Keywords
Highlights
Abstract
Graphical abstract
120
Review Paper
Received 2016-05-10 Revised 2016-11-14 Accepted 2016-11-24 Available online 2016-11-24
Membrane separations
Biorefinery
Bioproducts
Bioenergy
Membrane fouling
Membrane technology
• Membrane technologies for value-added chemicals recovery and bioenergy production in IFBR are reviewed
• Challenges and opportunities of membrane technologies in IFBR are discussed • Membrane fouling and its control in IFBR are highlighted
A Review of Membrane Technologies for Integrated Forest Biorefinery
1 Department of Chemical Engineering, Lakehead University, 955 Oliver Road, Thunder Bay, ON, Canada P7B 5E1 2 College of Geography and Environmental Sciences, Zhejiang Normal University, Jinhua, 321004, PR China
A. Bokhary
1, L. Cui
1, H.J. Lin
2, B.Q. Liao
1,*Article info
© 2017 MPRL. All rights reserved.
* Corresponding author at: Phone/fax: + (807) 343-8437 E-mail address: bliao@lakeheadu.ca (B. Liao)
DOI: 10.22079/jmsr.2016.22839 Contents
1. Introduction………...………....……….121
2. Membrane separation processes for IFBR………...………..121
3. Recovery of value-added chemicals in IFBR………....………..……….……..122
3.1.Hemicelluloses recovery…………...………...122
3.1.1. Microfiltration (MF)………...…….……….………122
3.1.2. Ultrafiltration (UF)………....……….………123
3.1.3. Nanofiltration (NF)………....……….………...124
Journal of Membrane Science & Research
journal homepage: www.msrjournal.com
More recently, the concept of integrated forest biorefinery (IFBR) has received much attention as a promising solution for the struggling forest industry in North America and Europe to overcome its difficult financial period and competes globally. This new business paradigm offers a broad range of potentially attractive products, from bioenergy to value-added green organic chemicals in addition to traditional pulp and paper products. However, it also implies adoption of different types of appropriate separation technologies. Recent advancements in membrane technologies and their valuable applications have resulted in numerous breakthroughs in IFBR. The review of the implementation of membrane technologies for the separation of the value-added chemicals in the integrated forest biorefinery could contribute to the knowledge required for the large-scale adoption of membrane technologies in the forest industry. This paper aims to present a state-of-the-art review on the applications and the recent advancements of membrane technologies in IFBR, and their capacities to produce value-added chemicals and bioenergy. The emphasis is given to the focus areas of IFBR, particularly: the recovery of value-added chemicals, black liquor concentration, product recovery from Kraft evaporator condensates, tall oil recovery, inorganic and inorganic compounds recovery, fermentation inhibitors removal, enzyme recovery, biobutanol and bioethanol production and recovery. The paper also discusses the challenges and opportunities of this new business paradigm of forest industries.
A. Bokhary et al. / Journal of Membrane Science and Research 3 (2017) 120-141
3.1.4. Diafiltration (DF)……….………...……….…………125
3.2. Lignin recovery………...……….……….……….……125
3.3. Wood extractives recovery………..……….….…127
4. Black liquor concentration……….. ………..128
5. Products recovery from Kraft evaporator condensates……….….…...128
5.1. Methanol and TRS recovery……….………...……….………128
5.2. Turpentine removal………...………..….…….…129
6. Tall oil recovery………..…..…129
7. Inorganic and organic compounds recovery……….……..…..…129
7.1. Inorganic compounds recovery………...……….….………129
7.2. Organic compounds recovery………..…….….…129
7.2.1. Organic acids recovery……….………...………129
7.2.2. Xylitol recovery……….……….….……129
8. Second generation biofuel production………..………130
8.1. Bioethanol production and recovery………...……….….………130
8.1.1. Fermentation inhibitors removal……….……….……..….…130
8.1.2. Enzyme recovery……….……….……..….…131
8.1.3. Membrane bioreactor (MBR) for bioethanol production……….…...…131
8.1.4. Bioethanol recovery and dehydration……….….…132
8.1.4.1. Membrane distillation (MD)……….……….………….……....132
8.1.4.2. Pervaporation (PV)………..…………..……….………132
8.2. Biobutanol recovery………...……….……..133
8.2.1. Pervaporation (PV)……….…….…133
8.2.2. Reverse osmosis (RO)………...……….……….134
9. Challenges and opportunities………..…..134
9.1. Challenges………...………..….134
9.1.1. Membrane-related challenges...……….134
9.1.2. IFBR-related challenges with corresponding applied solutions……….…135
9.2. Opportunities………...……….….136
10. Conclusions……….………138
References……….………..138
1. Introduction
Forests and forest products industries are one of the most important industries in North America and Europe. However, more recently they are facing reduced profit margins and a globally competitive market because of the new shift from newsprint to electronic media, increasing competition from low-cost countries, restricted environmental laws, and high energy, and water consumption. These issues have resulted in plant closures, layoffs, and mergers. Accordingly, pulp and paper companies have begun looking for new opportunities to improve revenue streams and profitability. Therefore, the notion of repurposing pulp and paper mills into an integrated forest biorefinery (IFBR) was created [1,2]. Implementing IFBR would improve the profitability and competitiveness of a stagnant pulp and paper industry by diversifying the industry’s products and generating new revenues.
The biorefinery concept is similar to today’s petroleum refinery, which generates different fuels and oil products. However, compared to the oil refinery, biorefinery products are environmentally friendly (non-toxic, biodegradable, reusable, and recyclable). Biorefining, defined by the International Energy Agency (Biorefinery, task 42), is the sustainable processing of biomass into a spectrum of marketable food & feed, products, and energy [3]. Biorefinery processes diverse bio-based feedstocks into a wide spectrum of products and biofuels.
IFBR is precisely required to process forest biomass materials into a spectrum of biofuel and bioproducts, similar to the operation of conventional petroleum refineries while maintaining cellulosic fibers for pulp and paper production [4]. It intends to implement biorefinery units into an existing pulping mill called receptor Kraft. The IFBR has multiple production platforms (e.g., cellulose, hemicelluloses, lignin, and extractives) that can be used in an integrated manner for the manufacturing of a wide range of potentially attractive products, from biofuels to value-added chemicals, as summarized in Figure 1.
In implementing IFBR, highly developed separation technologies that are cost efficient and environmentally responsible, are needed. Recent advancements in membrane technologies and their practical applications have resulted in numerous breakthroughs in IFBR. Membrane technologies have the potential to be a promising avenue of research and innovation for such applications because of their capabilities to offer excellent fractionation and separation, short processing steps, reduced chemicals utilization and considerable energy saving [5].
This paper discusses the applications and the recent development of membrane technologies related to IFBR and their capacity to produce value-added chemicals from forest biomass and wastes. The emphasis is given to the focus areas of IFBR, particularly: the recovery of value-added chemicals,
black liquor concentration, product recovery from Kraft evaporator condensates, tall oil recovery, inorganic and organic compounds recovery, fermentation inhibitors removal, enzyme recovery, and bioethanol and butanol production and recovery. This paper also discusses the advantages and limitations of membrane technologies for IFBR applications.
2. Membrane separation processes for IFBR
In the last few years, membrane filtration has been widely considered and implemented because it works simply and efficiently. Membrane separation processes allow the passage of one component more readily than the other because of the differences in physical and/or chemical characteristics of the membrane and the permeating components. Most membranes fall into one of two broad categories: microporous membranes and solution-diffusion membranes (as illustrated in Table 1). Membrane processes can be classified further as symmetrical or asymmetrical membranes.
Membrane separation processes, such as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverses osmosis (RO), pervaporation (PV), membrane distillation (MD), and diafiltration (DF) have special values in bioenergy and biomaterials production [5]. MF process is of great interest in the solid particles removal and large molecules separation. It does not develop significant osmotic pressures like the other membrane processes. In the arena of IFBR, UF has been utilized to concentrate and purify hemicellulose solutions with different molecular weight distributions, whereas NF has been used for the lignin recovery from prehydrolysate solutions and black liquor (BL). PV and MD are commonly used for dehydration and separation of organic compounds mixtures, while DF is usually used as downstream processing step for product concentration and purification.
Polymeric membranes, which utilize polysulfone (PS), polyethersulfone (PES), polyvinylidene fluoride (PVDF), regenerated cellulose (RC), polytetrafluoroethylene (PTFE), and fluoropolymers (FP), are the dominant membranes used for hemicellulose recovery. Whereas, ceramic membranes are the primary membranes used for lignin recovery from BL due to BL harsh conditions. Many configurations of membrane modules, such as tubular, flat sheet/plate-and-frame, spiral wound, and hollow fiber, have been used in the forest biorefinery. In IFBR, membrane separation processes are applied as a means of separation, recovery, purification, and dehydration, although they also have many other applications beyond the scope of this review.
Fig. 1. Simplified chart for the potential products from integrated forest biorefinery.
Table 1
Comparison between the main membrane separations processes related to IFBR modified from [5].
Membrane Microfiltration (MF)
Ultrafiltration (UF)
Nanofiltration (NF)
Reverse Osmosis (RO)
Pervaporation (PV)
Membrane Distillation (MD)
Driving force Pressure < 2 bar
Pressure 1-10 bar
Pressure 5-35 bar
Pressure 15-150 bar
Partial vapor Pressure
Vapor pressure difference
Thickness (μm) 10-150 micron 150-250 micron 150 micron 150 micron ~0.1 to few (Top layer)
20–100
Separation principle Sieving mechanism Sieving mechanism Solution-diffusion Solution-diffusion Solution-diffusion Vapor–liquid equilibrium
Membrane structure Symmetric porous
Asymmetric porous
Composite Composite Homogeneous
or composite
Symmetric or asymmetric porous
Pore size (nm) ~50–10,000 ~1–100 < 2 0.1-1 Nonporous
(dense)
~200–1000
Module Configuration
Flat sheet/plate-and-frame, tubular, hollow fiber
Flat sheet/plate-and-frame, tubular, spiral wound, hollow fiber, capillary
Flat sheet/plate-and-frame, tubular, spiral wound
Flat sheet Tubular Spiral wound Hollow fiber
Flat sheet/plate-and-frame, tubular, hollow fiber
Flat sheet/plate-and-frame, tubular, capillary
Membrane material Polymeric, ceramic Polymeric, ceramic Polymeric Organic polymers (cellulosic & polyamide derivatives)
Polymeric, ceramic, organic–inorganic
Hydrophobic polymer
Applications Lignin and hemicelluloses recovery and enzyme recovery
Lignin and hemicelluloses recovery, enzyme recovery
Lignin and hemicelluloses recovery and fermentation inhibitors removal
Lignin and hemicelluloses recovery and fermentation inhibitors removal
Bioethanol recovery Bioethanol recovery
3. Recovery of value-added chemicals in IFBR
Lignocellulosic biomass consists of four major components: cellulose, hemicellulose, lignin, and extractives. Proportions of these components vary depending on the biomass materials [6]. The relative ratio of cellulose and lignin is one of the important factors determining the suitability of plant species for any processing program. Biorefinery takes advantage of these different components and their intermediates, therefore maximizing the values derived from them.
In pulp and paper mills, cellulose is mainly devoted to the production of pulp and paper instead of bioenergy and biochemical, while the fate of hemicelluloses and lignins are depolymerized, de-branched, and solubilized in the cooking liquor. These components represent about half of the woody material [5]. Hemicellulose and Lignin can be recovered as value-added materials. Reportedly, many applications exist for lignin and hemicellulose, ranging from upgraded biofuels to high-value specialized chemicals [7,8]. Thus, these amounts of hemicellulose and lignin can be better used to increase the revenue margin of pulp and paper industry, as summarized in Figure 1. 3.1. Hemicelluloses recovery
Hemicelluloses represent one of the most abundant renewable resources on the earth. They belong to a group of complex polysaccharides which are formed through biosynthetic paths different from that of cellulose. Hemicelluloses are commonly divided into four groups of structurally different oligomer types: xyloglucans, mannans, xylans, glucomannans, and β-(1→3, 1→4)-glucans [9]. The composition and structure of hemicellulose vary considerably depending on the plant species. The molecular weight distribution of hemicelluloses varies widely, depending on the raw materials, and hemicellulose has a much lower molecular weight than cellulose. Like
cellulose, hemicelluloses function as supporting material in the cell wall. During the pulping, hemicelluloses and lignin are extracted from wood chips and partially end up in the black or prehydrolysis liquor. The fate of the hemicellulose is traditionally, and unfortunately, limited to discharge in wastewater streams for aerobic/anaerobic biological treatment [10]. Preferably, it can be burned in the mill’s recovery boiler as BL to regenerate energy. Since the heat value that can be generated of hemicelluloses (13.6 MJ/kg) is nearly half of that of lignin ((26.9 MJ/kg)), using hemicelluloses as a fuel source is unjustifiable [2].
Recently, many applications for hemicellulosic materials have emerged, including the production of hydrogels [11] and the fabrication of oxygen barrier film for food packaging [12]. Also, it can be used as a source of sugar that can be fermented to ethanol [13] or utilized as an emulsion stabilizer in food and feed [14]. Figure 2 illustrates some uses of hemicellulose. Moreover, hemicelluloses are used as a raw material for the production of furfural, hydroxymethylfurfural (HMF), and lactic acid [15]. On the other hand, hemicelluloses oligomers are highly bioactive and can be used as growth hormones for trees.
A. Bokhary et al. / Journal of Membrane Science and Research 3 (2017) 120-141
UF, NF, and DF for hemicellulose recovery and purification.
3.1.1. Microfiltration (MF)
MF is a membrane separation process that can be used to separate particles or biological entities in the range of ~ 0.05 μm to 10.0 μm from fluids by passage through a microporous membrane filter. Many studies were conducted to examine the suitability of MF to remove particles and suspended matter from the process water originating from the pulp and paper industry, as summarized in Table 2. MF effectively removed all the suspended matter. However, hemicelluloses are partly retained.
Persson et al. [16] extracted hemicelluloses from process water of the production of Masonite using a method involving three steps: removal of high molecular species by MF, pre-concentration of hemicelluloses by UF, and reduction of the salts concentration and monosaccharides by DF. Their results show that MF could efficiently remove high molar mass compounds. The total dry solid content (TDS) was 0.6 wt% in the primary process water, 0.35 wt% in the permeate, and 5.2 wt% in the retentate. Andersson et al. [17]
confirmed that particles and suspended matters in the process water could be
removed by MF. In their study, turbidity decreased from 960 nephelometric turbidity units (NTU) in the process water to 4 NTU in the permeate. Moreover, Hasan et al. [18] reported that colloidal and particle removal, using MF, is feasible and can significantly reduce turbidity when they examined the separation of sugar maple wood extracts by ceramic membrane of two different pore sizes: 0.2 μm and 0.01 μm.
Krawczyk and Jönsson [14] investigated the influence of membrane pore size (0.2, 0.4 and 0.8μm), cross-flow velocity, and back pulsing on membrane performance during the MF of thermomechanical pulping (TMP) process water. The results revealed the challenges of recovering the pure water flux of the 0.4 and 0.8μm membranes. However, increasing cross-flow velocity and back pulsing had a positive effect on the flux, but only a minor influence on the retention. Also, MF followed by UF was found to be a suitable combination, providing a concentrated and purified hemicellulose fraction
[19,20]. MF has shown high capabilities to clarify the process water and successfully remove all suspended matters. However, there is a need for feasibility studies to evaluate the cost-effectiveness of currently applied MF in the separation and purification of hemicelluloses.
Hemi source
Hardwood
Softwood
Extraction
methods
Xyloglucans
Xylans
Mannans
Glucomannans
β - glucans
Separation &
purification
methods
Hydrogels
Barrier film in food packaging
Feedstock for xylitol production
Thickening agent in ice-cream formulation Stabilization of emulsion
and foams
Strengthening agent in paper
Applications Types of hemis
Mannans gum lower the blood and liver cholesterol Acid hydrolysis
Fermentation
Bioethanol
Acid hydrolysis
Distillation
Furfural Animals feed and nutrients
Biochemical routes
Fig. 2. Sources and types of hemicelluloses with some of their uses in industry.
Table 2
Summary of MF membrane process for hemicelluloses recovery.
Raw material
Characteristics of membrane Operating conditions Performance Reference
Pore size Material Configuration Scale VRc
Hemicellulose conc. (g/L)
Temp (°C)
TMP (bar)
Cross flow velocity
Flux (L·m−2·h−1)
Hemi rejection (%) Spruce softwood
process water
0.2, 0.4, 0.8 μm
Ceramic Tubular Lab
scale
– 1.04/0.05/0.12 80 0.5 4 m/s 60 - 350 >50 [14]
Sugar maple wood extracts
0.2 /0.01 μm Ceramic –b Lab
scale
– – 20 1.5-2 1.3, 2, 2.6
m/s
– – [18]
Spruce wood process water
0.2 μm Ceramic Tubular Lab
scale ~0.9 8
0.8 60 0.7 5m/s 60 - 260 80 [19]
Spruce wood process water
0.2 μm PTFEa flat
sheet/plate-and-frame
Lab scale
– 1-1.5 20
-25
4 8.5 L/min – – [21]
Spruce wood process water
0.2 μm Ceramic Tubular Lab
scale
0.98 1.16 80 0.7 4 m/s 80-380 55-90 [21]
a PTFE = polytetrafluoroethylene b Indicates value not reported or not available.
c Volume Reduction (VR) is the ratio between the volume of the permeate and the initial volume of the feed
3.1.2. Ultrafiltration (UF)
UF is a membrane process that operates on a physical sieving separation process. It is best used to retain macromolecules. The range of molecules sizes that can be retained is from 0.001 µm to 0.1 µm. The UF process requires low trans-membrane pressure to operate, and driving pressure is usually between 1 and 10 bars. Currently, the UF membrane is widely used for various applications because of its high throughput, low operation cost, excellent selectivity, and requires no chemicals additives, thereby minimizing
the extent of denaturation and degradation of biological products.
In the pulp and paper industry, UF membrane is an effective method for the treatment of pulp and paper effluent. It removes most of the polluting substances, consisting of high molecular mass compounds, efficiently [22]
and recycles valuable materials [13,21,23]. Koivula et al. [24] stated that recovery, purification, concentration, and fractionation of hemicelluloses from wood hydrolysates are most attractive characteristics of UF membrane.
membrane has been investigated intensively by many researchers [10,16, 17,20,21,25,26]. Table 3 summarized the results of recent studies. Persson et al. [21] investigated four filtrations and membrane filtration process – namely, drum filtration, MF, UF, and NF – to fractionate the process water from a TMP mill. The permeate from the MF stage was ultrafiltered to concentrate and purify the hemicelluloses. They concluded that UF recovered about 95% of the hemicelluloses. Persson and Jönsson, [25] isolated galactoglucomannan (GGM) from a TMP mill process water using UF membrane. They suggested that, to retain the hemicelluloses, a UF membrane with a molecular weight cut-off between 1 and 10 kDa should be used.
A comparable study was conducted by Al Manasrah et al. [27]. They recovered GGM from wood hydrolysate using regenerated cellulose UF membranes with different molecular weight cut-off values 5 kDa, 10 kDa, and 30 kDa. 5 kDa membrane achieved 88% GGM retention, 63% purity, and 70% recovery rate at a VR of 86%, whereas, cut-off values 10 and 30 kDa have partly separated GGM. However, Persson et al. [16,20] achieved 80% purity when separated hemicelluloses TMP process water using hydrophilic UF membranes. Another study on the recovery of hemicellulose was reported by Jun et al. [28], who extracted hemicellulose from aspen chips before kraft pulping utilizing kraft white liquor by UF. They were able to recover xylan at levels of up to 48 g kg-1 of dry chips.
Other studies compared UF with their counterparts. For example, Liu et al. [29] compared the performance of UF and NF for hemicelluloses concentration. The results of this study showed that NF gave much better rejection rates on organic compounds than UF. In the same way, Ajao et al.
[30] conducted experiments to screen and select suitable organic membranes among three membranes separation processes – RO, NF, and UF – to concentrate and detoxify Kraft prehydrolysate. The three membranes demonstrated high sugar retentions compared to inhibitor removal. They were, however, not effective for the removal of the phenolic compounds.
During hemicellulose isolation, several studies have proved that a hydrophobic membrane has a higher fouling tendency than a hydrophilic membrane [10,16,20,24], while most of the foulants, exhibited in the pulp and paper effluents, are of phenolic and hydrophobic nature. However, pretreatment methods, such as pH adjustment, ion-exchange resin, use of MF, and activated carbon (AC) adsorption had positive impacts on the filtration capability of UF membrane [24,29]. Accordingly, to avoid fouling, high operational cost, and membrane lifetime shortening, the most suitable membrane and pretreatment method should be applied. Also, from these results, it was demonstrated that effective pretreatment method not only decreases membrane fouling but also enhances the efficiency of membrane cleaning.
Table 3
Summary of UF membrane process for hemicelluloses recovery.
Raw material
Characteristics of membrane Operating conditions Performance Reference
MWCO (kDa)
Materiala
Configuration Scale VRc
Hemi conc. (g/L)
pH Temp. (°C)
TMP (bar)
Cross flow velocity
Flux (L·m−2·h−1)
Hemi rejection (%) Spruce wood
process water
5/10 PS/PS Spiral wound Lab
scale 0, 0.70/ 0.97
14/53. 5
– b 60 3/5 20/21.6
L/min
32 - 170 70–96 [19]
Spruce wood process water
5 PES Spiral wound Lab
scale
0.99 0.83 – 80 6 25
L/min
10-135 93-99 [21]
Birch hydrolysate and Spruce hydrolysate
5/10 PS/RC – Lab
scale
0.66 – 7 -
8
60/55 3/5.5 1.5m/s 3 – 55/30 – 179/ 20 - 190
61–76 [24]
Spruce wood process water
10 PVDF Spiral wound Lab
scale 0.75-0.99
0.5-0.9
– 60
0.5-2.5 20 L/min
20-160 72-94 [25]
Spruce wood process water
1 PVDF Spiral wound Lab
scale 0-0.95
0.5-0.9
– 60 4-10 10L/min <105 <90 [25]
Spruce wood process water
5 PS Spiral wound Lab
scale
– 0.7 – 75 2-6 20
L/min
40-90 <90 [25]
spruce sawdust extract liquor
30/10/5 RC flat
sheet/plate-and-frame
Lab scale
0.86 4.7 – 65 1/3/
3.5
2 m/s ~ 107 - 245 88 [27]
aspen wood chips extracted liquor
10 PS – Lab
scale
– – 13.9
8
70 - 90 3.1- 3.45
– – – [28]
a
RC = regenerated cellulose, PS = polysulfone, PES = polyethersulfone, PVDF = polyvinylidene fluoride. b
– indicates value not reported or not available.
c Volume Reduction (VR) is the ratio between the volume of the permeate and the initial volume of the feed.
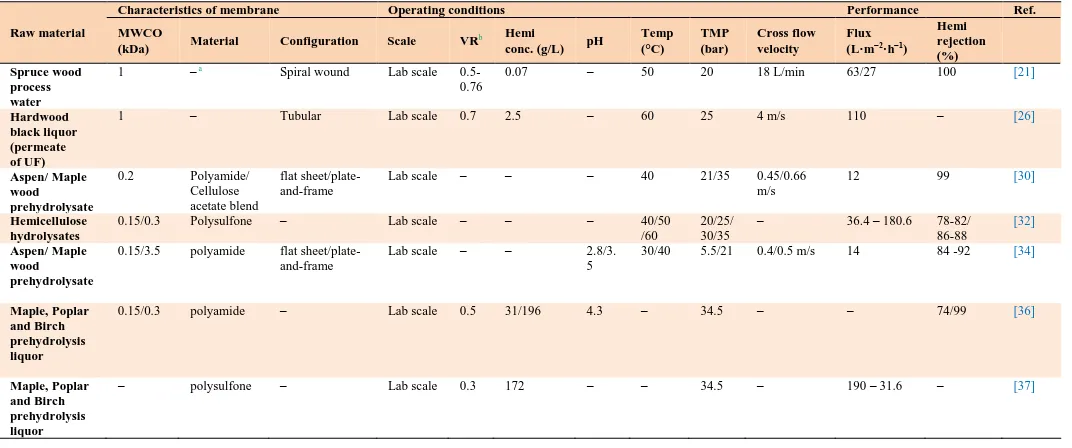
3.1.3. Nanofiltration (NF)
NF has been implemented effectively in a wide range of applications, such as the removal of organic contaminants from the aqueous solutions [31]
and partial fractionation and concentration of the sugars [32]. NF has been used widely for a long time, and while its industrial application has been limited to moderate pH for sometimes [33]. More recently, NF membranes for a broad range of pH have become commercially available.
In the forest industry, NF of effluents, from the alkaline extraction stage, has been investigated extensively using both polymeric and ceramic membranes [21,26,33]. Table 4 summarizes the results of recent studies. Schlesinger et al. [33] investigated the performance of five polymeric NF and tight UF membranes during the separation of hemicellulosic materials from process liquors containing 200 g/L sodium hydroxide. The experimental data showed that hemicellulose is almost quantitatively retained at molar masses above 1,000 g/mol. Also, Liu et al. [29] compared the performance of UF and NF membranes during hemicellulose concentration. Their results indicated that NF gave much better rejection rates on organic compounds than UF. Furthermore, Ajao et al. [34] evaluated the performance of six organic NF
membranes with different MWCOs and polymer materials to examine their ability to concentrate hemicellulosic sugars from prehydrolysate solution. 200 Da membrane was superior compared with others membranes’ cut-offs and achieved 99% sugars retention. Sjoman et al. [32] carried out another NF study on hemicelluloses recovery to recover d-xylose in the permeate from a hemicelluloses hydrolyzate stream. According to the results, NF showed a promising ability in recovering xylose from hemicellulose hydrolyzate permeate.
A. Bokhary et al. / Journal of Membrane Science and Research 3 (2017) 120-141
pressure compared with membranes with a low contact angle. High fouling was also observed in the filtrations made at an acidic pH solution, as compared to a neutral pH. Additionally, the retention of organic substances was significantly better at a neutral pH and the hydrophilicity of the membrane increased with the increase in pH.
NF has been used in a wide range of prehydrolysate solutions. It showed good performance regarding pure permeate product and low energy
consumption. However, the fouling phenomenon is one of the main shortcomings facing NF’s commercialization. Therefore, treatment of PHL, prior to the NF step, might be an effective way to improve filterability of the NF membrane process. Also, future research should include pilot scale experiments to enable a detailed economic analysis of the membrane system in a biorefinery process as few studies have examined the feasibility of using NF membranes for the filtration of paper mill process water.
Table 4
Summary of NF membrane process for hemicelluloses recovery.
Raw material
Characteristics of membrane Operating conditions Performance Ref.
MWCO
(kDa) Material Configuration Scale VR
b Hemi
conc. (g/L) pH
Temp (°C)
TMP (bar)
Cross flow velocity
Flux (L·m−2·h−1)
Hemi rejection (%) Spruce wood
process water
1 – a
Spiral wound Lab scale 0.5-0.76
0.07 – 50 20 18 L/min 63/27 100 [21]
Hardwood black liquor (permeate of UF)
1 – Tubular Lab scale 0.7 2.5 – 60 25 4 m/s 110 – [26]
Aspen/ Maple wood prehydrolysate
0.2 Polyamide/
Cellulose acetate blend
flat sheet/plate-and-frame
Lab scale – – – 40 21/35 0.45/0.66
m/s
12 99 [30]
Hemicellulose hydrolysates
0.15/0.3 Polysulfone – Lab scale – – – 40/50
/60
20/25/ 30/35
– 36.4 – 180.6 78-82/
86-88
[32]
Aspen/ Maple wood prehydrolysate
0.15/3.5 polyamide flat sheet/plate-and-frame
Lab scale – – 2.8/3.
5
30/40 5.5/21 0.4/0.5 m/s 14 84 -92 [34]
Maple, Poplar and Birch prehydrolysis liquor
0.15/0.3 polyamide – Lab scale 0.5 31/196 4.3 – 34.5 – – 74/99 [36]
Maple, Poplar and Birch prehydrolysis liquor
– polysulfone – Lab scale 0.3 172 – – 34.5 – 190 – 31.6 – [37]
a – indicates value not reported or not available.
b Volume Reduction (VR) is the ratio between the volume of the permeate and the initial volume of the feed.
3.1.4. Diafiltration (DF)
DF is a technique that selectively utilizes permeable (porous) materials to separate the components of solutions and suspensions based on their molecular size. Recent technological advances in membrane technologies and system design have created a new opportunity for efficient DF of various organic polymers and inorganic chemicals. However, the success of a DF for performing fractionation and concentration processes is largely associated with the selection of an appropriate membrane.
In general, DF is commonly used as downstream processing steps for product concentration and purification. For example, Andersson et al. [17]
used DF to recover hemicelluloses from process water of a TMP mill using spruce. They found that the purity of hemicellulose was increased from 57 to 77% after DF when they compared the UF retentate before and after DF. Also, González-Muñoz et al. [38] assessed the performance of DF as a method for purification of oligosaccharides obtained by auto-hydrolysis of Pinus pinaster wood. Continuous DF has been observed to result in an increased weight percent of substituted oligosaccharides, from 79.2% up to 94.7%. Further, the selective removal of monosaccharides rose from 4.3 up to 17.8 as a substituted oligosaccharides to monosaccharides ratio.
Al Manasrah et al. [27] also reported that DF can achieve a partial removal of xylan and a complete removal of monosaccharides from the GGM rich concentrate. González-Muñoz et al. [39] studied the fractionation of extracted hemicellulosic saccharides from Pinus pinaster wood by a multistep membrane process. The solution from the hydrothermal process was subjected to consecutive steps of DF using membranes of 0.3, 1, 3, 5, and 10 kDa. This work resulted in streams containing poly- and oligosaccharides (POHS) of different molecular mass distribution with decreased contents of monosaccharides.
The DF approach is suitable for obtaining a product with a high purity, depending on the molecules of interest and process conditions. Thus, selecting the most compatible membrane processes for overall effectiveness is important.
3.2. Lignin recovery
Lignin is a complex natural amorphous polymer. It acts as the essential glue that gives plants their structural integrity and holds the fibers of
polysaccharide together. The structure of lignin changes according to the biomass source and the isolation technique. In general, softwoods contain more lignin (25–35%) than hardwoods (20–25%) [40]. Lignin consists of three hydroxycinnamoyl alcohol monomers (C9), differing in their degree of methoxylation: p-coumaryl, coniferyl, and sinapyl alcohols [41]. The molecular weight of kraft lignin extracted from the black liquor range from 1000–2000 Da [8].
In the forest products industry, lignin is usually isolated from other components of wood chips (delignification) through the application of different extraction methods. These extraction methods result in various types of lignin with different characteristics (spent liquor), as showed in Figure 3. This pulping liquor cannot be utilized in pulp production because of its inferior quality, but is typically concentrated and then fired in a recovery boiler for the generation of steam and production of inorganic chemicals [42]. Compared to hemicellulose, lignin also offers several opportunities for IFBR to enhance their revenue streams and operation because it contributes as much as 30% of the weight and 40% of the energy content of lignocellulosic biomass. Besides being burned to produce energy in recovery boilers [43], lignin can also be used for different applications. For example, Kraft lignins have been utilized to produce binders, resins, carriers for fertilizers and pesticides, thermoplastic polymers, asphalt, lead storage batteries, and activated carbon [44]. Also, they can be used as a chelating agent for the removal of heavy metals from industrial wastewater, or as a dispersant in cement and gypsum blends [5]. Some examples of lignin applications in industry are shown in Figure 3.
compared to traditional separation methods.
Lignin source
Hardwood (Dicotyledono us/Angiosperm
lignin)
Softwood (Gymnosperm
lignin)
Delignification
Soda lignin Hydrolysis lignin
Kraft lignins
Organosolv lignin
Sulphite lignin
Separation & purification
Binders Carbon Fibers
Dispersants
Phenols Thermoplastic Activated carbon
Sorbents
Surfactants
Vanillin P-coumaryl
alcohol
Sinapyl alcohol Lignin compounds
Coniferyl alcohol
Types of lignin based on treatment
Chloro-lignins
Applications
Combustion Fermentation
Biofuel Energy
Asphalt emulsifier
Fig. 3. Sources and types of lignins with some of their applications in industry.
Membrane processes have been applied since the late sixties, but most of the applications have focused on the treatment of bleach plant effluent and fractionation of spent sulfite liquor [26]. More recently, much effort has been spent, and many in-depth investigations have been carried out, to reduce the organic load to the recovery boiler. Consequently, a wide range of pressure-driven liquid phase membrane separation processes, including MF, UF, NF, RO, or a combination of them, has been studied. UF and NF membranes were the most common types among them. This can be attributed to the size of the lignin molecules. UF and NF have been applied at both lab- and pilot-scale studies.
Many studies investigated the applications of ceramic UF membranes to isolate lignin from cooking liquors [8,26,46-48]. Table 5 summarizes the results of recent studies. Wallberg and Jonsson [46] and Toledano et al. [47]
investigated the performance of UF membranes for lignin isolation from black liquor (BL) solutions. It was found that UF membranes were an effective method to fractionate lignin. However, Holmqvist et al. [48] found that membrane flux for treating hardwood cooking liquor was much higher, and the retention was much lower compared to that for treating softwood liquor.
Likewise, some studies examined the use of polymeric UF membranes for lignin recovery [8,26]. The results of these studies indicated that the extraction of lignin from kraft cooking liquor, taken directly from a continuous digester without adjustment of pH and temperature, is possible. On the other hand, NF membranes were also applied to separate the dissolved lignin from pulp and paper mills BL. As shown in Table 5, both ceramic and polymeric NF membranes were studied. Arkell et al. [45] reported that polymeric NF membranes with a MWCO of 1 kDa had the best performance when compared to the other membranes.
Alternatively, several studies investigated hybrid membrane systems that apply combinations of UF with NF to treat BLs. Sjodahl et al. [49] and Dafinov et al. [50] reported that NF and UF were technically feasible ways to remove the organic materials from kraft pulping BLs and could achieve a higher flux in a batch process. Furthermore, Jonsson et al. [26] studied the performance of hybrid UF/NF and evaporation/UF processes for lignin extraction from BLs and cooking liquor. UF/NF process achieved a higher product concentration and purity compared to evaporation/UF process. Most of these studies have reported that there are certain ranges of the molecular masses that have a negative impact on filtration capacity. It is recommended that, to decrease membrane fouling, the focus should be not only on the highest removal of lignin and hemicellulose, but also on the sizes of hemicelluloses and lignin molecules.
Polymeric and Ceramic membranes for lignin recovery and purification
were the focus of the majority of the reported studies. Ceramic membranes have been shown to possess better performance over polymeric membranes
[45]. Compared to polymeric membranes, ceramic membranes can withstand BLs without pH and temperature adjustment because of their high thermal and chemical stability. Moreover, ceramic membranes are slower to foul and can be recovered utilizing more extreme membrane performance recovery systems that polymeric membranes are not able to handle. Although the ceramic membranes exhibit a higher capital cost compared to polymeric membranes, they are capable of achieving a high productivity as a result of their inherent hydrophilicity, which leads to reduced organic fouling.
The performance of membrane technologies also depends on the application of appropriate module design and configurations. There are two main types of modules used in BL treatment: plate and frame module and tubular module. The plate and frame module is the simplest configuration, comprising two end plates, a flat sheet membrane, and spacers; whereas in the tubular module configuration, the membrane is often cast on the inside of a tubular support, the feed will be pumped through the tube, and the permeate will be collected on the outer surface of the tube into a shell which could hold one or many tubes packed in a bundle.
The feasibility of membrane technology for lignin recovery from BL has been widely studied. Jonsson et al. [26] studied UF and a hybrid UF/NF process of cooking liquor and BL. The production cost of UF of extracted BL and the hybrid UF/NF process was € 33 per ton of lignin produced. Holmqvist et al. [48] conducted a cost estimate of lignin fuel extraction from BL. The cost was approximately € 20 per MWh of the calorific value of the lignin fuel. Also, Jönsson and Wallberg [51] estimated recovery costs of hardwood lignin by a ceramic UF membrane with a MWCO of 15 kDa from two process streams in a Kraft pulp mill processing 200 m3/h pulping liquor. Lignin was concentrated to 100 g/L and the estimated cost was about € 60 per ton of lignin. This cost could be further reduced if high membrane fluxes could be achieved.
A. Bokhary et al. / Journal of Membrane Science and Research 3 (2017) 120-141
MF
UF
Lignocellulosic
biomass
Hemicelluloses
Recovery
UF
P
ulping
Black liquor
concentration
Suspended solids
removal
Evaporation
Precipitation
NF
Lignin
NaOH recovery
Or Air flotation
Pulp
Fig. 4. Separation and recovery of hemicelluloses and lignins from product streams.
Table 5
Summary of UF and NF membrane processes for lignin recovery.
Raw
material Classification
Characteristics of membrane Operating conditions Performance
Ref. MWCO
(kDa)
Material Configuration Scale VRb Lignin
conc. (g/L)
pH Temp (°C)
TMP (bar)
Cross flow velocity
Flux
(L·m−2·h−1) Lignin Rejection (%)
Hardwood kraft black liquor (permeate of UF)
NF 1 –a Tubular Lab 0.7 54 – 60 25 4 m/s 110 80 [26]
Softwood kraft black liquor
UF 20 Al2O3/TiO2 Tubular – - 57.6 13 90 2 5 m/s – – [45]
Softwood kraft black liquor
NF 1 TiO2 Tubular – 0.85 252 13 90 2 - 20 4-2 m/s 159 ~78 [45]
Softwood kraft black liquor
NF 0.2/0.6/
1
– Tubular – 0.70 282 13 70 5 - 35 4-2 m/s 81 ~90 [45]
Hardwood black liquor
NF 1 ZrO2 – Lab – 48.8 – 25 2-6 1.5m/s 1-5 67.4 [49]
Black liquor NF 1 TiO2 Tubular Lab – – 12 30 3-7 2.1m/s 12-22 – [50]
Birch wood chips extract
UF 5 Polysulphone flat sheet Lab 0.66 2.2 –
4.4
– 60 5.5 1.5 m/s 15 - 120 – [52]
Woody spent liquor
NF/UF 1/3 TiO2 and
ZrO2
flat sheet/ Tubular
Lab – 16-24 – 25 5 2 m/s 30-139 75-94 [53]
Spruce wood pressurized hot water extract
UF 5/1 TiO2 and
ZrO3
– Pilot – – – 40 2.6/2.
2
1.7m/s – – [54]
Olive tree clippings Black liquor
UF 300/150/
15/5
Ceramic Tubular Lab – – – – – – – – [55]
Spent sulphite liquors (SSL)
UF 1/5/15 Ceramic
TiO2
– Pilot 0.78
/0.4 5/ 0.15
44.25 / 39.57/ 40.67
3.3 20 1.8 - 2 8 m/s 55.45/ 5.77/
6.00
45.67/ 65.38/ 41.69
[56]
a – indicates value not reported or not available.
b Volume Reduction (VR) is the ratio between the volume of the permeate and the initial volume of the feed
3.3. Wood extractives recovery
Extractives substances can be defined as lipophilic compounds that are soluble in various neutral organic solvents [57]. These compounds include simple sugars, turpentine, resin acids, fatty acids, waxes, and complex phenolic compounds, as shown in Table 6. Composition and content of these extractives are quite variable depending on the plant species [57]. Wood extractives are valuable compounds, which could be utilized as preservatives in food or raw materials for pharmaceutical and chemical industry [58]. Another new application for wood extractives is used as biocides. However, during the pulping processing, the majority of the wood extractives would be dissolved in the BL and either burnt to produce energy or discharged in wastewater.
Different methods have been investigated to separate wood extractives from BL. One of the possible alternatives for separation of wood extractives
is membrane separation processes [57]. Membrane-based techniques are known to offer a high level of purification coupled with a relatively low level of energy consumption. However, only a few studies were conducted for the use of the membrane technology in the removal of wood extractives, because membrane fouling hinders the wide adoption of this technology.
Leiviska et al. [60] characterized wastewater (influent and effluent) from integrated kraft pulp and paper mill by MF (8, 3, 0.45 and 0.22 µm) and UF (100, 50, 30 and 3 kDa) membranes, based on different size fractions. It was found that the sieved influent contained about 14.3 mg/L of wood extractives, of which 12.8 mg/L were resin and fatty acids and 1.5 mg/L sterols. The sieved effluent contained 1.7 mg/L of wood extractives, of which 1.45 mg/L were resin and fatty acids and 0.26 mg/L sterols. Leiviska et al. [60] also reported that MF with a large pore size (8 µm) was able to remove 30–50% of wood extractives.
Table 6
Classification of the common wood extractives (Compiled from [57, 59]).
Types Components Types Components
Lipid extractive components Aliphatics and alicyclic: Fatty acids
Carbohydrates Arabinose
Fats (fatty acid esters of glycerol) Galactose
Fatty alcohols Glucose
Waxes (esters of other alcohols) Xylose
Suberin (polyester) Raffinose
Terpenoids:
Monoterpenes (turpentine)
Starch
Diterpenes Pectic material
Triterpenes
(including resin acids and steroids)
Phenolic extractive components Simple Phenolics Other compounds Cyclitols
Stilbenes Tropolones
Flavonoids Amino acids
Isoflavones Alkaloids
Condensed tannins Coumarins
Hydrolyzable tannins Quinones
Lignans protein
In another study, Puro et al. [58] achieved 90% extractives retention from two chemo thermomechanical (CTMP) pulp mill process waters using one regenerated cellulose (RC) UF membrane and two polyethersulphones (PES) UF membranes. Kilulya et al. [61] revealed that the utilization of an ionic liquid, in the extraction of lipophilic wood extractives, coupled with liquid membrane extraction systems, offered a possible separation method. Pinto
[62] tested different NF membranes and UF membranes with a lower molecular weight cut-off for the concentration of polyphenolic compounds (gallic, tannic acids and maltose) and carbohydrate from an ethanolic extract of Eucalyptus globulus bark. All the tested membranes exhibited selective retention of polyphenolic compounds.
4. Black liquor concentration
Kraft pulp mills have utilized BLs as an energy source since the 1930s. Weak black liquor (WBL) is typically 15% dry solids and 85% water. It is usually concentrated to 65 - 80% by multi-effect evaporators and burned in a recovery boiler for the energy production. This technique consumes much energy during the evaporation of the water in the large steam-heated multiple-effect evaporators. Membrane filtration has been proposed as possible techniques to reduce this amount of water prior to evaporation. Pre-concentration of BL streams by membrane processes reduces power consumption rate in the evaporation stages.
However, unlike conventional membrane separation processes for water purification/desalination applications – the membranes required for concentrating BL must withstand the high pH (>12), high temperature (80-90°C), and different fouling species in BL. Technically, ceramic membranes appear to be an appropriate choice for this task. Liu et al. [63] studied BL concentration conditions using UF ceramic membrane technology. They concluded that BL could be concentrated up to 4-6 times by UF membrane. However, ceramic membranes are characterized by higher capital costs. In contrast, polymeric membranes are available at reasonable prices, but without pretreatment, are not withstand the harsh natures of the BL. To overcome these problem, the U.S. Department of Energy developed sacrificial protective coating materials that can be regenerated in-situ to enable high-performance membranes. They believe that this layer will prevent foulants in the WBL from adhering to the membranes, while also giving protection from BL harsh conditions [64]. Also, New Logic International developed “Vibratory Shear Enhanced Process” (VSEP™) for the treatment of BL streams without causing the fouling problems exhibited by conventional membrane systems. VSEP can be used with polymer MC, UF, NF and RO membranes [65]. This technology applies vibration technique to reduce fouling, and it has been studied in the pulp and paper industry for applications in black liquor concentration, paper mill effluent, paper coating effluent, whitewater treatment, and paperboard mill effluent [65].
5. Products recovery from Kraft evaporator condensates
5.1. Methanol and TRS recovery
Kraft pulping condensates are rich in methanol and totally reduced sulfur (TRS) compounds. Kraft pulp mills have the potential to produce around 15 kg of bio-methanol per ton of pulp from their evaporator condensates, depending on the type of wood and the pulping conditions [66]. During the BL evaporation to an adequate level of dry solids suitable for the combustion in the recovery boiler or a dedicated incinerator, the liquor releases volatile materials, such as sulfur compounds, terpenes, and methanol. These volatile compounds must be removed, either because they have economic value or because they cause pollution problems if discharged directly to the environment. For instance, recovery of methanol would become beneficial by reducing the cost of purchasing methanol that pulp mills use in the production of chlorine dioxide or as a reducing agent in the manufacture of bleach. Furthermore, TRS compounds can be utilized effectively as a reducing agent during the manufacturing of chlorine dioxide as well [67,68].
Many technologies have been suggested, evaluated, and applied for methanol and TRS recovery. These methods are based either on desorption or decomposition and are associated with high capital and operating costs. Currently, the majority of kraft pulp mills are using the air stripping technique to separate TRS compounds or the steam stripping method to isolate both methanol and TRS from evaporator condensates. Air stripping needs tall and costly columns and may cause operational problems in the form of foaming, flooding, or channeling. Steam stripping is characterized by high capital and operating costs because of living steam utilization in most cases. Among the advanced treatment processes, membrane technology has been also tested as shown below. Table 7 summarizes the patent information of membrane technology for methanol and TRS recovery. Jemaa et al. [67], Blume and Baker [69], and Savage and Piroozmand [70] developed methods and apparatuses for the treatment of kraft pulp mills’ condensate for TRS and methanol removal using membrane technology.
A. Bokhary et al. / Journal of Membrane Science and Research 3 (2017) 120-141
Table 7
Characteristics of membranes applied for methanol and TRS compounds recovery from black liquor condensate.
Membrane US Pat. No. 8,349,130 B2[67] US Pat. No. 4,952,751 [68] US Pat. No. 6,110,376[70]
Membrane type Hollow fiber contactors Pervaporation (PV) Reverse osmosis (RO)
Driving force Vapor pressure difference Partial vapor
Pressure 0.27 bar
Pressure 27.57 bar
Thickness (μm) – 1 micron 5 micron
Module configuration hollow fiber spiral-wound Spiral-wound
Membrane material polypropylene Silicone rubber polyamide
Separation principle Distribution coefficient Solution-diffusion Solution-diffusion
5.2. Turpentine removal
In the kraft process, turpentine is an important by-product which can be recovered from evaporator condensate as a saleable product or to burn as a fuel. It is also an inhibitor compound that can impede biofuel processing efficiency [71]. Many processes are known for the removal of terpenes, steam or air stripping is the technology commonly used to treat this condensate. Among these methods, membrane filtration process was also applied for treating kraft evaporator condensate. Minami et al. [71] studied the thermophilic anaerobic digestion of evaporator condensate obtained from a Kraft pulp mill production process. They examined a microfiltration (MF) membrane made of Teflon for terpene oils removal. Teflon membrane was selected because of its resistibility. The MF membrane was an effective method for the removal of terpene oil and the authors achieved about 90% removal of these oily substances. Alsuliman [72] examined the performance of Membrane bioreactors in the treatment of real kraft pulp mill evaporator condensate under different operational conditions (high temperature, short solids retention time, and low hydraulic retention time) and turpentine shock loads. The results of this study indicated that the biological removal of the main contaminants in evaporator condensate was feasible. However, turpentine shock loads exerted a significant impact on the overall removal kinetics of the main contaminants in evaporator condensate.
6. Tall oil recovery
In the pulping process, particularly kraft pulping of pine, spruce, and birch trees, tall oil soap is a major by-product. When the BL is concentrated and left to settle, the top layer of it is known as “tall oil soap." A typical composition of the tall oil is about 49% resin acids, 32% fatty acids, and 19% unsaponifiable [73]. Tall oil is a valuable by-product and can be recovered from spent BL and sold as a feedstock for special chemicals production [73]. Also, it can be toxic if discharged into the biological wastewater treatment system.
Therefore, the recovery of the tall oil is not only necessary to increase mill revenue streams, but also to decrease the toxicity of pulp mill effluents. A commonly used method for recovering the tall oil from concentrated liquor is flotation. However, through this process, a significant amount of tall oil may be lost as waste or, in best cases, burned for its heat value and not recovered for its much higher tall oil value. Membrane technology may contribute to the improvement of this method and reduce those losses. Under this context, Fremont [74] developed a process for enhancing the recovery of tall oil soap from BL. This process consists of two zones. In the first zone, the tall oil soap is collected on the surface of the BL and is skimmed from the tank. In the second zone, the remaining BL phase is subjected to a UF unit (Abcor HFJ and HFD membranes) with a MWCO of about 10kDa to concentrate the residual tall oil soap. This concentrate is recycled to the skim tank (first zone) to allow the tall oil soap to agglomerate. The tall oil soap is skimmed off while the permeate is disposed of. The author concluded that a continuous UF of the skim tank effluents could recover up to 55% more tall oil per ton of pine pulp produced.
7. Inorganic and organic compounds recovery
7.1. Inorganic compounds recovery
BL from the pulp and paper mills, referred to as “spent cooking liquor,” contains different compounds of degradation products of lignin and wood carbohydrates and numerous high-value inorganic chemicals which are used during the pulping process. These inorganic compounds can be recovered, and the active cooking chemicals (white liquor) can be reused in the cooking cycle. Many methods were investigated for the separation of inorganic
compounds from BL. Application of membrane processes is one such option which can recover inorganic chemicals and minimize the load on multi-stages evaporator system with no significant loss of inorganic compounds.
Mansour et al. [75] studied the separation of alkali from silica-rich BL with cellophane membrane dialysis. They concluded that less concentration and higher volume of the BL increase the efficiency of the dialysis. Also, a higher dialysis efficiency could be obtained at a higher temperature and shorter time. Moreover, the vibration of the BL during the dialysis process increased the effect of the surface area and reduced membrane fouling. In another study, the recovery of inorganic compounds, from the discharge water of the pulp and paper industry, was investigated by Mänttäri et al. [23]. In this study, the instantaneous chloride retention using a NF membrane varied between 26% and 10%. However, the retention of inorganic carbon was 60% to 70%. From these studies, it can be concluded that the membrane technology could be used to recover the alkaline chemicals from the process wastewater of pulp and paper mills.
7.2. Organic compounds recovery
7.2.1. Organic acids recovery
Spent Liquors from the kraft pulping process represent an unexploited source of various valuable organic compounds. The recovery of these organic compounds has rarely been studied for the reason that separating them from BL is challenging due to their low concentrations and the presence of inorganic compounds. Membrane technology has been introduced as a new approach for the separation and purification of these organic compounds.
Figure 5 shows a proposed process for carboxylic acids production from BL. Niemi et al. [76] studied the separation and fractionation of organic and inorganic compounds by membrane separation and crystallization under different operating conditions. Their outcomes demonstrated that a combination of membrane separation and crystallization is a productive strategy for recovering and purifying the valuable organic compounds in the BL. A similar study was carried out by Mänttäri et al. [77]. They studied the recovery and purification of organic acids from BL in two separation steps: UF for lignin removal from organic acids and NF for the organic acids purification after acidification. Their results indicated that UF, with a 1 kDa membrane, removed 75% of the lignin and that the total acid concentration in the permeate stream was 1.4 fold compared to the original BL. The purity of the organic acid from NF, after the acidification and cooling crystallization stages, was about 80% at the highest. Because of the Donnan exclusion and electro-neutrality principles, it was acid molecules permeated the membrane faster than water molecules. Also, Hellstén et al. [78] investigated the recovery and purification of hydroxyl acids from soda BL without neutralization, using UF, size-exclusion chromatography, ion-exchange, adsorption, and evaporation. They reported that a reduction of 99% in lignin content of the organic acid fraction was achieved and mixtures of hydroxyl acids at a high purity were produced. The typical purities of hydroxyl acids separated from softwood and hardwood black liquors were 81% and 63% on the mass basis, respectively.
7.2.2. Xylitol recovery
Xylitol is a natural sugar found in most plant materials. Currently, it can be commercially produced by either chemical reduction or microbial fermentation of xylose present in the spent sulfite liquor (SSL) and pre-hydrolysis liquor (PHL). Xylitol production is important because of its applicability as a diabetic sweetener (insulin is not needed to regulate its metabolism) [79]. According to Rafiqul and Mimi [80], the xylitol market is rising fast and is estimated to reach over US$ 340 million/year and is priced at US$ 6–7 per kg. However, xylitol recovery and purification is still the most challenge step in the xylitol production process.
of the potential energy savings and higher purity. Affleck [81] found that a 10 kDa molecular weight cutoff of polysulfone membrane is the most efficient membrane among 11 membranes tested for the separation and recovery of xylitol from a fermentation broth. Reportedly, the membrane allowed 82.2 to 90.3% of xylitol in the fermentation broth to pass through the membrane
while retaining 49.2 to 53.6% of impurities such as peptides and oligopeptides. The collected permeate from the 10 kDa membrane was crystallized and then analyzed by HPLC for xylitol and impurities. The results showed a high xylitol purity up to 90.3%.
Fig. 5. Proposed process for xylitol production from prehydrolysis liquor and carboxylic acids production from black liquor.
8. Second generation biofuel production
Recently, biofuel production from lignocellulosic biomass has received increased interest because of environmental pollution issues, depletion of global petroleum resources, and continuing price increase of crude oil. Production of the second-generation biofuels, based on forest biomass, could be beneficial to IFBR since it would add value to IFBR revenue streams as by-products. This review addresses the applications of membrane technologies for bioethanol and biobutanol production and recovery.
8.1. Bioethanol production and recovery
8.1.1. Fermentation inhibitors removal
The production of lignocellulosic-based biofuels is a challenging process because of the complicated nature of the raw materials. For this reason, harsh treatments, e.g., with chemicals and/or high temperatures, are needed to degrade lignocellulose to fermentable sugars. During the hydrolysis of these materials, a large group of compounds that are inhibitory to the fermentable microorganisms is formed or released [5]. In general, there are three main common types of fermentation inhibitors produced during biomass conversion: (1) weak acids (acetic, formic and levulinic acid), (2) furans (furfural and hydroxymethylfurfural (HMF)) (3) phenolic compounds (vanillin, phenol, and p-hydroxybenzoic acid). The types and concentrations of these inhibitors are determined by the pretreatment techniques, fermented feedstocks, and biomass sources [82].
The literature has applied and discussed a variety of detoxification methods for overcoming the inhibitors problems, including extraction, treatment with lime, zeolites adsorption, or using activated carbon [83,84]. However, most of these methods are accompanied by some disadvantages, such as high processing costs, the creation of additional waste, and/or losing sugars [83,84]. Thus, the development of effective pretreatment strategies to isolate these inhibitors from the forest biomass hydrolyzates is needed. In most cases, pretreatment optimization is the best way to prevent the formation of many of these compounds. Membrane separation technology seems a
capable candidate for this application. Accordingly, UF, NF, and RO processes have been examined for tackling these problems.
Han et al. [85] used adsorptive membranes and anion exchange resin for acetic acid removal from wood hydrolyzate. They concluded that membranes exhibited higher capacity and separation than ion exchange resins. Liu et al.
[86] reported that acetic acid, methanol, furfural, hydroxymethylfurfural, and formic acid could preferentially be removed to the permeates side if a NF membrane is used. Also, Choi et al. [87] and Weng et al. [88] tested NF membranes at different pH levels for acetic acid removal. They found that pH affects the separation performance of acetic acid and that the rejection rate of acetic acid is increased with increasing pH.
Qi et al. [89] investigated the removal of furfural by NF membranes. Furfural rejection rate decreased when the temperature and pH increased. However, permeation flux was increased for all membranes. Afonso [90]
assessed NF and RO for the concentration of acetic acid and furfural from the condensate of eucalyptus spent sulfite liquor. They reported that NF and RO demonstrated high retentions for both acetic acid and furfural. However, Zhou et al. [91] compared the NF and RO membranes’ performance for acetic acid separation under different operating conditions. The experimental results proved that RO membranes are much more efficient than NF membranes for retaining monosaccharides and decreasing the concentration of acetic acid. Different results were reported by Gautam and Menkhaus [92] when testing the efficiency of RO and NF for fermentation inhibitors removal. In this study, NF showed very promising results: sugars concentrated to more than 2.5 fold in the retentate and simultaneously separated more than 50% of the inhibitory components into permeate. Furthermore, Ajao et al. [30] studied the feasibility of NF membrane for a simultaneous concentration of acetic acid and sugars in the prehydrolysate solution from a Kraft dissolving pulp mill. The results of this study indicated that an NF membrane, with a MWCO of 200 Da, is capable of achieving about 99% sugar retention.
NF and RO membranes exhibited excellent efficiency in the removal of fermentation inhibitors and sugars concentration. However, the efficieny markedly depends on the operating conditions, such as feed concentration, feed pH, solute concentration, pressure, and temperature.
Black Liquor
BL
Lignocellulosic
biomass
Prehydrolysis
Liquor PHL
Steam or hot
water hydrolysis
Hydrolyzed
wood chips
Pulping
Acid or enzyme
hydrolysis
Detoxification
Xylose
hydrolysate
Fermentation
Membrane
Filtration
Hydroxy Acids
Size-exclusion
chromatography
UF/NF
Lignin recovery
Xylitol
NF
NaOH recovery
Adsorption
Purification oforganic acid fraction
Residual phenols removal
Inhibitors removal and pH adjustment





![Table 6 Classification of the common wood extractives (Compiled from [57, 59]).](https://thumb-us.123doks.com/thumbv2/123dok_us/8425196.1695647/9.595.89.522.84.305/table-classification-common-wood-extractives-compiled.webp)


