Lab 10: Determine the Equilibrium Constant for a Chemical Reaction
Driving Question (2. Purpose)
What is the equilibrium constant of the formation of iron(III) thiocyanate?
Background ( Purpose continued)
In many chemical reactions the products of the reaction can react to form the original reactants. The forward
reaction occurs rapidly at first, but slows as the reactants are consumed. The reverse reaction occurs slowly at
first and increases in rate as more products of the forward reaction become available. At some point the two
reactions occur at the same rate, resulting in a constant amount of reactants and products. The state in which the
concentrations of the reactants and products have no net change over time is known as chemical equilibrium.
The mathematical relationship between the concentrations of the reactants and products is given by the law of
mass action, which states that the rate of a chemical reaction is proportional to the concentration of the
reactants.
In general, for a reaction of the form,
aA + bB ⇌ cC + dD the equilibrium constant Keq is given by
In this experiment, you use a colorimeter to help determine the equilibrium constant for the formation of FeSCN2+.
In dilute solutions, iron (III) nitrate, Fe(NO3)3(aq), and potassium thiocyanate, KSCN(aq), are completely dissociated.
When these two solutions are mixed, the following equilibrium is established: Fe3+(aq) + SCN–(aq) ⇌ FeSCN2+(aq)
Pre-Lab Activity
Setting the stage for the activity
Of the five ions in solution, K+(aq), NO
3– (aq), and SCN– (aq) are colorless, Fe3+(aq) is nearly colorless, and FeSCN2+(aq) is deep red. Changes in the concentration of FeSCN2+(aq) are indicated by changes in the intensity of the color of the solution. The equilibrium expression for the reaction is
To calculate Keq for this reaction, you need to determine the molar concentration of Fe3+, SCN–, and FeSCN2+ at
equilibrium. The relationship between electromagnetic absorption and concentration of the absorbing species is given by Beer's law. Absorption of light is directly proportional to the distance that the light travels through an absorbing medium and the molar concentration of the absorbing species.
A = ε × l × c
where
A = absorption
ε = absorptivity coefficient (M–1cm–1)
l = path length that light travels through the solution (cm)
c = molar concentration of the absorbing species (M).
Note: The AP exam version of Equation 1 is written as “A = a b c” where a and b correspond to ε and l, respectively. The absorptivity coefficient εis a proportionality constant, and its value depends both on the nature of the absorbing species and on the wavelength of light chosen for the measurement. At a given wavelength and using a sample cell of constant path length, absorption is directly proportional to concentration. Thus, a measurement of A can be used to determine concentration. The red FeSCN2+ ion absorbs blue light and will be analyzed at 468 nm.
Example calculation to try
the solution was placed in a cell and the absorbance of the solution was measured. The cell path length l is 1.00 cm thick. The absorptivity coefficient εis 5302 M–1cm–1 for FeSCN2+.
The concentration of Fe3+ and SCN– in the reaction solution before the reaction between Fe3+ and SCN– occurs can be calculated by considering the dilution of the reactants.
M1V1 = M2V2 where
M1= initial concentration (M) V1 = initial volume (mL) M2 = final concentration (M)
V2= final volume after the dilution (mL)
Using this equation, the concentration of Fe3+ in the reaction solution before the reaction occurs is
The calculated [Fe3+]
0 is the concentration of iron(III) after the dilution (after mixing the reactants) but before the reaction starts. Solving for [Fe3+]
0 and doing the same tocalculate the initial concentration of SCN– results in the following:
To calculate the equilibrium constant, you need to calculate the equilibrium concentrations. The concentration of FeSCN2+ at equilibrium is determined using the absorbance of the solution:
The equilibrium concentrations of Fe3+ and SCN– are then the difference between their initial concentrations and the equilibrium concentration determined for FeSCN2+.
Using the calculated concentrations, the equilibrium constant is:
Pre-Lab Questions (3.):
1. What kind of mathematical relationship exists between the absorbance A and the concentration of the absorbing species c?
2. Looking at the equation (Equation 1) that describes the relationship between absorbance and the concentration, what would be the intercept on the plot of A versus c? Explain!
Materials and Equipment – for each group
Data collection system Pipet pump
Colorimeter and cuvette 0.01 M Iron (Fe3+), 20 mL
Extension cable 0.00300 M Potassium thiocyanate (KSCN), 20 mL
Beaker (3), 50-mL Kimwipes®
Test tube (5),15-mL Deionized water, 40 mL
Test tube rack Marker
Colorimeter
Light-tight cover
Calibration button
Extension cable (optional) Pasteur Pipets (5)
Procedure
Set Up
1. Start a new experiment on the data collection system. �(1.2)
2. Connect the colorimeter to the data collection system using a sensor extension cable. �(2.1) 3. Set the data collection system to monitor live data without recording. �(6.1)
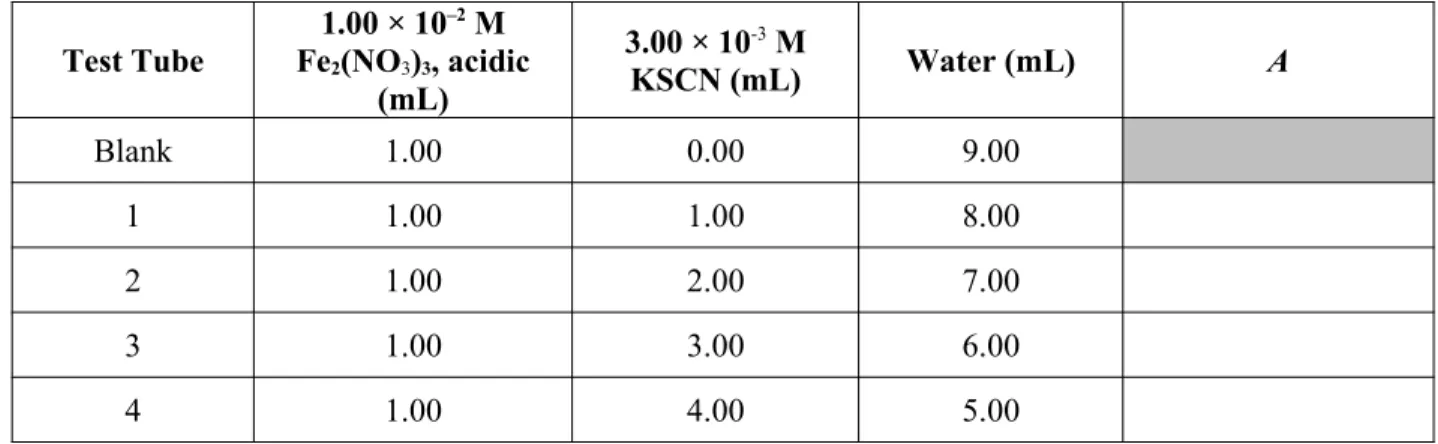
4. Label the first test tube “Blank” and the others from “1” to “4”.
5. Prepare the solutions in the labeled test tubes by combining the prescribed amounts of the reactants and water (see Table 1).
Table 1: Composition of solutions to study chemical equilibrium
6. Calibrate the colorimeter with the blank solution. �(3.2)
Important: Always make sure that the cell is clean and dry on the outside before placing it into the colorimeter.
7. Throughout this activity, collect data using blue light (468 nm) in the colorimeter. �(4.1)
Collect Data (4. & 5.)
8. Measure the absorbance of the four solutions following the steps below.
a. Rinse the cell with a small portion of the first solution and fill the cuvette two-thirds full. Wipe the cuvette clean and dry and place it into the colorimeter.
b. Why do you have to rinse the cell with some of the solution? (put answer in error analysis)
c. After the reading stabilizes, record the absorbance in Table 1 and Table 2. d. Dispose of the solution and rinse the cell thoroughly with water.
e. Why do you think it is important to rinse the cell thoroughly between measurements?
(put answer in error analysis)
9. Clean up according to your teacher's instructions. �(11.1)
Test Tube
1.00 × 10–2 M
Fe2(NO3)3, acidic
(mL)
3.00 × 10-3 M
KSCN (mL) Water (mL) A
Blank 1.00 0.00 9.00
1 1.00 1.00 8.00
2 1.00 2.00 7.00
3 1.00 3.00 6.00
Calculations and Graphs (6.)
For your calculations, consider the product of absorptivity ɛ and the cell thickness l to be ɛ × l = 5900 M–1
1. Calculate and record in Table 2 the initial concentrations of Fe3+ and SCN– in each test tube.
2. Calculate the concentration of the Fe(SCN)2+ ions from the absorbance measurements using Beer’s Law and the above value of ɛ × l. Record the values in Table 2.
3. Calculate the equilibrium concentration of the Fe3+ and SCN– ions from the initial concentration of the ions and the amount of ions used to establish the equilibrium concentration of FeSCN2+. Record the values in Table 2.
4. Calculate the equilibrium constant from the equilibrium concentration of the Fe(SCN)2+, Fe3+, and SCN– ions. Record the values in Table 2.
Table 2: Calculation of the equilibrium concentrations and the equilibrium constant
# [Fe3+]0
(M) [SCN–]0 (M) A [FeSCN
2+]
(M)
[Fe3+]
(M)
[SCN–]
(M) Keq
1
2
3
4
5. Calculate the average equilibrium constant and record the value below. Average value of
Keq:
Analysis Questions (7. Conclusion)
1. How did the absorbance change with increasing initial SCN– concentration while the initial concentration of Fe3+ was kept constant? Why?
2. Why do you think the equilibrium constant remained virtually constant, within experimental error, even though you were changing the concentrations? – (Can be part of the answer for 8. Error Analysis, but you must still write a separate section for part 8.)
3. How do you think your results would have been different if you used a cell with twice the path length?
4. Beer's Law (the linear relationship between concentration and absorbance) is accurate to about A = 1.5. How would you modify the experiment if the absorbance readings were higher than 1.5?
Post Lab Questions (9.)
Synthesis
1. Does the absorbance you measured come only from the FeSCN2+ ion? Explain your answer.
2. The Fe3+ ion can react with three SCN– ions according to the following equilibrium equations to form Fe(SCN) 2+ and Fe(SCN)3. These products are also red.
Fe+ + SCN–⇌ FeSCN2+ FeSCN2+ + SCN–⇌ Fe(SCN)
2+ Fe(SCN)2+ + SCN–⇌ Fe(SCN)3
In light of these reactions, propose an explanation as to why this experiment uses a large excess of Fe3+ ions.
Multiple Choice (more post-lab questions)
Select the best answer or completion to each of the questions or incomplete statements below.
1. The four measurements you made resulted in four substantially different equilibrium constants. Which statement could be correct?
A. The equilibrium constant is determined by the concentrations. We used different concentrations, so the resulting equilibrium constants should be the same.
B. The equilibrium of the second and third equations (SQ2 and SQ3) interfered with our measurements. C. The equilibrium constant depends on the temperature, so the fluctuation in room temperature might have
interfered with our measurements.
D. There was an error in mixing the solutions, or one (or both) of the stock solutions had the wrong concentration.
2. How do you think doubling the initial iron concentration of Fe3+ ions would affect the obtained value for K eq? A. Doubling the initial concentration of Fe3+ ions will double K
eq, according to Le Chatelier's Principle. B. Doubling the initial concentration of Fe3+ ions will not affect Keq.
C. Doubling the initial concentration of Fe3+ ions will result in half of the value for K eq.
D. The effect of doubling the Fe3+ initial concentration is not predictable; you would need to actually perform the experiments.
3. The FeSCN2+ ion is red, the other species are practically colorless. Would it interfere with your measurements if another species had color?
A. No, we measure only FeSCN2+.
B. If the other colored species does not absorb at 468 nm where we perform the experiment, it would not interfere with our measurements.