Inpatient Costs and Charges for Surgical Treatment
of Hypoplastic Left Heart Syndrome
WHAT’S KNOWN ON THIS SUBJECT: Hypoplastic left heart syndrome (HLHS) is a serious congenital heart defect that requires 3 staged palliative operations or cardiac
transplantation. Mortality rates have improved significantly over the past decade, but patients continue to consume extensive inpatient hospital resources.
WHAT THIS STUDY ADDS: Improved survival rates for patients born with HLHS have led to increased hospital stays and costs. Whether treated with 3 staged palliative operations or transplantation, patients require resources similar to those needed for prematurity or other major birth defects.
abstract
OBJECTIVE:Hypoplastic left heart syndrome (HLHS) is one of the most serious congenital cardiac anomalies. Typically, it is managed with a series of 3 palliative operations or cardiac transplantation. Our goal was to quantify the inpatient resource burden of HLHS across multiple academic medical centers.
METHODS:The University HealthSystem Consortium is an alliance of 101 academic medical centers and 178 affiliated hospitals that share diagnostic, procedural, and financial data on all discharges. We exam-ined inpatient resource use by patients with HLHS who underwent a staged palliative procedure or cardiac transplantation between 1998 and 2007.
RESULTS:Among 1941 neonates, stage 1 palliation (Norwood or Sano procedure) had a median length of stay (LOS) of 25 days and charges of $214 680. Stage 2 and stage 3 palliation (Glenn and Fontan procedures, respectively) had median LOS and charges of 8 days and $82 174 and 11 days and $79 549, respectively. Primary neonatal transplantation had an LOS of 87 days and charges of $582 920, and rescue transplantation required 36 days and $411 121. The median inpatient wait time for primary and rescue transplants was 42 and 6 days, respectively. Be-tween 1998 and 2007, the LOS for stage 1 palliation increased from 16 to 28 days and inflation-adjusted charges increased from $122 309 to $280 909, largely because of increasing survival rates (57% in 1998 and 83% in 2007).
CONCLUSIONS:Patients with HLHS demand considerable inpatient re-sources, whether treated with the Norwood-Glenn-Fontan procedure pathway or cardiac transplantation. Improved survival rates have led to increased hospital stays and costs. Pediatrics 2011;128: e1181–e1186
AUTHORS:Peter N. Dean, MD,aDiane G. Hillman, DHA,b
Kimberly E. McHugh, MD,cand Howard P. Gutgesell, MDa
Departments ofaPediatrics andbPublic Health Sciences,
University of Virginia Health System, Charlottesville, Virginia; andcDepartment of Pediatrics, Medical University of South
Carolina, Charleston, South Carolina
KEY WORDS
hypoplastic left heart syndrome, health care costs, congenital heart disease/defects, heart surgery, heart transplantation
ABBREVIATIONS
HLHS—hypoplastic left heart syndrome LOS—length of stay
S1P—stage 1 palliation S2P—stage 2 palliation S3P—stage 3 palliation
UHC—University HealthSystem Consortium
www.pediatrics.org/cgi/doi/10.1542/peds.2010-3742
doi:10.1542/peds.2010-3742
Accepted for publication Jun 29, 2011
Address correspondence to Howard P. Gutgesell, MD, Department of Pediatrics, University of Virginia Health System, PO Box 800386, Charlottesville, VA 22908-0386. E-mail: hpg@virginia.edu
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2011 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE:The authors have indicated they have no financial relationships relevant to this article to disclose.
is defined by underdevelopment of the left ventricle, aortic and mitral steno-sis or atresia, and hypoplasia of the ascending aorta.2HLHS is considered
one of the most serious congenital heart defects and, without interven-tion, the defect is typically fatal in the first week of life.2
In the 1980s, staged palliative repair and cardiac transplantation arose as treatment options for neonates born with HLHS. Staged palliative repair in-volves 3 cardiac procedures that typi-cally are performed before school age. Stage 1 palliation (S1P), which is performed in the first few days of life, involves the creation of a neo-aorta (Norwood procedure3) and shunt
placement to provide pulmonary blood flow (Blalock-Taussig shunt or Sano procedure4). Stage 2 palliation (S2P),
which is performed between 4 and 6 months of age, involves formation of a superior vena cava-pulmonary artery connection (Glenn5 or hemi-Fontan6
procedure). Stage 3 palliation (S3P), which is performed at 2 to 4 years of age, involves formation of a total cava-pulmonary artery connection (Fontan procedure7). Cardiac transplantation
most commonly is used as “rescue” therapy if these procedures do not yield acceptable results, but it also has been used as primary treatment, as an alternative to the staged palliation pathway.
Outcomes have improved greatly over the past 3 decades, which has allowed many patients to survive into adult-hood.8–11These improvements have led
to more patients undergoing surgical palliation and fewer patients being of-fered or requesting comfort care.12–15
We suspect that this change has caused increases in the resources and dollars spent on children born with HLHS.
There have been analyses of the costs of initial hospitalization for patients
multicenter studies analyzing the costs of the staged palliation pathway and cardiac transplantation. Our goal was to determine resource use by pa-tients with HLHS across multiple aca-demic medical centers throughout the United States in the current era.
METHODS
The University HealthSystem Consor-tium (UHC) is an alliance of 101 aca-demic medical centers and 178 affili-ated hospitals sharing information, including demographic, diagnostic, procedural, and financial data for each hospital admission. Encoded hospital identifiers were provided for individ-ual institutions.
The database was queried for data on all patients who were discharged be-tween 1998 and 2007 with the diagno-sis of HLHS. Patients were deemed to have HLHS on the basis ofInternational Classification of Diseases, Ninth Revi-sion, Clinical Modification, code 746.7 being included as any one of their coded diagnoses. Procedures during each hospitalization were determined by using the listed procedure codes, as described previously.8Patient
mortal-ity rates were determined on the basis of survival to discharge.
Patients undergoing transplantation were divided into 2 categories, namely, primary transplantation and rescue transplantation. Patients were consid-ered to have undergone primary trans-plantation if they were admitted to the hospital at younger than 30 days and the only cardiac procedure coded was cardiac transplantation (code 37.5). Patients were considered to have un-dergone rescue transplantation if they were admitted when they were older than 30 days and had a procedure code for cardiac transplantation.
To determine whether average daily hospital costs were related to surgical
tions were compared through 1-way analysis of variance (SPSS 18 [SPSS Inc, Chicago, IL]). Institutions that per-formed ⬍20 procedures during the studied time period were considered small volume, those that performed 21 to 64 procedures were considered me-dium volume, and those that per-formed⬎64 procedures were consid-ered large volume, as in our study of surgical mortality rates.8
Hospital length of stay (LOS) and hos-pital charges were reported by each hospital. Hospital costs are estimated by the UHC by using Medicare cost/ charge ratios and further adjustments in labor costs according to geographic area. LOS data were available for all patients, whereas charge and cost data were available for most. We ex-tracted this information for each hos-pitalization during which patients un-derwent S1P, S2P, S3P, or cardiac transplantation. We did not include ad-missions that did not include 1 of the aforementioned procedures; however, it is likely that hospitalizations before or after the surgical admissions were related to the surgical procedures, which would result in underestimation of the full inpatient costs of those pro-cedures. To adjust for inflation, dollar amounts were converted to 2007 equivalents by using the Consumer Price Index inflation calculator from the US Bureau of Labor Statistics.20
RESULTS
Primary cardiac transplantation was performed for 28 neonates, and res-cue cardiac transplantation was per-formed for 62 patients.
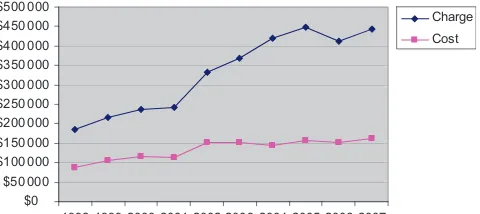
The LOS, costs, and charges were greatest for S1P (Tables 1 and 2). Dur-ing the decade studied, the median LOS, costs, and charges were 25 days, $99 070, and $214 680, respectively, for S1P. From 1998 to 2007, the LOS for S1P increased from 16 days to 28 days and inflation-adjusted costs increased from $65 041 to $113 827. Costs and charges increased progressively be-tween 1998 and 2007 (Fig 1). This was likely attributable to increasing sur-vival rates (rates were 57% in 1998 and
82% in 2007), because the median LOS for survivors was 28 days, compared with 13 days for patients who died. The median LOS for the 556 neonates who died after surgery was only 13 days, compared with 28 days for the 1265 neonates who were discharged.
The estimated costs for hospitalization for S1P remained constant at $4065 per day from 1998 to 2007, but charges increased from $7644 to $10 032 per day. Average daily costs were similar for small-, medium-, and large-volume institutions ($5148 ⫾ 2382, $4889⫾ 2976, and $4995⫾2507, respectively; P⬎.05). Several institutions exhibited marked variability of costs because of
very high resource utilization for a few patients with very short hospital stays.
The LOS, costs, and charges for S2P (Glenn procedure) and S3P (Fontan procedure) were similar and were ap-proximately one-third of those for S1P (Tables 1 and 2). The cumulative me-dian LOS, costs, and charges for 3-stage palliation were 44 days, $171 672, and $376 403, respectively.
Cardiac transplantation was associ-ated with the greatest use of re-sources (Tables 1 and 2). Primary transplantation as an alternative to S1P had LOS, costs, and charges of 87 days, $289 292, and $582 920, respec-tively. Rescue transplantation was as-sociated with a shorter hospital stay (36 days), costs were 23% lower ($222 509), and charges were 29% lower ($411 121). The median number of inpatient days before a heart be-came available was 42 days (range: 0 –164 days) for primary transplanta-tion and 6 days (range: 0 –145 days) for rescue transplantation.
The average costs for the procedures are skewed by the very long hospital-izations of a few patients. For example, the median costs and charges for S1P were $99 070 and $214 680, respec-tively, but the averages were $135 278 and $335 457. Charges exceeded $1 million for 3% of the procedures in the palliative pathway, 21% of primary transplants, and 25% of rescue transplants.
DISCUSSION
By using a large multicenter database, we determined the LOS, costs, and charges for surgical procedures for HLHS across the United States during a recent 10-year period. We demon-strated the significant inpatient finan-cial and resource demands made by patients with HLHS, whether treated with staged palliative operations or transplantation. As expected, S1P de-manded more resources than S2P or
TABLE 1 LOS for HLHS
n LOS, Median (Range), d S1P (Norwood or Sano procedure) 1941 25 (0–312)
S2P (Glenn procedure) 936 8 (1–326)
S3P (Fontan procedure) 840 11 (0–373)
Primary transplantation 28 87 (16–285)
Rescue transplantation at⬎30 d 62 36 (2–255)
TABLE 2 Costs and Charges of 3-Stage Palliation and Cardiac Transplantation for HLHS
Median Mean Range
Costs, $
S1P (Norwood or Sano procedure) 99 070 135 278 6499–2 025 387 S2P (Glenn procedure) 35 674 65 962 1252–992 958 S3P (Fontan procedure) 36 928 61 058 2712–1 143 363 Primary transplantation 289 292 361 724 80 854–1 118 266 Rescue transplantation at⬎30 d 222 509 283 398 16 181–1 064 411 Charges, $
S1P (Norwood or Sano procedure) 214 680 335 457 9932–6 856 341 S2P (Glenn procedure) 82 174 164 827 3340–2 401 738 S3P (Fontan procedure) 79 549 146 186 7033–4 407 888 Primary transplantation 582 920 827 574 182 058–4 024 679 Rescue transplantation at⬎30 d 411 121 683 566 26 720–3 781 342
$0 $50 000 $100 000 $150 000 $200 000 $250 000 $300 000 $350 000 $400 000 $450 000 $500 000
1998 1999 2000 2001 2002 2003 2004 2005 2006 2007
Charge Cost
FIGURE 1
Mean costs and charges of S1P between 1998 and 2007.
charge, for S1P over the decade re-sulted in increased LOS, costs, and charges. Cardiac transplantation (pri-mary or rescue) was more costly than any of the 3 palliative operations. As noted by Gajarski et al,18however,
pal-liative surgery followed by transplan-tation was the most costly.
Our previous analysis of this database demonstrated a decrease in mortality rates for S1P, from 43% in 1998 to 18% in 2007.8 Others also demonstrated
mortality rates ranging from 7% to 26%,21–23with significant improvement
over the past decades.10,11The recent
report from the Pediatric Heart Net-work showed either death or a need for transplantation for 26% and 36% of survivors of S1P with the Sano or Nor-wood technique, respectively.24 Rates
of survival after S2P and S3P were sig-nificantly better than that after S1P, and mortality rates ranged from 2% to 5% for S2P8,25,26and from 2% to 4% for
S3P.8,27–29
The decrease in mortality rates for S1P has come with significant increases in financial and resource costs. Despite adjustment for inflation, the costs, charges, and LOS after S1P were con-siderably greater in 2007 than in 1998. This increase is largely attributable to the increase in LOS produced by the increased survival rate. Interestingly, between 1998 and 2007 the charges per day increased for each stage, whereas the estimated costs per day remained constant (Table 3). Hospital costs seemed to be unrelated to insti-tutional surgical volume.
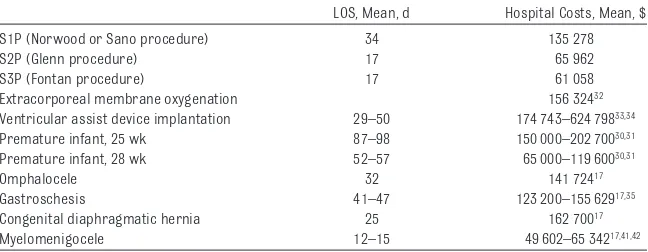
Although the resource utilization for surgical management of HLHS is con-siderable, it is comparable to that for other major congenital malforma-tions, as well as prematurity30,31(Table
4). It also is similar to that of highly technical interventions such as ex-tracorporeal membrane oxygenation
and ventricular assist device
placement.32–34
The data presented include only the in-patient hospital costs and charges for the major surgical procedures used in the management of HLHS and thus rep-resent only a fraction of the total costs of this malformation. Inpatient physi-cian charges were not included in our study and might be expected to add 15% to these costs.35Additional
hospi-talizations are frequent; it should be noted that our database included 9197 admissions for patients with HLHS but only 3806 admissions involved the 5 surgical procedures analyzed here. Survivors of the surgical procedures face a future of lifelong outpatient vis-its, cardiac catheterizations, imaging studies, and medication use. A portion of those on the 3-stage palliative path-way will ultimately require cardiac transplantation along with the requi-site repeated myocardial biopsies and lifelong immunosuppression. After completion of the Fontan procedure, patients remain with long-term com-plications and have a 10% to 13%
chance of death or transplantation within 10 years.36,37Despite the
periop-erative and long-term complications
after the Fontan procedure, however, the majority of patients are able to lead relatively normal lives and are satisfied with their quality of life.38–40
HLHS is an example of a disease entity that is changing the financial land-scape of health care in the United
States. Before the middle 1980s, chil-dren born with HLHS had minimal health care resource use because the only available option was comfort care and most patients died shortly after birth. With the success of surgical pal-liation, comfort care is offered less of-ten and most parents now are coun-seled that surgery is a feasible option.12–15As the operative and
peri-operative care of patients with HLHS continue to improve and mortality
rates improve, we predict that costs, charges, and hospital stays will con-tinue to increase.
There are several limitations to this study. The UHC database is an
admin-1998 2007 1998 2007 1998 2007
LOS, d 16 28 7 9 10 11
Costs, median, $ 65 041 113 827 25 183 44 067 27 286 39 611 Charges, median, $ 122 309 280 909 57 527 112 197 42 026 97 165 Costs per d, $ 4065 4065 3598 4896 2729 3601 Charges per d, $ 7644 10 032 8218 12 466 4203 8833 Cost/charge ratio 0.53 0.41 0.44 0.39 0.65 0.41
Mortality rate, %8 43 18 5 3 3 3
TABLE 4 Costs for HLHS Compared With Other Pediatric Diseases
LOS, Mean, d Hospital Costs, Mean, $
S1P (Norwood or Sano procedure) 34 135 278
S2P (Glenn procedure) 17 65 962
S3P (Fontan procedure) 17 61 058
Extracorporeal membrane oxygenation 156 32432
Ventricular assist device implantation 29–50 174 743–624 79833,34
Premature infant, 25 wk 87–98 150 000–202 70030,31
Premature infant, 28 wk 52–57 65 000–119 60030,31
Omphalocele 32 141 72417
Gastroschesis 41–47 123 200–155 62917,35
Congenital diaphragmatic hernia 25 162 70017
istrative database with the inherent limitations of such data sources. We were able to analyze only single hospi-talizations, and we were unable to track patients across subsequent hos-pitalizations or as outpatients. Data on survival to discharge were the only outcome data available; therefore, we were unable to assess quality of life or rates of survival after discharge. Ac-tual reimbursement rates were not available and undoubtedly vary widely depending on third-party payer
agree-ments. Cost-effectiveness calculations were not attempted because of the long-term uncertainty of survival for pa-tients with a single right ventricle and the frequent neurodevelopmental defi-cits found among patients who have un-dergone staged palliative operations. In addition, the considerable related inpa-tient, outpainpa-tient, and physician costs were not available for this population, which indicates that these results repre-sent a conservative estimate of the total costs of treating HLHS.
CONCLUSIONS
We present the results of a large mul-ticenter study that examined the inpa-tient costs of HLHS in the current era.
Improved survival rates have led to in-creased hospital stays and costs. Whether treated with 3-stage pallia-tion or transplantapallia-tion, patients with
HLHS demand extensive and expensive inpatient resources, similar to those for prematurity or other major birth defects.
REFERENCES
1. Gordon BM, Rodriguez S, Lee M, Chang R. Decreasing number of deaths of infants with hypoplastic left heart syndrome.J Pe-diatr. 2008;153(3):354 –358
2. Noonan JA, Nadas AS. The hypoplastic left heart syndrome: an analysis of 101 cases.
Pediatr Clin North Am. 1958;5(4):1029 –1056 3. Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia: hypoplastic left heart syndrome.N Engl J Med. 1983;308(1): 23–26
4. Sano S, Ishino K, Kawada M, et al. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome.J Thorac Cardiovasc Surg. 2003; 126(2):504 –509
5. Bridges ND, Jonas RA, Mayer JE, Flanagan MF, Keane JF, Castaneda AR. Bidirectional cavopulmonary anastomosis as interim palliation for high risk Fontan candidates.
Circulation. 1990;82(suppl IV):IV-170 –IV-176 6. Norwood WI. Hypoplastic left heart syn-drome.Ann Thorac Surgery. 1991;52(3): 688 – 695
7. Farrell P Jr, Chang A, Murdison K, Baffa J, Norwood W, Murphy J. Outcome and assess-ment after the modified Fontan procedure for hypoplastic left heart syndrome. Circu-lation. 1992;85(1):116 –122
8. McHugh KE, Hillman DG, Gurka MJ, Gutgesell HP. Three-stage palliation of hypoplastic left heart syndrome in the University Health System Consortium.Congenit Heart Dis. 2010;5(1):8 –15
9. Mahle WT, Spray TL, Wernovsky G, Gaynor JW, Clark BJ III. Survival after reconstruc-tive surgery for hypoplastic left heart syndrome: a 15-year experience from a sin-gle institution. Circulation. 2000;102(19 suppl 3):III-136 –III-141
10. Tibballs J, Kawahira Y, Carter BG, Donath S, Brizard C, Wilkinson J. Outcomes of surgical
treatment of infants with hypoplastic left heart syndrome: an institutional experience 1983–2004.J Paediatr Child Health. 2007; 43(11):746 –751
11. Pigula FA, Vida V, del Nido P, Bacha E. Con-temporary results and current strategies in the management of hypoplastic left heart syndrome.Semin Thorac Cardiovasc Surg. 2007;19(3):238 –244
12. Gutgesell HP, Gibson J. Management of hyp-oplastic left heart syndrome in the 1990s.
Am J Cardiol. 2002;89(7):842– 846 13. Chang RR, Chen AY, Klitzner TS. Clinical
man-agement of infants with hypoplastic left heart syndrome in the United States, 1988 –1997. Pediatrics. 2002;110(2): 292–298
14. Prsa M, Holly CD, Carnevale FA, Justino H, Rohlicek CV. Attitudes and practices of car-diologists and surgeons who manage HLHS.
Pediatrics. 2010;125(3). Available at: www. pediatrics.org/cgi/content/full/125/3/e625
15. Wernovsky G. The paradigm shift toward surgical intervention for neonates with hy-poplastic left heart syndrome.Arch Pediatr Adolesc Med. 2008;162(9):849 – 854 16. Mishra V, Lindberg H, Seem E, et al. A
com-parison of hospital costs with reimburse-ment received for patients undergoing the Norwood procedure for hypoplasia of the left heart. Cardiol Young. 2005;15(5): 493– 497
17. Centers for Disease Control and Prevention. Hospital stays, hospital charges, and in-hospital deaths among infants with se-lected birth defects: United States, 2003.
MMWR Morb Mortal Wkly Rep. 2007;56(2): 25–29
18. Gajarski RJ, Towbin JA, Garson A. Fontan palliation versus heart transplantation: a comparison of charges.Am Heart J. 1996; 131(6):1169 –1174
19. Williams DL, Gelijns AC, Moskowitz AJ, et al. Hypoplastic left heart syndrome: valuing the survival. J Thorac Cardiovasc Surg. 2000;119(4):720 –731
20. Bureau of Labor Statistics. CPI inflation cal-culator. Available at: http://data.bls.gov/cgi-bin/cpicalc.pl. Accessed December 1, 2010
21. Tweddell JS, Hoffman GM, Mussatto KA, et al. Improved survival of patients undergoing p a l l i a t i o n o f h y p o p l a s t i c l e f t h e a r t syndrome: lessons learned from 115 con-secutive patients.Circulation. 2002;106(12 suppl I):I-82–I-89
22. Berry JG, Cowley CG, Hoff CJ, Srivastava R. In-hospital mortality for children with hyp-oplastic left heart syndrome after stage I surgical palliation: teaching versus non-teaching hospitals.Pediatrics. 2006;117(4): 1307–1313
23. Hirsch J, Gurney J, Donohue J, Gebre-mariam A, Bove E, Ohye R. Hospital mortality for Norwood and arterial switch operations as a function of institutional volume. Pedi-atr Cardiol. 2008;29(4):713–717
24. Ohye RG, Sleeper LA, Mahony L, et al. Com-parison of shunt types in the Norwood pro-cedure for single-ventricle lesions.N Engl J Med. 2010;362(21):1980 –1992
25. Scheurer MA, Hill EG, Vasuki N, et al. Survival a f t e r b i d i r e c t i o n a l c a v o p u l m o n a r y anastomosis: analysis of preoperative risk factors.J Thorac Cardiovasc Surg. 2007; 134(1):82– 89.e2
26. Douglas WI, Goldberg CS, Mosca RS, Law IH, Bove EL. Hemi-Fontan procedure for hyp-oplastic left heart syndrome: outcome and suitability for Fontan.Ann Thorac Surg. 1999;68(4):1361–1367
27. Azakie A, McCrindle BW, Benson LN, et al. Total cavopulmonary connections in chil-dren with a previous Norwood procedure.
diopulmonary bypass with aortic cross-clamping.Ann Thorac Surg. 2006;82(5): 1611–1620
29. Hirsch JC, Goldberg C, Bove EL, et al. Fontan operation in the current era: a 15-year sin-gle institution experience.Ann Surg. 2008; 248(3):402– 410
30. St John EB, Nelson KG, Cliver SP, Bishnoi RR, Goldenberg RL. Cost of neonatal care ac-cording to gestational age at birth and sur-vival status.Am J Obstet Gynecol. 2000; 182(1):170 –175
31. Gilbert WM, Nesbitt TS, Danielsen B. The cost of prematurity: quantification by gesta-tional age and birth weight.Obstet Gynecol. 2003;102(3):488 – 492
32. Mahle WT, Forbess JM, Kirshbom PM, Cuadrado AR, Simsic JM, Kanter KR. Cost-utility analysis of salvage cardiac extracor-poreal membrane oxygenation in children.
J Thorac Cardiovasc Surg. 2005;129(5): 1084 –1090
33. Mahle WT, Ianucci G, Vincent RN, Kanter KR.
86(5):1592–1597
34. Morales DLS, Zafar F, Rossano JW, et al. Use of ventricular assist devices in children across the United States: analysis of 7.5 mil-lion pediatric hospitalizations.Ann Thorac Surg. 2010;90(4):1313–1319
35. Sydorak RM, Nijagal A, Sbragia L, et al. Gastroschisis: small hole, big cost.J Pediatr Surg. 2002;37(12):1669 –1672
36. Stamm C, Friehs I, Mayer JE, et al. Long-term results of the lateral tunnel Fontan opera-tion.J Thorac Cardiovasc Surg. 2001;121(1): 28 – 41
37. Khairy P, Fernandes SM, Mayer JE Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery.Circulation. 2008;117(1): 85–92
38. Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmen-tal outcome and lifestyle assessment in school-aged and adolescent children with
39. Saliba Z, Butera G, Bonnet D, et al. Quality of life and perceived health status in surviving adults with univentricular heart.Heart. 2001;86(1):69 –73
40. Anderson PAW, Sleeper LA, Mahony L, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network mul-ticenter study.J Am Coll Cardiol. 2008;52(2): 85–98
41. Ouyang L, Grosse SD, Armour BS, Waitz-man NJ. Health care expenditures of chil-dren and adults with spina bifida in a pri-vately insured U.S. population. Birth Defects Res A Clin Mol Teratol. 2007;79(7): 552–558
DOI: 10.1542/peds.2010-3742 originally published online October 10, 2011;
2011;128;e1181
Pediatrics
Peter N. Dean, Diane G. Hillman, Kimberly E. McHugh and Howard P. Gutgesell
Syndrome
Inpatient Costs and Charges for Surgical Treatment of Hypoplastic Left Heart
Services
Updated Information &
http://pediatrics.aappublications.org/content/128/5/e1181
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/128/5/e1181#BIBL
This article cites 40 articles, 8 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/cardiology_sub
Cardiology
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2010-3742 originally published online October 10, 2011;
2011;128;e1181
Pediatrics
Peter N. Dean, Diane G. Hillman, Kimberly E. McHugh and Howard P. Gutgesell
http://pediatrics.aappublications.org/content/128/5/e1181
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.