Childhood Hepatitis B Virus Infections in the United States Before
Hepatitis B Immunization
Gregory L. Armstrong, MD*; Eric E. Mast, MD, MPH*; Mary Wojczynski, MPH‡; and Harold S. Margolis, MD*
ABSTRACT. Objective. To estimate the number of
hepatitis B virus (HBV) infections among US children younger than 10 years before implementation of routine childhood hepatitis B immunization.
Methods. Incidence of HBV infection in children was modeled from existing prevalence data by means of re-gression analysis. Sources of data for the models in-cluded published and unpublished surveys that deter-mined the prevalence of HBV infection in US-born children. The number of nonperinatal HBV infections in children younger than 10 years was estimated by apply-ing these infection rates to 1991 population data accord-ing to maternal race, ethnicity, and birthplace.
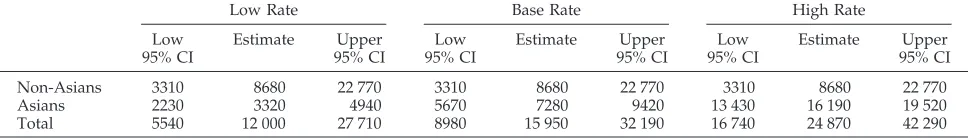
Results. Estimated annual rates of infection ranged from 24 per 100 000 in non-Asian children to 2580 per 100 000 in children of Southeast Asian immigrant moth-ers. These rates indicate that by the early 1990s, HBV was infecting 16 000 children who were younger than 10 years (8700 non-Asian children and 7300 Asian-American chil-dren) annually. The total estimate, not including perina-tal infections, ranged from 12 000 (95% confidence inter-val: 5500 –27 700) to 24 900 (95% confidence interinter-val: 16 700 – 42 300) infections and depended on how the esti-mated rates were applied to the population data.
Conclusion. Thousands of US children were infected each year with HBV before routine infant hepatitis B immunization, placing them at high risk of death from cirrhosis or hepatocellular carcinoma later in life.
Pediatrics 2001;108:1123–1128; hepatitis B, incidence, prevalence, immunization.
ABBREVIATIONS. HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; anti-HBs, antibody to hepatitis B surface antigen; anti-HBc, antibody to hepatitis B core antigen; NHANES, National Health and Nutrition Examination Survey; CI, confidence inter-vals.
H
epatitis B virus (HBV) infection in young children rarely produces symptoms that are recognizable as hepatitis1–3 but frequently leads to a chronic infection that can progress to end-stage liver disease. Adults who have had chronic HBV infection since childhood develophepatocellu-lar carcinoma at a rate of 5% per decade, which is 100 to 300 times the rate observed among uninfected people in the general population.4 – 6Because the risk of chronic infection increases with decreasing age,2,7 people who are infected in early childhood experi-ence a disproportionately large burden of disease attributable to HBV.
HBV infection is now almost completely prevent-able by immunization. In populations in which the infection is highly endemic, routine childhood im-munization has led to dramatic decreases in the prevalence of chronic infection8 –10as well as declines in childhood mortality from hepatocellular carcino-ma.11
In the United States, the Advisory Committee on Immunization Practices first recommended hepatitis B vaccination for infants who were born to HBV-infected mothers in 198212and expanded this recom-mendation in 1988 to include all infants in racial/ ethnic groups with high rates of chronic HBV infection. By the early 1990s, accumulating data showed that many children remained at risk for HBV infection because of incomplete maternal screening13 and because a substantial proportion of infections occurred in children of hepatitis B surface antigen (HBsAg)-negative mothers.14,15Furthermore, hepati-tis B surveillance data indicated that adults and ad-olescents who were at high risk of infection were not being vaccinated and that 30% of cases in adults were associated with no recognizable risk factors.16 With these data and a growing body of evidence that hepatitis B vaccination was safe, the Advisory Com-mittee on Immunization Practices expanded its hep-atitis B immunization recommendations in 1991 to include all infants, primarily to stop HBV transmis-sion among children14,15,17–19 and eventually also to prevent HBV infections in adolescents and adults.20 Recently, critics of routine infant immunization have asserted that HBV infection is rare and of little consequence in children.21,22 The low priority ac-corded to hepatitis B immunization also contributed to a 1999 recommendation to delay the initiation of infant hepatitis B immunization until thimerosal pre-servative-free vaccine was available.23 To quantify better the burden of HBV from infections acquired during childhood, we analyzed published and un-published data to estimate the annual number of nonperinatal childhood infections that occurred in the United States before routine immunization.
From the *Hepatitis Branch, Division of Viral and Rickettsial Diseases, National Center for Infectious Diseases, and ‡Immunization Services Divi-sion, National Immunization Program, Centers for Disease Control and Prevention, Atlanta, Georgia.
Received for publication Feb 28, 2001; accepted May 8, 2001.
Reprint requests (G.L.A.) Mailstop G-37, 1600 Clifton Rd, NE, Atlanta, GA 30333. E-mail: garmstrong@cdc.gov.
METHODS Incidence Model
The incidence of HBV infection was estimated from age-specific prevalence data by means of “catalytic” models,24,25the
mathe-matical details of which are specified in the appendix. The model functioned much like a simple linear regression model in which the dependent variable was the prevalence of serologic markers of HBV infection and the independent variable was age. The inter-cept (at age⫽0) was fixed at a proportion equal to the rate of perinatal HBV infection, and the slope of the line, an estimator of the incidence of infection, was determined by regression analysis. This simple linear model was used to estimate the average inci-dence over the lifetimes of the children in each study population because there were not enough data to apply more complicated models capable of estimating age-specific incidence. This linear model also assumed that HBV infection was not associated with increased mortality in children and that infected children retained serologic evidence of infection (HBsAg, antibody to HBsAg [anti-HBs], or antibody to hepatitis B core antigen [anti-HBc]).
Prevalence Data Sources
The incidence of HBV infection among non-Asian Americans was modeled from prevalence data obtained in the second and third National Health and Nutrition Examination Surveys (NHANES II, 1976 –1980,26 and NHANES III, 1988 –199427 [the
“NHANES model”]). Analysis was restricted to US-born non-Asian participants who were 0 to 11 years of age. Only those who tested positive for anti-HBc and at least 1 other marker of HBV infection (anti-HBs or HBsAg) were considered to have been in-fected. To estimate the rate of perinatal HBV infection in this population, we assumed that the prevalence of HBsAg in mothers of these children was 0.17% on the basis of a meta-analysis of 18 studies of 26 959 pregnant, non-Asian women in the United States (M. Wojczynski, unpublished data) and that in the absence of postexposure prophylaxis 42% of children of HBsAg-positive mothers would be infected during the perinatal period,28giving
an overall perinatal infection rate of 0.07%.
Four different prevalence studies were used to construct 3 incidence models for Asian-American children. The “Southeast Asian model” used data from 2 studies: a survey of US-born children of Hmong refugees living in Wisconsin15and a survey of
US-born children of Vietnamese immigrants living in Louisiana.29
No perinatal HBV transmission was assumed in this model be-cause in the studies used to derive this model, children who were born to HBsAg-positive mothers were excluded. The criteria for HBV infection were as described in the original articles (anti-HBc-positive in the Louisiana study29and anti-HBc- or HBsAg-positive
in the Wisconsin study15).
The “Chinatown model” used data from a 1996 survey of students who were attending 2 public schools in New York City’s Chinatown (Centers for Disease Control and Prevention, unpub-lished data). Students who were foreign-born or non-Asian or who had received hepatitis B vaccine were excluded from the analysis. Students were considered to be HBV-infected if they tested posi-tive for HBsAg or for both anti-HBs and anti-HBc. Because an effective program to prevent perinatal HBV infection had been in place for this population since the mid-1980s,30we assumed no
perinatal infection in unvaccinated children.
The “Honolulu model” was based on a survey of students who were attending 48 of Honolulu’s 54 public schools in 1989.31Only
Hawaiian-born participants were included in the model, and the criteria for HBV infection were as described for the Chinatown model. However, children in this study were too old to have participated in Hawaii’s perinatal hepatitis B prevention program, which began in 1985. We assumed that 1.2% of these children would have been born to HBsAg-positive mothers (P. Effler, Ha-waii Department of Health, unpublished data), and 42% of these,28
or 0.5%, would have been infected perinatally.
Population Estimates
The number of children who were younger than 10 years in the United States in 1991 was estimated from the Current Population Survey.32The race, ethnicity, and birthplace of the mothers of
these children were obtained by reviewing computerized natality records for the previous 10 years, in which Asian-American eth-nicity was specified as Chinese, Japanese, Hawaiian, Filipino, or
“other Asian, Pacific Islander.” Natality data from 1997,33which
included more categories of Asian-American ethnicity than data from previous years, was used to divide further the category of “other Asian, Pacific Islander” into Guamanian, Vietnamese, Ko-rean, Asian Indian, and Samoan.
Calculation of Race/Ethnic-Specific Infection Rates
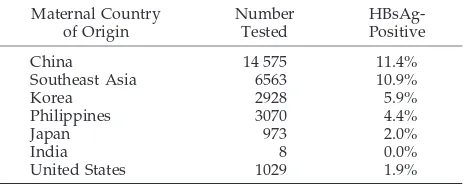
To determine the number of early childhood HBV infections in each racial/ethnic group, we applied the infection rates estimated from the models to the 1991 US population aged 0 to 9 years according to maternal race and birthplace (US-born vs foreign-born). The infection rate from the NHANES model was applied to children of white, black, Hispanic, and American Indian mothers, and the rate from the Honolulu model was applied to children of Hawaiian mothers. Rates of infection in other Asian-American children were assumed to vary in proportion to the prevalence of HBsAg in their mothers, and these rates were estimated from published data (Table 1).34The HBV infection rate from the
Chi-natown model was applied directly to children of Chinese women. For children of Filipino and Korean mothers, 50% of the China-town model infection rate was used and one fifth of this infection rate was applied to children of Indian and Japanese women.34The
rate from the Southeast Asian model was applied to children of Vietnamese and Pacific Islander women.34,35The weighted
aver-age infection rate of children whose mothers were of known Asian or Pacific Island origin was applied to children whose mothers were of unspecified Asian or Pacific Island origin. The HBV in-fection rate was assumed to be 5 times lower for children of Asian or Pacific Island descent whose mothers had been born in the United States compared with children whose mothers were for-eign-born.36
Sensitivity Analysis
We considered 2 alternatives to the “base rate” scenario de-scribed above. In the “low rate” scenario, rates from the China-town model were applied to the population data in place of the rates from the Southeast Asian model. In the “high rate” scenario, the reverse was done (ie, rates from the Southeast Asian model were applied in place of rates from the Chinatown model).
RESULTS
The incidence of childhood HBV infection varied considerably among the different population models (Table 2 and Fig 1). Among non-Asians (the NHANES model), the incidence estimate with the most conservative criteria for HBV infection (anti-HBc positive and either anti-HBs or HBsAg positive) was 23.7 per 100 000 (95% confidence interval [CI]: 9.0 – 62.3). Using a less conservative definition of HBV infection (the presence of any of the 3 HBV infection markers), the estimated incidence rate was 102.7 per 100 000 (95% CI: 65.7–160.5).
When modeled separately, the data from Hurie et al15 and Mahoney et al29 produced incidence esti-mates of 3280 (95% CI: 2420 – 4460) and 2290 per 100 000 (95% CI: 1810 –2900), respectively. Because these rates were not different statistically, the 2
stud-TABLE 1. Prevalence of HBsAg Among Pregnant Women Delivering in the United States
Maternal Country of Origin Number Tested HBsAg-Positive
China 14 575 11.4%
Southeast Asia 6563 10.9%
Korea 2928 5.9%
Philippines 3070 4.4%
Japan 973 2.0%
India 8 0.0%
United States 1029 1.9%
ies were combined into a single model, the Southeast Asian model (Table 2). Rates in the Chinatown and Honolulu models were approximately one fifth and one seventeenth that of the Southeast Asian model, respectively (Table 2).
By application of the HBV infection rates from the respective models to the 1991 US population data, it is estimated that 15 950 (95% CI: 8980 –32 190, Tables 3 and 4) children who were younger than 10 years were infected annually. In the sensitivity analysis, the estimated number of infections per year de-pended on how the rates from the Chinatown and Southeast Asian models were applied to the racial/ ethnic distribution of children in the United States (Table 4).
DISCUSSION
On the basis of prevalence of markers of HBV infection in US children, we estimated that 16 000 children who were younger than 10 years were in-fected in 1991, with the number of infections almost equally divided between Asian American and non-Asian children. Most of these infections would have been clinically silent and unrecognized, because in-fection at a young age rarely results in symptoms that are suggestive of hepatitis.1,2 This estimate is consistent with a previous, less refined estimate of early childhood infection in the United States37 and does not include perinatal infections in the approxi-mately 15 000 children who were born to HBsAg-positive mothers in 1991 (M. Wojczynski, unpub-lished data).
Since 1991, the number of US children who are born to HBsAg-positive women has increased sub-stantially attributable in large part to increases in foreign-born Asian-American women of childbear-ing age. Applychildbear-ing the estimated maternal prevalence of HBsAg (M. Wojczynski, unpublished data) and the rates of childhood infection from our model to 1998 births,38there would have been 6800 perinatal infections and an additional 18 700 infections in the first 10 years of life among this birth cohort had no vaccination taken place. Chronic infection would oc-cur in 90% of those infected in the perinatal period, 60% of those infected before 2 years of age, and 25% of those infected between ages 2 and 9 years.2,7Thus, 12 100 children would have been chronically in-fected, half in the perinatal period and the other half after birth but before their 10th birthday. Many of the chronically infected children would develop chronic liver disease later in life, and approximately 25%,4,5 or 3000, would eventually die from cirrhosis or hep-atocellular carcinoma. However, according to the
most recent National Immunization Survey, approx-imately 90% of children are now fully immunized with 3 doses of the hepatitis B vaccine by their sec-ond birthday. Thus, as a direct effect of vaccination, the 1998 birth cohort can expect at least 2700 fewer deaths from chronic liver disease as it grows to adult-hood.
Although the estimates of infection incidence for Asian-American children of foreign-born mothers were the most robust, they also highlight the limita-tions of the prevalence studies conducted before in-troduction of routine childhood hepatitis B immuni-zation in the United States. These included small sample size and, for some, lack of information about maternal HBsAg status as a surrogate for risk of perinatal infection. In addition, because of the lack of data, precise estimates of HBV infection rates could not be made for the respective Asian-American eth-nic groups. However, the 17-fold difference in inci-dence estimates most likely reflects the true hetero-geneity in HBV infection incidence found among Asian immigrant communities.34
Estimates of childhood HBV infection in black, Hispanic, and white children are less precise than those derived from Asian Americans for 2 reasons. First, because HBV infection rates were lower among non-Asian children, the 2 NHANES surveys, al-though large, did not have serologic data from enough children to yield age-specific prevalence or incidence estimates that were as precise as those obtained from studies of Asian children. Second, the true rate of perinatal infection was unknown and had to be estimated from external sources of data. However, if the rate of perinatal HBV infection were lower than the assumed 0.07%, then the true rate of early childhood infection would have been higher than that estimated by the NHANES model.
We were not able to estimate directly the propor-tion of childhood HBV infecpropor-tions that occurred among children who were born to HBsAg-positive mothers and among children who were born to HB-sAg-negative mothers because maternal HBsAg sta-tus was not available for all studies. However, in 2 population-based studies conducted among Asian-Pacific Islander children who were born before peri-natal hepatitis B prevention programs were widely implemented in the United States, 61% to 66% of chronic HBV infections were among children who were born to HBsAg-negative mothers.15,29 Many of these chronic infections among children who were born to HBsAg-negative mothers would not be pre-vented by identification and immunization of infants who were born to HBsAg-positive mothers. Thus,
TABLE 2. Estimated Annual Incidence of HBV Infection Determined From Age-Specific Prevalence Data for Children in Different Racial/Ethnic Groups Born in the United States
Model Source of Prevalence Data Number of
Participants in Analysis
Ages of Participants
in Analysis
Annual Incidence per 100 000 (95% CI)
NHANES NHANES II26and III27 4793 2–11 23.7 (9.0–62.3)
Southeast Asian Hurie et al,15Mahoney et al29 823 1–12 2580 (2140–3110)
Chinatown Centers for Disease Control and Prevention, unpublished data
541 4–12 517 (346–771)
both prevention of perinatal hepatitis B and routine infant hepatitis B immunization are required to pre-vent all early childhood HBV infections.8
Horizontal HBV transmission between children in households has been well documented in the United States14,15,29 as well as transmission from adults to children.14,39 – 41In addition, transmission may occur outside the household setting. For example, studies in Louisiana,29 Wisconsin,15 and Georgia14 found that approximately one third of HBV infections among children who were born to HBsAg-negative mothers occurred in households where no other chronically infected household member was identi-fied. The mechanisms of horizontal HBV transmis-sion are not completely defined but presumably in-volve inapparent percutaneous or permucosal
exposure to blood or body fluids that contain HBV. In addition to blood, HBV has been found in impe-tiginous exudates and saliva, albeit at concentrations lower than in blood.42 HBV also survives in the environment for at least 7 days, and HBsAg has been detected on environmental surfaces of households of chronically infected individuals.43,44 Risk factors for horizontal HBV transmission in household settings include contact with dermatologic lesions,45 premas-tication of food, and sharing washcloths or other articles contaminated with blood or body secre-tions.43,46
The need for infant hepatitis B immunization in the United States has been questioned in the past47 and again more recently21,22 because of misconcep-tions about the importance of childhood HBV
infec-Fig 1. In each of the 4 graphs, the circles represent the data points used to model incidence. The areas of the circles are proportional to the number of observations at each point (the scale is different in each graph). The solid line represents the modeled incidence, and the dotted lines represent the 95% CIs. Note that the scale of theyaxis is different in each graph.
TABLE 3. Number of Children Ages 0 to 9 Years in the United States in 1991 and the Estimated Number of Nonperinatal HBV Infections That Would Have Occurred in These Children Without Vaccination
Maternal Race Population Rate Category* Rate (Infections per 100 000 Population)
Number of HBV Infections
US-Born
Foreign-Born
US-Born
Foreign-Born
US-Born
Foreign-Born
US-Born
Foreign-Born
Total
Non-Asian 32 588 790 3 977 393 N N 23.7 23.7 7733 944 8677
Hawaiian 56 278 1022 H H 153 153 86 2 88
Chinese 21 933 161 551 C/5 C 103 517 23 835 858
Asian Indian, Japanese 51 385 235 802 C/25 C/5 21 103 11 244 255
Filipino, Korean 36 507 277 733 C/10 C/2 52 259 19 718 737
Vietnamese, Samoan, Guamanian 7865 156 906 S/5 S 516 2581 41 4050 4091
Other Asians/Pacific Islanders 21 231 174 763 103 702 22 1227 1249
Total 32 783 990 4 985 170 24.2 161 7934 8019 15 953
tion in United States. The lack of appreciation that HBV infection occurs among infants who are born to HBsAg-negative mothers contributed to a recent rec-ommendation that, until thimerosal preservative-free hepatitis B vaccine became available, immuniza-tion be delayed until after 6 months of age.23,48This recommendation, although intended to be tempo-rary, has had a lasting effect on immunization rates despite the current availability of preservative-free vaccine.49
A recent study of teenage, first-time blood donors showed that of 2061 HBsAg-positive people identi-fied, half were Asian American and 13% of the Asian Americans had been born in the United States.50 Continued childhood HBV infection has implications both for personal health outcomes and for public health, including the adequacy of the nation’s blood supply. If hepatitis B immunization were to have ceased in the 1990s, then thousands of children would have become chronically infected each year, which in the long-term would lead to a large number of deaths from end-stage liver disease.28Our analysis of widely available data support the need for univer-sal infant immunization to prevent chronic hepatitis B and the substantial burden of hepatitis B-related chronic liver disease that would be acquired during infancy and early childhood.20
APPENDIX
The incidence of HBV infection was assumed to be constant with respect to time and age such thatP(A), the prevalence of HBV infection in a cohort of ageA, is given by:
P共A兲⫽1⫺e⫺共*A⫹P0兲
whereP0is the rate of perinatal infection andis the incidence in the susceptible population.Lambdaand its standard error were estimated by maximum like-lihood methods, except for analyses involving the NHANES surveys, in which standard errors were estimated by means of the balanced repeat replicate method.51 Ninety-five percent CIs were calculated assuming that the logarithm of incidence was nor-mally distributed.
REFERENCES
1. Beasley RP, Hwang LY, Lin CC, et al. Incidence of hepatitis B virus infections in preschool children in Taiwan.J Infect Dis. 1982;146:198 –204 2. McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and sub-sequent development of the carrier state.J Infect Dis. 1985;151:599 – 603 3. Krugman S, Overby LR, Mushahwar I, et al. Viral hepatitis, type B.
Studies on natural history and prevention re-examined.N Engl J Med. 1979;300:101–106
4. Beasley RP. Hepatitis B virus—the major etiology of hepatocellular carcinoma.Cancer. 1988;61:1942–1956
5. Beasley RP, Hwang LY. Overview on the epidemiology of hepatocellu-lar carcinoma. In: Hollinger FB, Lemon SM, Margolis HS, eds.Viral Hepatitis and Liver Disease. Baltimore, MD: Williams & Wilkins; 1991: 532–535
6. Alward WL, McMahon BJ, Hall DB, et al. The long-term serological course of asymptomatic hepatitis B virus carriers and the development of primary hepatocellular carcinoma.J Infect Dis. 1985;151:604 – 609 7. Edmunds WJ, Medley GF, Nokes DJ, et al. The influence of age on the
development of the hepatitis B carrier state.Proc R Soc Lond B Biol Sci. 1993;253:197–201
8. Hsu HM, Lu CF, Lee SC, et al. Seroepidemiologic survey for hepatitis B virus infection in Taiwan: the effect of hepatitis B mass immunization.
J Infect Dis. 1999;179:367–370
9. Chen HL, Chang MH, Ni YS, et al. Seroepidemiology of hepatitis B virus infection in children: ten years after mass vaccination in Taiwan.
JAMA. 1996;276:906 –908
10. Harpaz R, McMahon BJ, Margolis HS, et al. Elimination of new chronic hepatitis B virus infections: results of the Alaska immunization pro-gram.J Infect Dis. 2000;181:413– 418
11. Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children.
N Engl J Med. 1997;336:1855–1859
12. Centers for Disease Control and Prevention. Inactivated hepatitis B virus vaccine. Recommendations of the Immunization Practices Advi-sory Committee (ACIP).MMWR Morb Mortal Wkly Rep. 1982;31:317–328 13. Silverman NS, Darby MJ, Ronkin SL, Wapner RJ. Hepatitis B prevalence in an unregistered prenatal population. Implications for neonatal ther-apy.JAMA. 1991;266:2852–2855
14. Franks AL, Berg CJ, Kane MA, et al. Hepatitis B virus infection among children born in the United States to Southeast Asian refugees.N Engl J Med. 1989;321:1301–1305
15. Hurie MB, Mast EE, Davis JP. Horizontal transmission of hepatitis B virus infection to United States-born children of Hmong refugees. Pe-diatrics. 1992;89:269 –273
16. Alter MJ, Hadler SC, Margolis HS, et al. The changing epidemiology of hepatitis B in the United States. Need for alternative vaccination strat-egies.JAMA. 1990;263:1218 –1222
17. McMahon BJ, Rhoades ER, Heyward WL, et al. A comprehensive pro-gramme to reduce the incidence of hepatitis B virus infection and its sequelae in Alaskan natives.Lancet. 1987;2:1134 –1136
18. Schreeder MT, Bender TR, McMahon BJ, et al. Prevalence of hepatitis B in selected Alaskan Eskimo villages.Am J Epidemiol. 1983;118:543–549 19. Wong DC, Purcell RH, Rosen L. Prevalence of antibody to hepatitis A
and hepatitis B viruses in selected populations of the South Pacific.Am J Epidemiol. 1979;110:227–236
20. Centers for Disease Control and Prevention. Hepatitis B virus: a com-prehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Im-munization Practices Advisory Committee (ACIP).MMWR Morb Mortal Wkly Rep. 1991;40:1–25
21. Hepatitis B Vaccine: Helping or Hurting Public Health. Hearing Before the Subcommittee on Criminal Justice, Drug Policy and Human Re-sources, Committee on Government Reform, 106th Congress; May 18, 1999
22. Vaccines—Finding the Balance Between Public Safety and Personal Choice. Hearing Before the Committee on Government Reform, 106th Congress; August 3, 1999
TABLE 4. Sensitivity Analysis: Estimated Number of HBV Infections in US Children Who Were Younger Than 10 Years in 1991*
Low Rate Base Rate High Rate
Low 95% CI
Estimate Upper 95% CI
Low 95% CI
Estimate Upper 95% CI
Low 95% CI
Estimate Upper 95% CI
Non-Asians 3310 8680 22 770 3310 8680 22 770 3310 8680 22 770
Asians 2230 3320 4940 5670 7280 9420 13 430 16 190 19 520
Total 5540 12 000 27 710 8980 15 950 32 190 16 740 24 870 42 290
23. American Academy of Pediatrics, Committee on Infectious Diseases and Committee on Environmental Health. Thimerosal in vaccines—an interim report to clinicians.Pediatrics. 1999;104:570 –574
24. Meunch H.Catalytic Models in Epidemiology. Cambridge, MA: Harvard University Press; 1959
25. Ades AE, Nokes DJ. Modeling age- and time-specific incidence from seroprevalence: toxoplasmosis.Am J Epidemiol. 1993;137:1022–1034 26. McQuillan GM, Townsend TR, Fields HA, et al. Seroepidemiology of
hepatitis B virus infection in the United States. 1976 to 1980.Am J Med.
1989:87:5S–10S
27. McQuillan GM, Coleman PJ, Kruszon-Moran D, et al. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994.Am J Public Health.
1999;89:14 –18
28. Margolis HS, Coleman PJ, Brown RE, et al. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations.JAMA. 1995;274:1201–1208
29. Mahoney FJ, Lawrence M, Scott C, et al. Continuing risk for hepatitis B virus transmission among Southeast Asian infants in Louisiana. Pediat-rics. 1995;96:1113–1116
30. Henning KJ, Pollack DM, Friedman SM. A neonatal hepatitis B surveil-lance and vaccination program: New York City, 1987 to 1988.Am J Public Health.1992:82:885– 888
31. Pon EW, Ren H, Margolis H, et al. Hepatitis B virus infection in Honolulu students.Pediatrics. 1993;92:574 –578
32. Current Population Survey, March. Washington, DC: Bureau of the Census; 1991 (machine readable data file)
33. 1997 United States Natality Public Use Data File. Hyattsville, MD: National Center for Health Statistics; 2000 (machine readable data file) 34. Tong MJ, Hwang SJ. Hepatitis B virus infection in Asian Americans.
Gastroenterol Clin North Am. 1994;23:523–536
35. Brindle RJ, Eglin RP, Parsons AJ, et al. HTLV-1, HIV-1, hepatitis B and hepatitis delta in the Pacific and South-East Asia: a serological survey.
Epidemiol Infect. 1988;100:153–155
36. Zuckerman AJ, ed.Prevention of Perinatal Hepatitis B Virus Infection With Hepatitis B Immune Globulin and Hepatitis B Vaccine. New York, NY: Wiley; 1988
37. Margolis HS, Alter MJ, Hadler SC. Hepatitis B: evolving epidemiology and implications for control.Semin Liver Dis. 1991;11:84 –92
38. 1998 United States Natality Public Use Data File. Hyattsville, MD: National Center for Health Statistics; 2001 (machine readable data file) 39. Szmuness W, Harley EJ, Prince AM. Intrafamilial spread of
asymptom-atic hepatitis B.Am J Med Sci. 1975;270:293–304
40. Bernier RH, Sampliner RE, Gerety R, et al. Hepatitis B infection in households of chronic carriers of hepatitis B surface antigen: factors associated with prevalence of infection.Am J Epidemiol. 1982;116: 199 –211
41. Hess G, Born H, Dormeyer H, et al. Hepatitis B virus markers among family contacts of asymptomatic HBsAg carriers.Scand J Gastroenterol. 1979;14:373–378
42. Jenison SA, Lemon SM, Baker LN, Newbold JE. Quantitative analysis of hepatitis B virus DNA in saliva and semen of chronically infected homosexual men.J Infect Dis. 1987;156:299 –307
43. Pattison CP, Boyer DM, Maynard JE, Kelly PC. Epidemic hepatitis in a clinical laboratory. Possible association with computer card handling.
JAMA. 1974;230:854 – 857
44. Heathcote J, Cameron CH, Dane DS. Hepatitis B antigen in saliva and semen.Lancet. 1974;1:71–75
45. Whittle HC, Bradley AK, McLauchlan K. Hepatitis B virus infection in two Gambian villages.Lancet. 1983;1:1203–1206
46. Leichtner AM, LeClair J, Goldmann DA, et al. Horizontal nonparenteral spread of hepatitis B among children.Ann Intern Med. 1981;94:346 –349 47. Sepkowitz S. Estimates and reported cases of hepatitis B infection in
children.Pediatr Infect Dis J. 1993;12:542–544
48. Centers for Disease Control and Prevention. Thimerosal in vaccines: a joint statement of the American Academy of Pediatrics and the Public Health Service.MMWR Morb Mortal Wkly Rep. 1999;48:563–565 49. Centers for Disease Control and Prevention. Impact of the 1999 AAP/
USPHS joint statement of thimerosal in vaccines on infant hepatitis B vaccination practices.MMWR Morb Mortal Wkly Rep.2001;50:94 –97 50. Watanabe KK, Williams AE, Schreiber GB, Ownby HE. Infectious
dis-ease markers in young blood donors.Transfusion. 2000;40:954 –960 51. Rust KF. Variance estimation for complex surveys using replication
techniques.Stat Methods Med Res. 1996;5:283–310
ABSTRACT
Harman JS, Kelleher KJ. Pediatric length of stay guidelines and routine practice.
Arch Pediatr Adolesc Med.2001;155:885– 890
“Background.Guidelines for inpatient length of stay (LOS) have been developed by Milliman and Robertson (M&R) and are widely applied by health plans. This study was designed to compare LOS for several pediatric conditions with the M&R LOS criteria using recent data and to determine if concordance of actual practice with M&R LOS criteria varied between children and adults.
Conclusions. The M&R LOS criteria were divergent from routine practice for both children and adults. Greater divergence of adult discharges illustrates the need to consider comorbid conditions when implementing these guidelines. Thus, patient care may suffer if guidelines are implemented in an uninformed way. These findings emphasize the importance of using the best possible science when pro-ducing guidelines such as these.”
DOI: 10.1542/peds.108.5.1123
2001;108;1123
Pediatrics
Gregory L. Armstrong, Eric E. Mast, Mary Wojczynski and Harold S. Margolis
Immunization
Childhood Hepatitis B Virus Infections in the United States Before Hepatitis B
Services
Updated Information &
http://pediatrics.aappublications.org/content/108/5/1123 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/108/5/1123#BIBL This article cites 41 articles, 4 of which you can access for free at:
Subspecialty Collections
b
http://www.aappublications.org/cgi/collection/infectious_diseases_su
Infectious Disease
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.108.5.1123
2001;108;1123
Pediatrics
Gregory L. Armstrong, Eric E. Mast, Mary Wojczynski and Harold S. Margolis
Immunization
Childhood Hepatitis B Virus Infections in the United States Before Hepatitis B
http://pediatrics.aappublications.org/content/108/5/1123
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.