Environmental Tobacco Smoke and Serum Vitamin C Levels in Children
Richard S. Strauss, MD
ABSTRACT. Background. High levels of free radicals in tobacco smoke are thought to be responsible for de-creased levels of serum ascorbic acid in smokers and adults exposed to environmental tobacco smoke (ETS). The association of ETS to serum ascorbic acid in children is unknown.
Methods. Data were analyzed from the Third Na-tional Health and Nutrition Examination Survey, a na-tionally representative sample of children and adoles-cents (nⴝ 2968). Comprehensive data including serum cotinine levels and family smoking patterns allowed for analysis of relationship of ETS to serum ascorbic acid levels. Data from 24-hour dietary recall also allowed for the control of vitamin C intake. Children were divided into categories of low and high ETS exposure based on levels of serum cotinine above or below 2 ng/mL. Smok-ers were defined by either self-report or serum cotinine
>15 ng/mL.
Results. Although there was a trend for lower levels of vitamin C intake in children with higher levels of ETS exposure, this trend did not reach statistical significance. Among all children, serum ascorbic acid levels were lin-early related to serum cotinine levels (rⴝ0.19). In addi-tion, a dose-response relationship was observed between levels of tobacco exposure and serum ascorbic acid levels. After adjusting for age, gender, vitamin C intake, and multivitamin use, environmental tobacco exposure re-mained significantly associated with lower levels of se-rum ascorbic acid in children who were exposed to both high and low levels of ETS.
Conclusion. Exposure of children to ETS leads to sig-nificant alterations in serum ascorbic acid levels. There-fore, this study further highlights the potential dangers of ETS to children.Pediatrics2001;107:540 –542; environ-mental tobacco smoke (ETS), children, adolescents, vita-min C, ascorbic acid.
ABBREVIATIONS. NHANES III, Third National Health and Nu-trition Examination Survey; ETS, environmental tobacco smoke; OR, odds ratio; CI, 95% confidence interval.
I
n 1994, approximately 15 million children were exposed to second hand smoke.1 Data from thefirst phase of the Third National Health and Nu-trition Examination Survey (NHANES III) confirm that 43% of children live in a home with at least 1 smoker.2 In children, there is strong evidence that
exposure to environmental tobacco smoke (ETS) is
associated with increased risk of respiratory illness-es,3 asthma,4 anesthesia complications,5 sudden
in-fant death syndrome,6 low birth weight7, and
ad-verse lipid profiles.8As a result, position statements
warning of the serious effects of ETS have been is-sued by both the American Academy of Pediatrics9
and the American Heart Association.10
In addition, ETS is associated with lower levels of serum vitamin C (ascorbic acid) in adults.11 In a
small study of 28 children exposed to ETS, serum ascorbic acid levels were also significantly lower in ETS-exposed children compared with control chil-dren.12 Decreased serum ascorbic acid in those
ex-posed to ETS is most likely related to the extremely high levels of free radicals in tobacco smoke that leads to depletion of biological stores of antioxi-dants.11,13 For instance, Kallner and colleagues14
have demonstrated over a 40% increase in ascorbic acid turnover from free radical induced depletion in smokers compared with nonsmokers.
To address the association of ETS to serum ascor-bic acid in children, data were analyzed from the Third National Health and Nutrition Examination Survey (NHANES III), a nationally representative sample of children and adults. Comprehensive data including serum cotinine levels and family smoking patterns allowed for analysis of relationship of ETS to serum ascorbic acid levels. Data from 24-hour dietary recall also allowed for the control of vitamin C intake.
METHODS Sample
The first phase of the NHANES III examined a nationally representative sample of children and adults between 1988 and 1991.aThe sample included 2968 children ages 4 through 18 with serum cotinine levels.
Smoking and Nutritional Assessment
Parents and adolescents were asked about frequency of smok-ing and number of cigarettes smoked per day. The total number of cigarettes smoked by household members each day were then calculated. Nutritional intake was assessed using a 24-hour diet recall for all children. Parents provided information on nutritional intake for children 11 years or younger, while children⬎12 years old provided their own intake. Intake of vitamin C was calculated using the US Department of Agriculture’s Survey Nutrient Data Base based on the 24-hour dietary record. Interviews were con-ducted privately, by trained study staff, and staff performance was monitored routinely.
From the Department of Pediatrics, UMDNJ–Robert Wood Johnson School of Medicine, New Brunswick, New Jersey.
Received for publication Apr 5, 2000; accepted Jun 29, 2000.
Reprint requests to (R.S.S.) Department of Pediatrics, UMDMJ–Robert Wood Johnson Medical School, One Robert Wood Johnson Pl, CN-19, New Brunswick, NJ 08903-0019. E-mail: strausrs@umdnj.edu
PEDIATRICS (ISSN 0031 4005). Copyright © 2001 by the American Acad-emy of Pediatrics.
aSerum cotinine levels were not measured in the second phase of NHANES
III (1992–1994).
Laboratory Testing
Serum cotinine levels were measured using an isotope dilution, liquid chromatography, tandem mass spectrometry method. Chil-dren were categorized according to level of ETS as follows:
1. Nonexposed: No one in the household reported smoking. 2. Low exposure (ETS-Low): At least 1 parent reports smoking
and serum cotinine levels⬍2 ng/mL.
3. High exposure (ETS-High): At least 1 parent reports smoking in the household and serum cotinine levels between 2 ng/mL and 15 ng/mL.
4. Smokers: Either self-reported smoking by child/adolescent or serum cotinine level⬎15 ng/mL.
Previous studies using the NHANES III data have demon-strated a 96% concordance between self-reported smoking status and serum cotinine levels above or below 15 ng/mL.15Serum
levels of ascorbic acid were measured by isocratic high-perfor-mance liquid chromatography (Waters HPLC System, Waters Chromatography Division, Milford, MA).
Statistics
The NHANES III study oversampled blacks, Hispanics, and younger adolescents. By using sample weights provided by NHANES III, the data were adjusted to account for unequal selection. Differences in proportions were assessed using2. Odds
ratios (ORs) were calculated using logistic regression. Multivariate logistic regression analysis was used to assess the independent effects of smoking on nutritional intake after adjusting for age and gender. To adjust for complex sample design and clustering effects in the NHANES III sample, statistical significance was assessed using the balanced repeated replication method using the soft-ware package WesVarPC (Westat Inc, Rockville, MD).
RESULTS
In the NHANES III survey, 35.3% of nonsmoking children were exposed to household smoking. An additional 11.6% of children were classified as smok-ers. Among children 12 years and older, 22.1% were smokers of which 55.9% were boys. Among children classified as smokers, 98.4% were 12 years or older. Serum cotinine levels were⬎2 ng/mL in 2.6% of children whose parents reported no household expo-sure to cigarettes compared with 34.0% for nonsmok-ing children whose parents reported smoknonsmok-ing (OR: 13.07; 95% confidence interval [CI]: 9.04 –18.67). Of children who reported at least occasional smoking, serum cotinine levels were⬎2 ng/mL in 93.8% (OR: 94.63; 95% CI: 51.95–172.39). As expected, parental levels of smoking were significantly higher in dren with high ETS exposure compared with chil-dren with low ETS exposure (number of cigarettes smoked by members of household/d: 25.9⫾ 1.5 vs 14.2 ⫾ 0.6;P ⬍ .001). Among children who did not report smoking, serum cotinine levels were linearly related to number of cigarettes smoked by family members (r ⫽ 0.19; P ⬍ .05). Among children who reported smoking, serum cotinine levels were also linearly related to number of cigarettes that they reported smoking per day (r⫽0.42;P ⬍.0001).
Although there was a trend for low levels of vita-min C intake in children with higher levels of ETS exposure or smoking, this trend did not reach statis-tical significance (vitamin C intake per day (mg)– nonexposed: 111.3⫾ 3.3, ETS-low: 109.6⫾ 5.4, ETS-high: 104.8⫾ 5.0, smokers: 95.8⫾ 7.2,F⫽ 0.16). In addition, after adjusting for age and gender there was no difference between vitamin C intake per day between those children with no exposure to ETS and
those with either low exposure (P ⫽ .82) or high exposure (P⫽.31). However, after adjusting for age and gender, vitamin C intake was lower among chil-dren who smoked compared with nonsmokers (P⬍
.001). In addition, fruit intake was similar among nonsmoking children with and without ETS expo-sure (servings/day: 0.96⫾ 0.03 vs 0.86 ⫾ 0.06;P ⫽
.11). However, fruit intake was lower among chil-dren who smoked compared with those who did not (servings/day 0.52⫾ 0.04 vs 0.92⫾0.03;P⬍ .001). Among all children, serum ascorbic acid levels were linearly related to serum cotinine levels (r ⫽
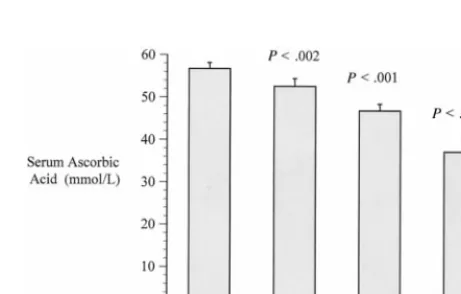
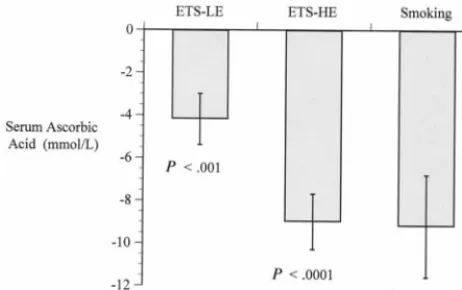
0.19;P⬍.001). In addition, a dose-response relation-ship was observed between levels of tobacco expo-sure and serum ascorbic acid levels (Fig 1). After adjusting for age, gender, vitamin C intake, and mul-tivitamin use, environmental tobacco exposure re-mained significantly associated with lower levels of serum ascorbic acid in children who were exposed to both high and low levels of ETS (Fig 2).
DISCUSSION
This study demonstrates significantly lower levels of serum ascorbic acid levels in children exposed to ETS. Furthermore, a dose-response relationship was observed between ETS exposure and serum ascorbic acid with the lower levels of serum ascorbic acid in children with high levels of exposure compared with children with higher levels of exposure. Overall, se-rum ascorbic acid levels were approximately 20% lower in children exposed to ETS compared with those who are not exposed. Tribble and colleagues have demonstrated that adults exposed to ETS show similar results.11 In addition, Jendrycczko and
col-leagues12demonstrated 50% lower ascorbic acid
lev-els in children exposed to ETS compared with non-exposed children; however, only 28 non-exposed children were studied.
Decreased serum ascorbic acid in children exposed to ETS may be a result of increased rates of ascorbic acid metabolism associated with environmental to-bacco exposure.12 Decreased serum levels may also
be attributable to altered dietary preferences in
chil-Fig 1. Serum ascorbic acid levels in children with A, no exposure to ETS; B, low exposure (ETS-LE): at least 1 smoker in house and serum cotinine⬍2 ng/mL; C, high exposure (ETS-HE): at least 1 smoker in house and serum cotinine ⬎2 ng/mL; D, smoking: either self-reported smoking by child/adolescent or serum cotin-ine level⬎15 ng/mL. Mean⫾standard error of the mean.
ARTICLES 541
at Viet Nam:AAP Sponsored on August 30, 2020
www.aappublications.org/news
dren and families exposed to ETS. Schiffman and colleagues16 have documented that ETS exposure
attenuates both taste and smell leading to a prefer-ence for fatty foods. Similarly, Emmons and col-leagues17 have demonstrated decreased vitamin C
intake as well as decreased fruit and vegetable intake in adults exposed to ETS in the workplace. In this study, children who smoked reported lower levels of fruit intake; nevertheless, serum ascorbic acid re-mained lower among children who smoked even after adjusting for total vitamin C intake. In addition, the findings of lower levels of serum vitamin C in ETS exposed children could not be accounted for by lower levels of dietary intake because reported levels of vitamin C intake and fruit consumption were not significantly different among nonsmoking children with and without ETS exposure.
CONCLUSION
Exposure of children to ETS leads to significant alterations in serum ascorbic acid levels in addition to the previously described respiratory ailments as-sociated with ETS. This report is the first large study to document direct metabolic consequences of ETS in children. This study is, therefore, in direct contrast to continued assertions by the tobacco industry that ETS causes no damage.18,19 Because ascorbic acid
protects against plasma lipid and low-density li-poprotein oxidation,20and also appears to be
impor-tant in protecting DNA from oxidative damage,21
this report further highlights the potential dangers of ETS to children.
REFERENCES
1. The great American smokeout.MMWR Morb Mortal Wkly Rep.1997;46: 1037–1051
2. Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Mauer KR. Expo-sure of the US population to environmental tobacco smoke. NHANES III, 1988 –1991.JAMA.1996;275:1233–1240
3. Li JSM, Peat JK, Xuan W, Berry G. Meta-analysis on the association between environmental tobacco smoke (ETS) exposure and the preva-lence of lower respiratory tract infection in early childhood.Pediatr Pulmonol. 1999;27:5–13
4. Strachan DP, Cook DG. Health effects of passive smoking. VI. Parental smoking and childhood asthma: longitudinal and case control studies.
Thorax. 1998;53:204 –212
5. Skolnick ET, Vomvolakis MA, Buck KA, Mannino SF, Sun LS. Exposure to environmental tobacco smoke and the risk of adverse respiratory events in children receiving general anesthesia.Anesthesiology. 1998;88: 1144 –1153
6. Anderson HR, Cook DG. Passive smoking and sudden infant death syndrome: review of the epidemiological evidence. Thorax. 1997;52: 1003–1009
7. Mainous AG, Hueston WJ. Passive smoke and low birth weight: evi-dence for a threshold effect.Arch Fam Med. 1994;875– 878
8. Moskowitz WB, Schwartz PF, Schieken RM. Childhood passive smok-ing, race, and coronary artery disease risk.Arch Pediatr Adolesc Med. 1999;153:446 – 453
9. American Academy of Pediatrics, Committee on Environmental Health. Environmental tobacco smoke: a hazard to children.Pediatrics. 1997;99: 639 – 642
10. Committee on Atherosclerosis and Hypertension in Children, American Heart Association. Active and passive tobacco exposure: a serious pe-diatric health problem.Circulation.1994;90:2581–2590
11. Tribble DL, Giuliano LG, Formann SP. Reduced plasma ascorbic acid concentrations in nonsmokers regularly exposed to environmental to-bacco smoke.Am J Clin Nutr. 1993;58:886 – 890
12. Jendrycazko A, Szpyrka G, Gruszczynski J, Kozowicz M. Cigarette smoke exposure of children: effect of passive smoking and vitamin E supplementation on blood antioxidant status. Neoplasma. 1993;40: 199 –203
13. Church DF, Pryor WA. Free radical chemistry of cigarette smoke and its toxicological implications.Environ Health Perspect. 1985;64:111–118 14. Kallner AB, Harmann D, Hornig DH. On the requirements of ascorbic
acid in man: steady-state turnover and body pools in smokers.Am J Clin Nutr. 1981;34:1347–1355
15. Caraballo RS, Giovino GA, Pechacek TF, et al. Racial and ethnic differ-ences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988 –1991.JAMA.1998;280: 135–139
16. Schiffman SS, Nagel HT. Effect of environmental pollutants on taste and smell.Otolaryngol Head Neck Surg. 1992;106:693–700
17. Emmons KM, Thompson B, Feng Z, Hebert JR, Heimendinger J, Linnan L. Dietary intake and exposure to environmental tobacco smoke in a worksite population.Eur J Clin Nutr. 1995;49:336 –345
18. Coggins CRE (RJ Reynolds Tobacco Company). Estimating exposure to environmental tobacco smoke.JAMA. 1996;276:603
19. Repace JL, Lowrey AH. Issues and answers concerning passive smoking in the workplace: rebutting tobacco industry arguments.Tobacco Con-trol. 1992;1:208 –219
20. Frei B. Ascorbic acid protects lipids in human plasma and low-density lipoprotein against oxidative damage.Am J Clin Nutr. 1991;54(suppl 6):1113S–1118S
21. Fraga CG, Motchnik PA, Shigenaga MK, Helblock HJ, Jacob RA, Ames BN. Ascorbic acid protect against endogenous oxidative DNA damage in human sperm.Proc Natl Acad Sci U S A. 1991;88:11003–11006
Fig 2. Serum ascorbic acid levels in ETS exposed children and smoking children compared with nonsmoking, nonexposed chil-dren after controlling for age, gender, vitamin C intake, and multivitamin use. Mean⫾standard error of the mean.
DOI: 10.1542/peds.107.3.540
2001;107;540
Pediatrics
Richard S. Strauss
Environmental Tobacco Smoke and Serum Vitamin C Levels in Children
Services
Updated Information &
http://pediatrics.aappublications.org/content/107/3/540
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/107/3/540#BIBL
This article cites 19 articles, 7 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/smoking_sub
Smoking
http://www.aappublications.org/cgi/collection/substance_abuse_sub
Substance Use
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
Information about ordering reprints can be found online:
at Viet Nam:AAP Sponsored on August 30, 2020
www.aappublications.org/news
DOI: 10.1542/peds.107.3.540
2001;107;540
Pediatrics
Richard S. Strauss
Environmental Tobacco Smoke and Serum Vitamin C Levels in Children
http://pediatrics.aappublications.org/content/107/3/540
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.
the American Academy of Pediatrics, 345 Park Avenue, Itasca, Illinois, 60143. Copyright © 2001 has been published continuously since 1948. Pediatrics is owned, published, and trademarked by Pediatrics is the official journal of the American Academy of Pediatrics. A monthly publication, it
at Viet Nam:AAP Sponsored on August 30, 2020
www.aappublications.org/news