The Role of Protein C, Protein S, and Resistance to
Activated Protein C in Legg-Perthes Disease
John Eldridge, MD*; Anne Dilley, PhD‡; Harland Austin, DSc‡§; Muhydine EL-Jamil, MPH‡§; Lori Wolstein, MPH‡§; John Doris, MD储; W. Craig Hooper, PhD‡; Peter L. Meehan, MD储; and
Bruce Evatt, MD‡
ABSTRACT. Objectives. It has been hypothesized that Legg-Perthes disease is caused by repeated vascular interruptions of the blood supply to the proximal femur, which are precipitated by coagulation system abnormal-ities. To test this theory, we conducted a case-control study among 57 patients with Legg-Perthes disease and an equal number of community controls. We measured protein C and protein S and resistance to activated pro-tein C (APC-R) from plasma.
Study Design. Participants were placed into 1 of 3 mutually exclusive categories based on the control dis-tribution: 1) normal, defined as either above or within 1 standard deviation below the expected mean; 2) low nor-mal, defined as between 1 and 2 standard deviations below the expected mean; and 3) low, defined as >2 standard deviations below the expected mean. DNA was analyzed to determine the presence of a point mutation in the factor V gene that causes APC-R.
Results. We observed a statistically significant in-creased risk of Legg-Perthes disease with decreasing lev-els of protein C and a nearly significant increased risk with decreasing levels of protein S. The factor V gene defect was present in 5 (9%) of 55 cases and 3 (5%) of 56 controls (odds ratio 1.8, 95% confidence interval: 0.4 –7.7), but the mean level on the APC-R plasma test was similar for cases and controls. Nine cases and 1 control had 2 low normal or low test results (odds ratio 13.0, 95% confi-dence interval: 2.2–75).
Conclusions. Our results support the belief that ab-normalities of the coagulation system leading to a throm-bophilic state play a role in Legg-Perthes disease; how-ever, larger studies are needed before definitive recommendations for coagulation testing can be made. Pediatrics2001;107:1329 –1334;avascular necrosis, coagu-lation factors, genetics, Legg-Perthes disease, risk factors, thrombosis.
ABBREVIATIONS. APC, activated protein C; APC-R, resistance to activated protein C; APC-R ratio, activated protein C resistance ratio; OR, odds ratio.
L
egg-Perthes disease is a common pediatric hip disorder causing pain and decreased hip mo-tion, and may cause deformity of the femoral head. Although traumatic, developmental, and in-flammatory causes have been postulated, the cause of Legg-Perthes disease is largely unexplained. The most widely accepted theories involve repeated vas-cular interruptions of the blood supply to the prox-imal femur.1 Sanchis et al2 were able to producepathologic changes consistent with Legg-Perthes dis-ease after 2 experimental infarctions of the femoral heads of laboratory animals. Such observations sug-gest that thrombotic events that result in venous occlusion of the femur with subsequent venous hy-pertension and bone death may be a cause of Legg-Perthes disease.
Protein C and protein S are circulating glycopro-teins that are part of the negative feedback system of blood clotting. Low levels of either protein C or protein S increase the tendency to clot (thrombophil-ia). Deficiencies of protein C and protein S can be acquired or inherited. The population frequency of heterozygous protein C deficiency is⬃0.5%, whereas that of heterozygous protein S deficiency is un-known.3However, protein C deficiency and protein
S deficiency occur with similar frequency in patients with thromboembolic disease (⬃5%–15%, depending on age of occurrence3).
Protein C is inactive while circulating and must be activated before acting as an anticoagulant. Acti-vated protein C (APC) binds to a receptor on the endothelial cell, thrombomodulin, and then begins the deactivation of factors V and VIII, as well as stimulating fibrinolysis. Resistance to activated pro-tein C (APC-R), a poor response to APC in an acti-vated partial thromboplastin time assay, is a recently discovered mechanism for thrombophilia.4The
mo-lecular basis of APC-R has been identified as a point mutation in the factor V gene where arginine at residual 506 is replaced by glutamine (Arg506-⬎Gln).5 Epidemiologic studies of APC-R indicate
that the Arg506-⬎Gln mutation is an important cause of thrombosis.6 – 8
In 1996, Glueck et al9 reported that among 44
children with Legg-Perthes disease, 19 had protein C deficiency and 4 had protein S deficiency.9The
fol-lowing year, these same researchers reported that 13% of their Legg-Perthes patients had the factor V defect10 compared with an expected population From the *Department of Orthopedics and Pediatrics, University of
Louis-ville School of Medicine, LouisLouis-ville, Kentucky; ‡Hematologic Diseases Branch, Division of AIDS, STD, and Laboratory Research, National Center for Infectious Diseases, Centers for Disease Control and Prevention, US Department of Health and Human Services, Atlanta, Georgia; §Department of Epidemiology, Rollins School of Public Health, Emory University, At-lanta, Georgia; and储Emory University School of Medicine, Atlanta, Georgia. Received for publication Apr 13, 2000; accepted Sep 20, 2000.
Address correspondence to John C. Eldridge, MD, 4001 Kresge Wy, Suite 324, Louisville, KY 40207.
prevalence of 5%.8 If a thrombophilia state is an
underlying cause of Legg-Perthes disease, the multi-ple infarction theory of the cause of Legg-Perthes disease is supported. The purpose of the present study was to explore further the roles of protein C, protein S, and APC-R in the cause of Legg-Perthes disease.
MATERIALS AND METHODS
One hundred fifty-two patients who had been diagnosed and treated for Legg-Perthes disease at an orthopedic clinic in Atlanta, Georgia between 1985 and 1995 were identified as being eligible for inclusion as cases in the study. These patients, or the parents of those who were children, were informed of the study by a letter and subsequent telephone call. Of the 152 eligible cases, 57 (37.5%) agreed to participate, 22 (14.5%) declined, and 73 (48%) were not located.
Controls were selected from among volunteers recruited by community advertising. Persons with a history of chronic ortho-pedic problems were not eligible as controls. One control was selected for each case, and the distribution of age (within 3 years), race, and gender among the controls was identical to the distri-bution of these variables in the case group. No case or control was currently taking antibiotics or steroids, and none had experienced a recent bone fracture, situations that can have a transient effect on the coagulation system.
The participant, or a parent of a child participant, completed a short questionnaire which elicited information on basic demo-graphics and medical history. Twenty milliliters of blood were obtained from each participant, and plasma levels of free protein S and functional protein C, as well as the anticoagulant response to APC, were measured. The presence of the factor V genetic mutation was determined from DNA extracted from the blood sample.
Fifty-seven cases and 57 controls were enrolled into the study (44 males and 13 females in each group). One male control with a history of a brain tumor, 1 male case with a history of leukemia, and 1 male case who was currently receiving Warfarin because of protein S deficiency were excluded from the analyses. Thus, the study included 55 cases and 56 controls. The mean age of cases and controls was 14 (range: 3–31) and 15 (range: 4 –32) years, respectively. All participants were white. As some participants were quite young, sufficient blood (20 mL) for the protein C and protein S assays from 4 cases and 3 controls was not obtained, and for the same reason, the APC-R assay was not done for 5 cases and 3 controls.
Laboratory Methods
Blood was drawn using standard procedures required for co-agulation studies. Anticoagulated blood was centrifuged at 1660 x g. Cells were saved at 4°C to 8°C for DNA extraction, and plasma was transferred to a polycarbonate tube and centrifuged at 30 900 x g. Platelet-poor plasma was dispensed in 0.5 mL aliquots into polystyrene tubes, snap-frozen, and stored at⫺70°C.
Functional protein C was determined with the Staclot Protein C kit (Diagnostica Stago, Asnienes-Sur-Siene, France)11on an ST4
coagulation instrument (Diagnostica Stago). Plasma diluted 1:10 in Owren-Koller buffer (Diagnostica Stago) was added to protein C deficient plasma and activated with Agkistrodon c. contortrix venom. Clotting was begun by the addition of 0.025 M calcium chloride. APC, produced by the venom, inhibited factors V and VIII (provided by the protein C deficient plasma) and thus pro-longed the clotting time obtained by the addition of calcium chloride. Plasma levels of protein C were expressed as percentage of normal adult values.
Free protein S was measured by enzyme-linked immunosor-bent assay technique with the Asserachrom S kit (Diagnostica Stago). First, protein S bound to C4b-binding protein was precip-itated by a 1:1.17 dilution of plasma with 25% polyethylene glycol provided in the kit. The mixture was then incubated on ice for 30 minutes and centrifuged at 8800 x g. Protein S remaining in the supernatant was captured in a sandwich of rabbit antihuman protein S coupled with peroxidase. Bound peroxidase was then detected with orthophenylenediamine/hydrogen peroxide.
Plasma levels of free protein S were expressed as percentage of normal adult values.
The APC-R screening test was done by an activated partial thromboplastin time test with and without the addition of APC on an ACL 3000 plus coagulation instrument (Instrumentation Lab-oratory, Lexington, MA and Milano, Italy). Test plasma was acti-vated with Platelin LS (Organon Teknika, Durham, NC) for 5 minutes at 37°C; clotting was begun with the addition of Calcium-BSA buffer containing 10 mM Tris HCl, 0.05 M NaCl, 30 mM CaCl2, pH 7.5, plus 0.1% bovine serum albumin and 2 g/mL APC
(Celsus Laboratories, Cincinnati, OH). A baseline test was per-formed without the addition of APC to Calcium-BSA solution. The activated protein C resistance ratio (APC-R ratio) was calculated as the clotting time of test plasma after APC addition/clotting time of test plasma with no APC.
The presence of the factor V genetic defect was determined from DNA extracted from 3 mL of whole blood using the Genera DNA Extraction Kit (Genera, Minneapolis, MN) per the manufac-turer’s instructions and was stored at⫺20°C. Polymerase chain reaction was used to amplify DNA fragments in factor V. Restric-tion enzyme analysis was conducted using MnlI.
Statistical Methods
Separate linear regression models were used to evaluate the relation between protein C, protein S, and the APC-R ratio and age and gender among the controls.12Protein C levels were directly
related to age (P⬍.01) but not to gender. Protein S levels were related directly to age (P⬍.01) and were higher among males (P⫽.05). The APC-R ratio was unrelated to both age and gender. Thus, protein C was regressed on age among the controls and used the model to obtain age-specific, expected mean protein C levels. Protein C levels for each participant were then expressed as the number of standard deviations above or below the expected mean level for a specific age. Participants were then placed into 1 of 3 mutually exclusive categories: 1) normal, defined as either above or within 1 standard deviation below the expected mean; 2) low normal, defined as between 1 and 2 standard deviations below the expected mean; and 3) low, defined as⬎2 standard deviations below the expected mean. For protein S, age- and gender-specific mean values were obtained from controls using a linear model that included terms for age and gender. Participants were classified as normal, low normal, or low as described for protein C. For the APC-R ratio, the classification was based on the unadjusted measurement. For the factor V defect, participants were classified either as homozygous for the absence of the mu-tation or as heterozygous for the defect (no participant was ho-mozygous for the mutation).
The odds ratio (OR) was used as a measure of association between Legg-Perthes disease and each clotting factor.13The
ref-erent for the ORs was the normal category. The ORs for the low normal and the low categories are interpreted as the relative risk of Legg-Perthes for that category compared with the normal cat-egory. Age and gender have been accommodated in the analysis by the method we used to define low normal and low for each clotting factor. Fisher exact P values were used for statistical testing, and exact confidence limits were obtained for each OR.14
Mantel’s trend test was used to test for an increase in the OR across the 3 ordinal categories of each clotting factor.15
RESULTS Protein C and Protein S
is increased almost fourfold for persons with levels lower than 2 standard deviations below the controls mean. This OR is imprecise because of the small number of participants in the lowest category of protein C.
The findings for protein S were similar. The trend test across the 3 ordinal categories was almost statis-tically significant (P ⫽ .06). Two cases and no con-trols had a protein S level lower than 2 standard deviations below the expected mean (Fig 2). Thus, the OR for this category relative to the normal cate-gory is infinite. The exact lower confidence limit for this OR is 0.2.
APC-R Assay and Factor V Arg506->Gln Mutation The mean value of the APC-R ratio was similar for cases (4.74) and for controls (4.88) (mean difference: ⫺0.14, P⬎ .20). The ORs relating Legg-Perthes dis-ease and the APC-R functional assay and the factor V mutation are displayed in Table 2. The risk of Legg-Perthes disease for persons with a low normal test result is comparable to that of persons in the normal category (OR 1.1). Although the OR relating the low category to the normal category is high (3.4), the finding is not statistically significant, nor is the trend of increased risk across the 3 ordinal categories. The distribution of the standard deviations of APC-R
among the cases and controls appears in Fig 3. The factor V defect was present in 5 of 55 cases (9%) and in 3 of 56 controls (5%) yielding a nonstatistically significant increased OR of 1.8. All participants with the factor V defect had a APC-R functional assay in the low normal or low range.
Presence of Low Protein C, Low Protein S, or APC-R Participants were classified according to the total number of low normal or low test results that they had. No participant was classified as low normal or low on all 3 clotting factors (protein S, protein C, and the APC-R test). As seen in Table 3, 9 cases and 1 control had 2 low normal or low test results, yielding an OR of about 13.
DISCUSSION
The results of this study suggest that abnormalities of the anticoagulant system leading to a thrombo-philic state are a risk factor for Legg-Perthes disease. An increase in the risk of Legg-Perthes disease with decreasing levels of protein C and protein S was observed. Our findings with respect to APC-R and the factor V Arg506-⬎Gln mutation are less striking, but nonetheless consistent, with the notion that thrombophilia is a risk factor for Legg-Perthes dis-ease. Furthermore, if 2 of the 3 results were at least 1
Fig 1. Distribution of standard deviations of protein C in cases and controls.
TABLE 1. Distribution of Cases and Controls and the ORs According to an Ordinal Classification of Protein C and Protein S Standard Deviation
Below Mean‡
Protein C* Protein S†
Case Control OR 95% Confidence Interval
Case Control OR 95% Confidence Interval
Normal 37 47 1.0 Referent 39 47 1.0 Referent
Low normal 11 5 2.8 0.8–11.1 10 6 2.0 0.6–7.3
Low 3 1 3.8 0.3–204 2 0 ⬁ 0.2–⬁
Total 51 53 51 53
Pvalue for trend⫽.04 Pvalue for trend⫽.06
* Protein C levels are adjusted for age.
† Protein S levels are adjusted for age and gender.
standard deviation below the mean, the odds of hav-ing Legg-Perthes disease is increased by about 13-fold. These findings suggest that the extent or sever-ity of the thrombophilic state is the relevant factor in the cause of Legg-Perthes disease and not the specific levels of protein C or S, per se.
Our protein C, protein S, and factor V mutation findings are consistent with those of Glueck,9,10
al-though much less striking. For example, Glueck10
found functional APC-R in 36% of cases, but in only 4% of controls. Some of the differences in the mag-nitude of the associations between proteins C and S and APC-R and Legg-Perthes disease reported in this study and in the study by Glueck may be because of the use of different cutpoints to determine defi-ciency. Alternatively, Glueck10,11found 13% of
Legg-Perthes patients had the factor V mutation compared with only 1% of controls (OR of about 14). It is difficult to reconcile this finding with ours (OR of 1.8) in terms of different cutpoints. Their OR may be too high because their control series, but not their cases, included some persons of black race. The prev-alence of the factor V Arg506-⬎Gln mutation is⬃5%
in white populations8 and ⬍1% in black
popula-tions.16The number of blacks in their control group
was not specified. The inclusion of blacks among their controls, but not cases, partially accounts for the low prevalence of the factor V mutation in their controls and their very large OR. We cannot explain why findings from our study and that of Glueck should differ so drastically in magnitude with re-spect to the clotting proteins and APC-R. Nonethe-less, we consider that, in general, our findings sup-port the observations of Glueck and, taken together, the studies provide strong evidence that clotting ab-normalities are a risk factor for Legg-Perthes disease. This study has some limitations. Because protein C and protein S levels are known to be lower in infants than in adults,11and because it is uncertain at what
age adult levels are attained, we could not use abso-lute cutpoints based on adult values to determine deficiency. Our study found that both protein C and protein S levels increase with age and that protein S levels are generally higher in males than females. We used our control group to obtain age-specific mean values for protein C and age- and gender-specific
Fig 2. Distribution of standard deviations of protein S in cases and controls.
TABLE 2. Distribution of Cases and Controls and the ORs According to an Ordinal Classification of the APC-R Functional Assay and the Presence of the Factor V Arg506 –⬎Gln Defect
Cases Controls OR 95% Confidence Interval
APCR plasma test–standard deviation below mean*
Normal 42 47 1.0 Referent
Low normal 5 5 1.1 0.2–5.2
Low 3 1 3.4 0.3–180
Total 50 53
Pvalue for trend⫽.35 Factor V defect
No 50 53 1.0 Referent
Yes 5 3 1.8 0.4–7.7
Total 55 56
mean values for protein S. Although this method is useful for case/control comparisons, the cutpoints for low normal and low may not have clinical signif-icance because levels as low as 30% of adult values have been shown to provide normal hemostasis in children17–19 Another limitation of the study is that
protein S, protein C, and plasma APC-R were mea-sured after the onset of the disease, in some cases by many years. We assume that in most situations, age normalized protein C and protein S levels would vary little with time. However, transient reduced levels of protein C may be brought on by infection or injury.20 Although we ascertained that all
partici-pants were free of clinical infection or injury at en-rollment into the study, we may have missed sub-clinical events. Also, participants who were normal for protein C and S at the time of this study may have previously experienced prolonged transient defi-ciency before the onset of Legg-Perthes disease. However, we emphasize that such misclassification in the present or in the past would attenuate the relation between these clotting factors and Legg-Per-thes disease and, thus, would not explain the associ-ations that we found. An added concern about the retrospective nature of the study is the possibility that the disease alters the coagulation system. In this situation, lowered protein C and protein S would be an outcome rather than a cause of the disease. We are not aware of any studies suggesting this
circum-stance as a possibility. Finally, an obvious limitation of the study is its small size and resultant impreci-sion.
Viral infections can induce acquired thrombophilia by stimulating antiphospholipid antibodies, termed the lupus anticoagulant. Thromboembolism and pur-pura fulminans were noted to occur after varicella infections and were associated with lupus anticoag-ulant formation and acquired protein S deficien-cy.21–23 Phospholipids are essential in the
deactiva-tion of factor V by APC. The lupus anticoagulant, an IgM antibody, seems to neutralize the effect of phos-pholipids in this reaction,24which would likely cause
acquired APC-R. Thus, a child who has no innate protein C or protein S deficiency or APC-R, could, after an infection, be transiently thrombophilic from the dysfunction of these systems. Similar antibodies have been noted after some viral pneumonia, viral hepatitis, measles, and scarlet fever.25Also, in
Legg-Perthes disease, the thrombotic event may be sepa-rated by several months from clinical presentation. The disease process in some Legg-Perthes patients may be attributable to transient decreases in protein C or protein S levels, which could make conditions right for thrombosis, but later resolve, causing a false-negative coagulation test.
Testing for coagulopathy in children with Legg-Perthes disease is a dilemma for the clinician. Many local laboratories lack the capability to perform
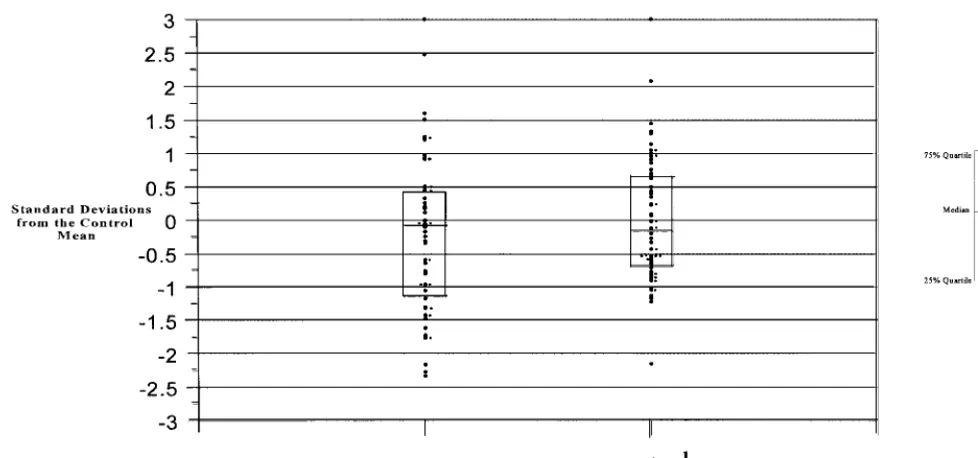
test-Fig 3. Distribution of standard deviations of APC-R functional assay in cases and controls.
TABLE 3. Distribution of Cases and Controls and the ORs According to the Total Number of Low Normal or Low Test Results
Number of Test Results (Protein C, Protein S, APCR Functional Test)⬎1
Standard Deviation Below the Expected Mean
Cases Controls OR 95% Confidence Interval
All results normal 25 36 1.0 Referent
1 result at least low normal 16 16 1.4 0.6–3.4 2 results at least low normal 9 1 13.0 2.2–75
Total 50 53
ing for protein C, protein S, or APC-R, and transport to regional centers requires careful packaging and handling. This study showed that age-based normal values are critical to identifying coagulopathy in chil-dren and suggests that the standard adult values may lead to false-positive results. However, Legg-Perthes disease may be the first sign of familial co-agulopathies that eventually result in deep venous thrombosis and stroke in family members. Identifi-cation of children with thrombophilia may result in prevention of future health problems in themselves and their family members. In the future, identifica-tion of genetic defects in the coagulaidentifica-tion system may identify children at risk from bacterial, viral, or en-vironmental challenges, perhaps reducing the inci-dence and morbidity or Legg-Perthes disease.
CONCLUSION
Low plasma levels of protein C, protein S, and/or APC-R seem to increase the risk of Legg-Perthes disease. APC-R is a common hereditary cause of thrombosis in adults, and it may play a role in the cause of Legg-Perthes disease. However, more data in the form of larger, prospective studies is needed before definitive conclusions can be drawn and rec-ommendations for testing made.
REFERENCES
1. Weinstein SL. Perthes disease.Curr Orthop. 1988;2:181–188
2. Sanchis M, Freedman MAR, Zahir A. Experimental stimulation of the blood supply to the capital epiphysis in the puppy.J Bone Joint Surg. 1973;55A:335
3. Kolodziej M, Comp PC. Hypercoagulable states due to natural antico-agulant deficiencies. In: Anderson JW, ed.Current Opinion in Hematol-ogy. 1993:301–307
4. Dahlback B, Carlsson M, Svensson PJ. Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagu-lant response to activated protein C: prediction of a cofactor to activated protein C.Proc Natl Acad Sci U S A. 1993;90:1004
5. Bertina RM, Koelman BPC, Koster T, et al. Mutation in blood coagula-tion factor V associated with resistance to activated protein C.Nature. 1994;369:64 – 67
6. Koster T, Rosendaal FR, de Ronder H, Brier E. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Throm-bophilia Study.Lancet. 1993;342:1503–1506
7. Svensson PJ, Dahlback B. Resistance to activated protein C as a basis for
venous thrombosis.N Engl J Med. 1994;330:517–522
8. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men.N Engl J Med. 1995;332:912–917
9. Glueck CJ, Crawford A, Roy D, Freiberg R, Glueck H, Stroop D. Asso-ciation of antithrombotic factor deficiencies and hypofibrinolysis with Legg-Perthes disease.J Bone Joint Surg. 1996;78:3–13
10. Glueck CJ, Brandt G, Gruppo R, et al. Resistance to Activated Protein C and Legg-Perthes Disease.Clin Orthop.1997;338:139 –152
11. Glueck CJ, Glueck HI, Greenfield D, et al. Protein C and S deficiency, thrombophilia, and hypofibrinolysis: pathophysiologic causes of Legg-Perthes disease.Pediatr Res. 1994;35:383–388
12. Andrew MB, Paes R, Milner M, Johnston L, Mitchell DM, Tollefen P. Development of the human coagulation system in the full term infant. Blood. 1987;70:165–172
13. Kleinbaum DG, Kupper LL, Muller KE.Applied Regression Analysis and Other Multivariate Methods. Boston, MA: PWS-KENT Publishing Company; 1988
14. Rothman KJ. Modern Epidemiology. Boston, MA: Little, Brown and Company; 1986
15. Mantel N. Chi-square tests with one degree of freedom: extensions of the Mantel-Haenszel procedure.J Am Stat Assoc. 1963;58:690 –700 16. Hooper WC, Dilley A, Ribiero MJ, et al. A racial difference in the
prevalence of the Arg506-⬎Gln mutation.Thromb Res. 1996;81:577–581 17. Griffin JH, Evatt B, Zimmerman TS, Kleiss AJ, Wideman C. Deficiency of protein C in congenital thrombotic disease.J Clin Invest. 1981;68: 1370 –1373
18. Griffin JH, Evatt B, Wideman C, Ferandez JA. Anticoagulant protein C pathway defective in majority of thrombophilic patients.Blood. 1993;82: 1989 –1993
19. Broekmans AW, Veltkamp JJ, Bertina RM. Congenital protein C defi-ciency and venous thromboembolism: a study of three Dutch families. N Engl J Med. 1983;309:340 –344
20. Mannucci PM, Vigano S. Deficiencies of protein C, an inhibitor of blood coagulation.Lancet. 1982;2:463
21. Manco-Johnson MJ, Nuss R, Key N, et al. Lupus anticoagulant and protein S deficiency in children with post-varicella purpura fulminans or thrombosis.J Pediatr. 1996;128:319 –323
22. D’angelo A, Della Valle P, Crippa L, Pattarini, E, Grimaldi LME, D’angela SV. Brief report: autoimmune protein S deficiency in a boy with severe thromboembolic disease.N Engl J Med. 1993;328:1753–1757 23. Levin M, Eley BS, Louis J, Cohen H, Young L, Heyderman RS. Post-infectious purpura fulminans caused by an autoantibody directed against protein S [review]. J Pediatr. 1995;127:355–363
24. Freyssinet JM, Wiessel ML, Gauchy J, Boneu B, Cazenave JP. An IgM lupus anticoagulant that neutralizes the enhancing effect of phospho-lipids on purified endothelial thrombomodulin activity—a mechanism for thrombosis.Thromb Haemost. 1986;55:309 –313
25. Vaarala O, Palosou T, Kleemola M, Aho K. Anticardiolipin response in acute infections.Clin Immunol Immunopathol. 1986;41:8 –15
DEPLORED AND DESPISED
Most Americans die horribly—and painfully. More than 80% die in hospitals or nursing homes far from their loved ones. Many die wracked by pain, unable to get the relief they need. It is, says a guru of dying well, Nicholas Christakis, a situation where Americans experience “deaths they deplore in situations they despise.”
Lagnado L. . . . Die.Wall Street Journal.November 11, 2000
DOI: 10.1542/peds.107.6.1329
2001;107;1329
Pediatrics
Doris, W. Craig Hooper, Peter L. Meehan and Bruce Evatt
John Eldridge, Anne Dilley, Harland Austin, Muhydine EL-Jamil, Lori Wolstein, John
Legg-Perthes Disease
The Role of Protein C, Protein S, and Resistance to Activated Protein C in
Services
Updated Information &
http://pediatrics.aappublications.org/content/107/6/1329 including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/107/6/1329#BIBL This article cites 22 articles, 3 of which you can access for free at:
Subspecialty Collections
skeletal_disorders_sub
http://www.aappublications.org/cgi/collection/rheumatology:musculo
Rheumatology/Musculoskeletal Disorders
following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
DOI: 10.1542/peds.107.6.1329
2001;107;1329
Pediatrics
Doris, W. Craig Hooper, Peter L. Meehan and Bruce Evatt
John Eldridge, Anne Dilley, Harland Austin, Muhydine EL-Jamil, Lori Wolstein, John
Legg-Perthes Disease
The Role of Protein C, Protein S, and Resistance to Activated Protein C in
http://pediatrics.aappublications.org/content/107/6/1329
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.