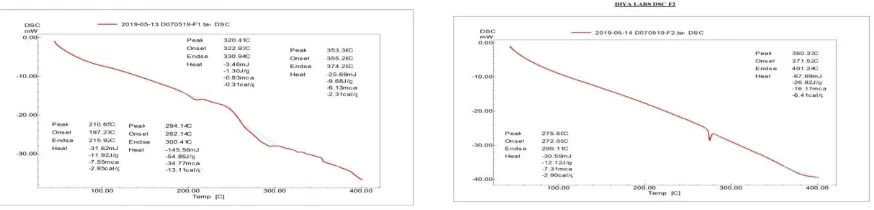
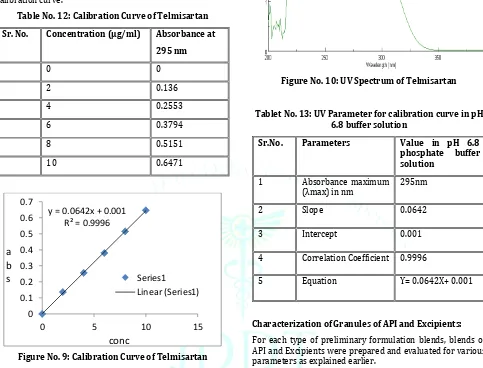
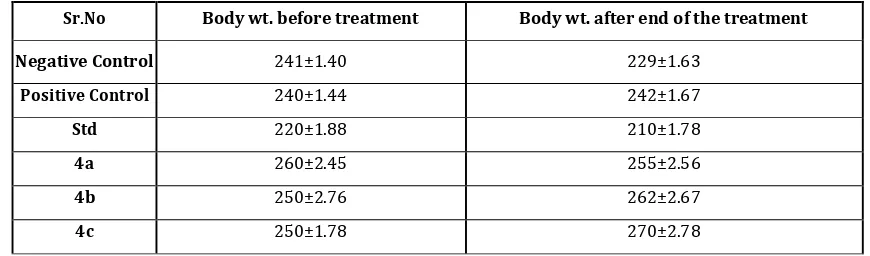
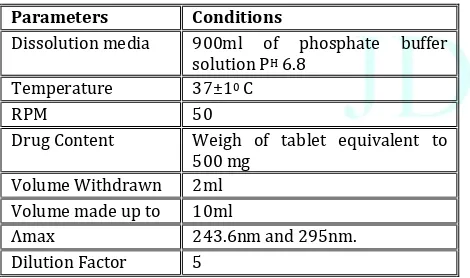
Formulation and Evaluation of Teneligliptin and Telmisartan Bilayer Tablets for the Treatment of Coexistent Type II Diabetes Mellitus and Hypertension
Full text
Figure



Related documents
Seventy-two patients with mixed hemorrhoids who had undergone hemorrhoidectomy were randomly assigned to the following 2 groups: the EA treatment group (EA) received surround
Anterior tibial translation (mean ± SD) in the intact, anterior cruciate ligament (ACL)-deficient, and anatomic double bun- dle and single posterolateral (PL) bundle ACL-reconstructed
In this systematic review, 4 studies of femoral or acetab- ular defects, 3 studies of tibial fractures, 2 studies of large bone defects, 2 bone tumors studies, 2 studies of
The factors that are considered include the time to initiate chest com- pressions, duration of chest compressions, initial arrest rhythm, age, comorbidities and any reversible causes
We also compared the AUC of machine learning using the network topology to the logis- tic regression based on the standard financial measures (non-network information): firm
The most important problem in our case was the emergency treatment of bleeding esophageal varices in a patient with portal hypertension secondary to portal vein thrombosis..
An English translation (accuracy confirmed by back translation) of the two ques- tionnaires on personal preference is shown in Table 1. Similar statements with appropriate
of coverage established in our exome sequencing, we did not identify any two patients who had de novo variants in the same gene, any de novo coding variants unique to the tumours,