case of test. They have reported that CO2 produced after mineralisation of polymer for 30 days
was found to be greater for vinyl acetate Poly (corn oil) fumarate –co-vinyl acetate (PCFVA) polymer was generator followed by CORVP,PCFMMA (Poly (corn oil) fumarate –co-methylmetacrylate) and PCFSTY (Poly (corn oil) fumarate-co-styrene) . Only few studies have founded on mineralisation of PET in soil and MSM.
Table 1Mean variation in CO2 evolution during of PET
degradation in MSM by Bacillus licheniformis
S.No Treatments CO2 (g/l)
1 Control 0.19 d
2 PET powder 0.70 a
3 PET flakes 0.46 c
4 UV treated
PET flakes
0.60 b
F 115.06***
S.No Weeks CO2 (g/l)
1 I 0.06c
2 II 0.54b
3 III 0.57b
4 IV 0.59b
5 V 0.67a
F 107.458***
***Significant at P < 0.001; In a column, figures having dissimilar letters differ significantly according to Duncan New Multiple Range Test (DMRT)
Identification was carried out by 16S rDNA sequence analysis and the strain was identified as Bacillus licheniformis. The 16s rDNA sequence was submitted to NCBI Gene Bank depository under the accession no AE017333. 16s rDNA sequence of isolated bacterium exhibited the homology with other bacterial Bacillus licheniformis ATCC14580 (T ) (98.92%).
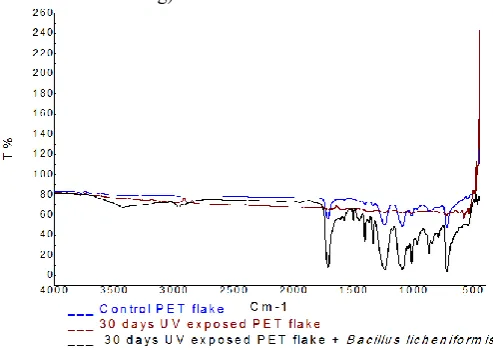
The differences in chemical composition of PET samples can be revealed by comparing the IR spectra. Compilation of FTIR spectral peaks band assignment of control PET flakes, 30 days UV exposed and bacterial treated PET flakes reveal appearance of new peaks. The signature peaks of PET flakes include 3781 cm-1 ( O-H bond stretching), 1716 cm-1 (C=O bond stretching), 1407 cm-1 (C-H bond stretching), 1339 cm-1 (C-H bond stretching ),1242 cm-1 (C-C-O) bond stretching),1093 cm-1 (O-C-C bond stretching), 1017 cm-1 (C-H bond stretching), 871 cm-1 (C-H bond stretching) and 723 cm-1 ( C-H bond stretching).
Fig 1 FTIRspectra of PET flake of control, 30days UV exposed and
inoculated with Bacillus licheniformis in MSM
On exposure of PET flakes to UV for 30 days, several new peaks appeared ( 3910 cm-1: O-H bond stretching; 3422 cm-1: O-H bond stretching; 2971 cm-1: C-H bond stretching; 2872 cm-1: C-H bond stretching; 1578 cm-1:C-C bond stretching; 1482 cm-1, 1432 cm-1, 1372 cm-1: C-H bond stretching ; 1237 cm-1: C-O bond stretching; 790 cm-1: C-H bond stretching).
On inoculation of PET flakes with Bacillus licheniformis new absorption peaks appeared at 3378 cm-1 (O-H bond stretching) and 1592 cm-1(C-C bond stretching). In addition, shift in absorptions peaks from 1716 to 1715 cm-1, 1407 to 1409 cm-1, 1242 to 1240 cm-1, 1093 to 1092 cm-1, 871 to 872 cm-1,723 to 724 cm-1 were observed. Disappearance of absorption bands occurred at 3781 cm-1 and 1339 cm-1. In general, decline in the intensity of the characteristic peaks of UV untreated PET was noticed on inoculation of PET with Bacillus licheniformis 1715 cm-1,1409 cm-1,1240 cm-1, 1092 cm-1,872
cm-1and 724 cm-1) . Interesting feature observed in Fig -1 (table-2) is the appearance of new bands at 1505 cm-1 and
1400 cm-1region (1453 cm-1 and 1408 cm-1), which have been assigned to C-C and C-H bond stretching, respectively. In addition, new absorption peaks, were evinced at 1339 cm-1 (C-H bond stretching), 1241 cm-1 (C-C-O bond stretching), 1175 cm-1 (C=O bond stretching), 1116 cm-1 (C-H bond stretching), 1044 cm-1 (C=O bond stretching), 970 cm-1 (C-H bond stretching) and 856 cm-1 (C-H bond stretching). Thus inoculation with Bacillus licheniformis could lead to different conformation changes in PET. Shift in absorption bands from 3422 to 3424 cm-1, 1709 to 1714 cm-1, 1372 to 1371 cm-1, 1093 to 1095 cm-1, 1015 to 1017 cm-1, 873 to 872 cm-1, 790 to 792 cm-1, 725 to 723 cm-1 have been evinced. Disappearance of band occurred at 3910 cm-1, 3784 cm-1, 2971 cm-1, 2872 cm-1, 1482 cm-1, 1432 cm-1 and 1237 cm-1. Fig 17 also indicates that sharp absorption bands occurred at 1714 cm-1,1241 cm-1,1095 cm-1, 1017 cm-1,872 cm-1,792 and 723 cm-1. Doublet occurred at 1408 and 1339 cm-1. Appearance of absorption peaks at 1408 cm-1 (C-H bond stretching) and 1241 cm-1 (C-C-O bond stretching), in the FTIR spectra of all the Bacillus licheniformis inoculated 30 days UV exposed PET flakes was a common phenomena observed in this study. Comparative analysis of the FTIR spectra of UV unexposed, UV exposed and UV exposed inoculated with bacteria reflect maximum number of absorption peaks in 30 day UV exposed PET inoculated with Bacillus licheniformis. which implies that UV irradiated PET on inoculation of Bacillus licheniformis elicits intensive modification in the chemical structure of PET and would mediate effective degradation of PET. On inoculation of PET with Bacillus lichniformic, peaks in the 4000 to 3000 cm-1 region became broadened after degradation, which was due to the formation of hydroxyl and carboxylic groups.
stable to light and are susceptible to rapid degradation during UV exposure. They have concluded that UV light modified the chemical structure of PU. The absorption of UV induces the degradation of PU and photo-oxidation of the CH2 groups[14].
Table 2 Band assignment of FTIR spectra of PET flake of Control PET, UV unexposed PET inoculated with Bacillus
licheniformis, 30days UV exposed and inoculated with Bacillus licheniformis in MSM
Control PET flake (Wave number
(cm-1)
PET flakes +
Bacillus licheniformis
(Wave number (cm-1)
30 days UV exposed PET flake Wave number
(cm-1)
30 days UV exposed PET flake + Bacillus
licheniformis
Wave number (cm-1)
3781 (O-H) 3378 ( O-H) 3910 (O-H) 3424 (O-H)
1716 ( C=O) 1715 ( C=O) 3784 (O-H) 1714 (C=O)
1407 ( C-H) 1592 ( C-C) 3422 (O-H) 1578 (C-C)
1339 (C-H) 1409 ( C-H) 2971 (C-H) 1505 (C-C)
1242 (C-C-O) 1240 (C-C-O) 2872 (C-H) 1453 (C-H)
1093 (O-C-C) 1092 (O-C-C) 1709 (C=O) 1408 (C-H)
1017 (C-H) 1017 ( C-H) 1578 (C-C) 1339 (C-H)
871 (C-H) 872 ( C-H) 1482 (C-H) 1371 (C-H )
723 (C-H) 724 ( C-H) 1432 (C-H) 1241 (C-C-O)
1372 (C-H) 1175 (C=O)
1237 (C-O) 1116 (C-H)
1093 (O-C-C) 1095 (O-C-C)
1015 (C-H) 1044 ( C=O)
873 (C-H) 1017 (C-H)
790 (C-H) 970 (C-H)
725 (C-H) 872 (C-H)
856 (C-H) 792 (C-H) 723 (C-H)
Plate- 1 SEM of PET flake of Control PET , UV unexposed PET inoculated with Bacillus licheniformis, UV exposed and inoculated with Bacillus licheniformis in MSM
This was also confirmed by the shift in absorption peak of C=O stretching vibration after degradation. This observation is well supported by the findings of Yuan –Xuan Weng et al., (2013)[15] who have also demonstrated that biodegradation of p(3 HB, 4 HB) films in soil were mainly caused by microorganism and many low molecular weight polymer were produced. Broadening of absorption peaks in the 4000 to 3000 cm-1 region was observed in the FTIR of PET flakes exposed to UV for 30 days. This observation is in line with the findings of Arkatkar et al., (2010)[16] who have opined that PP –SUV (Short UV treated Polypropylene) were more prone to hydrophilic in the presence of microorganisms. In additional, Chonde Sonal et al., (2012) have confirmed through FTIR spectroscopy Trametes versicolor NCIM 1086 mediated degradation of the synthetic polymer nylon 6 by the appearance of new groups like CH3, CONH2, CHO and
CHOOH and have reasoned at that it could be due to hydrolysis or oxidation. They have further attributed it to the cleavage of C-C bond in CH2-CH2 adjacent to nitrogen atom
and weakening C=N stretching. In addition, they have also noticed decrease in the strength of characteristic band of C(O) NH occurring around 3300 cm-1,1640 cm-1,1550 and 1018 cm
-1
after 90 days. In additional, Emergence of methylene (C-H), eater (C=O),ester linkage terephthalate (C-O-C) noticed in the study due to UV treatment in the FTIR spectra of PET exposed to UV for 30 day period gains supported form the observation , They have depicted in the FTIR spectra of photo-degraded LLDPE exposed to bacterial culture for 7 days, the bands at 1643 cm-1 and1550 cm-1 which are typical of amide I and II bands, respectively, and together with the broad band in the 3300-3100 cm-1, region of NH-amid absorption, can be assigned to the presence of a significant amount of protein material on the polymer surface. They have also observed broad band peaking at 1130 and 993 cm-1 which indicates the presence of polysaccharides, the usual exopolymers produced by microorganisms, which are the major constituents of the biofilm. In addition, they have noticed that on incubation of the
photo-oxidized polyethylene with B.borstelensis and MIX (Mixture of Bacillus cereus, B.megaterium and B.Subtilis). for
90 days resulted in a marked reduction in the amount of carbonyl residues estimated in terms of carbonyl index[17,18].
CONCLUSION
References
1. W. J.Rex, D.J .Tennant, Polymeric linear terephthalic esters. Google patents. 1949
2. Plasticsinsight.com. Global PET resin production capacity. https://www.Plasticsinsight.com/global-pet-resin-production-capacity accessed 01.12.2017 2017. 3. F. Awaja, F. Daver, E. Kosir, F. Cser. The effect of
chain extension on the thermal behavior and crystallinity of reaction extruded recycled PET. Journal of thermal analysis and Calorimerty. Vol.78, pp. 865-84, 2004 4. Awaja, F. Daver. Recycling of PET. European Polymer
Journal. Vol.41, pp. 1453- 77, 2004.
5. R. F. Vazzoler, Microbiologiae Saneamento Ambiental. EdUSP.2001.
6. H. D. Flemming, Relevance of biofilms for the biodeterioration of surfaces of polymeric materials. Polymer Degradation and Stability.Vol.59, no.pp.309-315. 1998.
7. A. C. Albertsson, S. Karlsson, Macromolecular Architecture- Natural as a model for degradable polymer. Journal of Macromolecular Science, Part A:Pure Applied chemicsty.Vol.33, no.10, pp.1571-1579, 1996.
8. P. H. A. Sneath, S.N. Mair, M, Elisabeth Sharp, G.J. Holt, Bergeys Manual of systematic Bacteriology, Williams ailkines, Baltimore USA.1994.
9. F. Pinzari, G. Pasquariello, A.D. Mico, Biodeterioration of Paper: A SEM Study of Fungal Spoilage Reproduced Under Controlled Conditions. Macromol. Symp Vol.238.pp.57-66. 2006.
10. R.J.Muller, J. Augusta, M. Pantke, An iterlaboratory investigation into biodegradation of plastics: Part I: A modified sturm-test. Mater organismen. 27 pp 179-189, 1992.
11. S. Umamaheswari, M. Murali, P. Ilayaraja, Micromorphological and chemical changes during biodegradation of Polyethylene terephthalate (PET) by Penicillium sp, J. Microbiol. Biotech. Res. Vol.3, no. 4, pp.47-53, 2013.
12. Chetna Sharon, Madhuri Sharon, Studies on Biodegradation of Polyethylene terephthalate: A synthetic polymer, J. Microbiol. Biotech. Res. Vol. 2, no. 2,pp. 248-257, 2012.
13. J. Shakina, K. Sthiys Lekshmi, G. Allen Gnana Raj. Microbial degradation of Synthetic polyesters form renewable resources. Indian journal of science. Vol.1, no.1, pp. 21-28. 2012
14. Dan Rosu, Liliana Rosu, N.Constantin Cascaval. IR-change and yellowing of polyurethane as a result of UV irradiation. Polymer Degradation and stability. Vol.94, pp. 591-596,2009
15. X. Y. Weng, J. Hrenovic, Min Zhang, Xiu-Li Wang, Yu-Zhong Wang, Biodegradation behavior of P(3HB,4HB)PLA blend in real soil environments, Polymer testing. 32, pp.60-70, 2013.
16. A. A. Arkatkar, S. Juwarkar,P.V. Bhaduri, M. Uppara, M. Doble, Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface, Inter Biodet Biodegra. Vol.64, pp.530-536, 2010.
17. G.Chonde Sonal, G.Chonde Sachin,R. Bhosale Pallavi, P.D Raut, Studies on degradation of synthetic polymer Nylon 6 by lignolytic fungus Phanerochaete Chrysosporium nci. J Envirm Reserch Develo. Vol. 6, pp.1073, (2012).
18. Alexandors Lions, Mahmoud, M. Berekaa, Rudolf Reichelt. and Alexander Stein buchel, Biodegradation of cis-1,4-Polyisoprene Rubbers by Distinct Actinomycetes: Microbial Strategies and Detailed Surface Analysis. Applied environmental microbiology. Vol.66 no.4,pp1639-49,2000
How to cite this article:
Murali Markandan et al.2019, Estimation of Cryptographic Approach on IoT Devices. Int J Recent Sci Res. 10(08), pp. 34310-34314. DOI: http://dx.doi.org/10.24327/ijrsr.2019.1008.3866