Evaluating the Success of Lake Trout Refuges
in the Great Lakes
by
Jennifer A. Kullgren
Advisors: Dr. Ed Rutherford Dr. Don Scavia Reader: Dr. Jim Diana
Program in the Environment Senior Thesis
Abstract
Lake trout rehabilitation efforts in the Great Lakes have developed as a result of
extirpated populations during the mid-20th century. Current lake trout restoration efforts of the lower four Great Lakes have been unsuccessful in producing self-sustaining populations and population size is considerably below historic harvest levels. Stocking efforts, control of sea lamprey, and protected species-based refuges have been used as management tools to improve population size and encourage natural recruitment. Four lake trout refuges have been created since 1985 to assist in the rehabilitation efforts of lake trout populations. I conducted a literature review and analyzed available data to evaluate the success of the Six Fathom Bank refuge in Lake Huron for restoring naturally reproducing populations of lake trout. Within the refuge, the major population bottleneck for lake trout natural reproduction appears to be predation of fry by invasive alewives. Poor quality of lake trout prey and predation of lake trout eggs also contribute to poor reproductive success. I used data on lake trout stocking, stage-specific survival rates, fecundity, and egg-to-predator ratios to estimate the minimum number of lake trout females needed for a successful population in Six Fathom Bank. Estimated numbers of females needed for successful reproduction are higher than currently exist, and range from approximately 122,000 lake trout at low egg predator densities and small amount (50 ha) of spawning habitat, to 1.5 million lake trout at high predator densities and a larger amount (300 ha) of spawning habitat. Managers concerned with restoring viable lake trout populations in Lake Huron should consider lowering sources of adult mortality, such as fishing and sea lamprey parasitism, to rebuild spawning stocks.
Table of Contents
1. Introduction... 4
1.1. Overview... 4
1.2. Lake Trout Refuges in the Great Lakes ... 5
1.3. Lake Trout Rehabilitation Stocking Efforts... 6
1.4. Current Status of Lake Trout Rehabilitation... 7
1.5. Current Threats to Lake Trout Rehabilitation... 9
1.6. Stakeholders... 10
1.7. Thesis Objectives ... 11
1.8. Hypotheses... 11
2. Methods... 12
2.1. Document Collection and Analysis ... 12
2.2. Case Study of Six Fathom Bank Refuge ... 13
2.3. Stocking Data Analysis... 13
2.4. Lake Trout Life Stage Analysis ... 14
2.5. Calculation of Minimum Viable Population... 14
2.6. Comparative Study of Refuge Network... 15
3. Results... 16
3.1. Lake Huron Lake Trout Stocking Strains ... 16
3.2. Stocking Trends in Lake Huron ... 17
3.3. Six Fathom Bank Case Study ... 20
3.4. Lake Trout Early Life History ... 23
3.5. Current Threats to Lake Trout Populations in Six Fathom Bank Refuge... 24
3.6. Number of Reproducing Females Needed... 28
3.7. Lake Trout Movement Study ... 33
3.8. Comparative Reserve Network ... 34
4. Discussion ... 36
Acknowledgements... 42
References... 43
1. Introduction
1.1. Overview
Overexploitation and habitat destruction in aquatic ecosystems around the world are current problems faced by fish populations. In the Laurentian Great Lakes, survival of many native species is challenged by the introduction of invasive species, habitat loss and fragmentation, ecosystem stresses, and overfishing. During the 1940s and 1950s, lake trout (Salvelinus namaycush) populations in the Great Lakes declined severely as a result of heavy fishing pressures and invasion of the sea lamprey (Petromyzon marinus), and became extirpated in the four lower Great Lakes (Coble et al. 1990). Since stock rebuilding efforts began in the 1960s, lake trout abundance has slowly increased, but is currently far below historic averages. Lake trout were one of the primary top native deepwater predators in the Great Lakes trophic pyramid (Hansen and Peck 2006) and were the only predator able to occupy all habitats from the surface to the lake bottom and from shore to shore (Eschenroder et al. 1995). Efforts to bring populations back to sustainable levels include sea lamprey control, restrictions on fishing, stocking lake trout yearlings, and the creation of lake trout refuges (Hansen and Peck 2006).
Marine Protected Areas (MPAs) have been used as a management tool to improve and restore aquatic ecosystems. The term MPA is broad, overarching, and can take on a meaning that encompasses many different goals and objectives. MPAs generally conserve biodiversity, reduce habitat degradation, protect severely depleted species, control exploitation rates, and reduce mortality rates for critical stages of a species life history (National Research Council 2001). Regulations are generally site-specific, but can
vary from strict no-take rules to multiple-use policies (National Research Council 2001). The main objective for a MPA is to reduce the anthropogenic impacts for a specific area and restore habitat or species populations through conservation and protection (Airame et al. 2003).
The overall goal of my honors thesis is to evaluate effectiveness of refuges in protecting and restoring sustainable lake trout populations in the Great Lakes. The specific research question that I would like to answer is: what are the main factors that explain the failure of lake trout refuges in protecting spawning populations? I will review trends and the current status of lake trout populations in the Great Lakes, and then analyze existing data on lake trout stocking rates and survival in the Six Fathom Bank refuge of Lake Huron to identify the impediments to lake trout reproduction in Lake Huron. Results of this analysis will inform appropriate management about actions for achieving successful reproduction in the Six Fathom Bank refuge.
1.2. Lake Trout Refuges in the Great Lakes
Lake trout refuges have been created in the Great Lakes to help protect and restore naturally reproducing lake trout populations (Stanley et al. 1987). Although these refuges were not legally designated as MPAs, they do share functions and objectives with many other legally selected MPAs. The goal for the lake trout refuges is to create protected critical habitat that can aid in increased reproductive success for the population.
Currently, there are a total of four refuges designated to protect lake trout in the Great Lakes (Appendix A). These sites were chosen as a result of their location and habitat type. Refuges in Lake Michigan were created in 1985 and 1986, and include the Milwaukee Reef and Fox Island refuge (Stanley et al. 1987). In Lake Huron, refuges were created in 1986 and 1997, and include the Drummond Island and Six Fathom Bank refuge (Stanley et al. 1987). Current management regulations for the lake trout refuges in the Great Lakes restrict the taking, harvesting, sport fishing, and commercial catch of lake trout within the refuge boundaries (Fisheries Orders 2005). Success of lake trout reproduction has been monitored through stocking and returns of fish marked with adipose fin clips and coded-wire tags. The data suggest that reproductive success of lake trout populations has not improved, despite increases in stocking numbers (Madenjian et al. 2006a).
1.3. Lake Trout Rehabilitation Stocking Efforts
Lake trout populations were nearly extirpated in the lower four Great Lakes in the late 1960s (Hansen and Peck 2006). Stocking of hatchery-reared lake trout and control of parasitic sea lamprey have been the primary tools used to rehabilitate lake trout populations. In Lake Huron, different stocking techniques, including the use of 5 genetically different lake trout strains, coded-wire tagging for monitoring and research purposes, and multiple stocking locations, have been used to increase the success of lake trout populations since 1973 (Madenjian et al. 2006a). Stocking efforts increased during the 1970s and 1980s and lake trout population size slowly increased during the late 1980s. However, lake trout numbers still have not reached 10 percent level of the average
population size in 1929-1943, a period when lake trout harvests and population sizes were considered sustainable (Hansen and Peck 2006). While lake trout stocking continues to increase, reproductive success of adults has failed to improve and self-sustaining populations have not developed.
1.4. Current Status of Lake Trout Rehabilitation
Overall, the current status is not favorable for the lake trout populations of the Great Lakes. In general, there is little to no natural recruitment for lake trout populations in the lower four Great Lakes (Bence et al. 2003). Lake trout refuges, coupled with intensive hatchery stocking programs, have been designated to protect lake trout populations. These areas provide a location to conduct research and perform trial-and-error procedures that may help the future success of lake trout populations.
Lake Superior
The goal of lake trout rehabilitation in Lake Superior is to maintain and achieve a diverse self-sustaining population (Horns et al. 2003). Lake trout have been declared restored in Lake Superior, and self-sustaining populations exist within its waters. However, these levels are still below the harvest objective and historic abundance for 1929-1940 of 2.0 million kg of annual catch (Hansen 1996; Hansen and Peck 2006). Lake Superior is the only lake that has experienced significant reproductive success as a result of major stocking efforts (Hansen and Peck 2006).
Lake Huron
Lake Huron has experienced intensive stocking attention since 1973 (Great Lakes Fish Stocking Database 2007) after populations were extirpated in the 1950s (Hansen and Peck 2006). The specific objective of “lake trout rehabilitation in Lake Huron is to
restore self-sustaining populations that are capable of yielding 1.4 to 1.8 million kg by the year 2020” (Ebener 1998). Total lake trout yield in 2004 is more than double that in 1999 and has reached 0.7 million kg (Bence 2005). The majority of this harvest is a result of lake trout stocking. During 2000 to 2004, an average of 3.4 million lake trout yearlings were stocked annually (Bence 2005). Recently, the estimated available reproducing females have increased by 90% from 1999 to 2004 in Lake Huron’s main basin (Bence 2005). Currently, there has been localized reproductive success in a limited number of areas in Lake Huron, including Parry Sound, where a native population of lake trout is thought to persist (Jonas et al. 2005b).
Lake Erie, Lake Michigan, and Lake Ontario
The lake trout populations of Lake Erie, Lake Michigan, and Lake Ontario were
extirpated in the 1940s and 1950s (Mills et al. 2005; Jonas et al. 2005a; Ryan et al. 2003). Rehabilitation efforts through stocking began as early as 1960 (Great Lakes Fish
Stocking Database 2007). In all three of these lakes, recovery efforts have failed to produce self-sustaining lake trout populations and there is no natural recruitment that has occurred from stocking efforts (Jonas et al. 2005a; Mills et al. 2005; Ryan et al. 2003). Recruitment rates are based exclusively on stocking efforts (Jonas et al. 2005a; Mills et al. 2005; Ryan et al. 2003).
1.5. Current Threats to Lake Trout Rehabilitation
Historically, the major threats that have led to the drastic decline in lake trout populations include a combination of mortality by sea lamprey predation and overfishing. The sea lamprey control program began in the early 1950s and has been successful in controlling lamprey populations. To date, control efforts have reduced sea lamprey populations by 90% in most areas of the Great Lakes (Great Lakes Fishery Commission 2007). Although sea lamprey populations have decreased significantly due to aggressive control efforts, lake trout mortality rates still are high and result from sea lamprey wounds and fishing (Bence et al. 2003). Fishing pressure has subsided due to governmental regulations created to protect the declining fisheries in the Great Lakes. Even though the
implementation of these restrictions is strictly enforced, recreational harvest continues to exceed maximum harvest limits (Bence et al. 2003).
In addition to mortality caused by sea lamprey predation and fishing, other factors may impede achievement of self-sustaining lake trout populations. One of the most severe threats to the success of the lake trout populations is predation or disease during the fry stage of growth. Early mortality syndrome is a disease that affects survival of lake trout eggs and fry, and results from low (< 1.0 nmol/g) thiamine levels in mature females who eat alewives and rainbow smelt, which are high in thiaminase (Bronte et al. 2003;
Honeyfield et al. 2005). Lake trout that primarily eat alewife and rainbow smelt produce eggs with thiamine concentration levels that are 1/9th to 1/17th the level of eggs from lake trout that lack these species in their diets (Bronte et al. 2003). Habitat degradation and
contaminants are additional factors that may play a role in low reproductive success of lake trout (Bronte et al. 2003).
Genetic and demographic factors also may impede the rehabilitation of lake trout. The number of lake trout stocked may be too low to increase numbers to adulthood (Bronte et al. 2003). Low spawner abundance, combined with low reproductive success and low genetic diversity can contribute to the lack of success in increasing lake trout population numbers (Bronte et al. 2003).
1.6. Stakeholders
There are many different perspectives and viewpoints that must be considered when analyzing the status and conservation efforts of lake trout. Stakeholders include private/recreational anglers, commercial fishers, tribal members,
government/management officials, scientists/researchers, and future generations.
Although recreational anglers may not consider lake trout to be the most valuable and desirable fish species in the Great Lakes to catch, they generally support the restoration efforts to increase lake trout population numbers (Kernen 1995). All lake trout refuges strictly prohibit any commercial fishing activity within the boundaries of the protected areas; however, commercial fisheries can target lake trout outside the border of the refuges. Management and government officials play an increasingly important role in the success of the lake trout conservation effort. The U.S. Fish and Wildlife Service and the Michigan Department of Natural Resources are the main government agencies
responsible of the restoration project, and make management decisions regarding stocking procedures, fishing regulations, habitat protection, and enforcement that will affect the success of establishing naturally reproducing lake trout populations. The re-establishment of lake trout populations to self-sustaining levels will positively benefit the overall biological diversity of the Great Lakes and help the overall ecosystem health (Kernen 1995).
1.7. Thesis Objectives
The overarching objective of my thesis research is to identify the main factors causing failure of refuges to protect spawning populations of lake trout in the Great Lakes. Specific study objectives include: evaluate the existing environmental and demographic threats to the lake trout population; compare the lake trout refuge network in the Great Lakes with other Marine Protected Areas to assess the limitations to the refuge system; and provide recommendations for how to improve the refuge system to more effectively create self-sustaining lake trout populations in the Great Lakes for future generations.
1.8. Hypotheses
I hypothesize that stocking efforts have been inadequate to support self-sustainable, wild lake trout populations. Specifically, I hypothesize that a population bottleneck exists in the fry stage, limiting natural reproduction and restoration of lake trout populations. I speculate that this bottleneck is due to predation of fry by alewives. I hypothesize that the refuge network created within the Great Lakes is not large enough to adequately protect lake trout due to their expansive seasonal movements during ontogeny.
2. Methods
2.1. Document Collection and Analysis
I began this study with an extensive review and analysis of existing literature on lake trout refuges in the Great Lakes, lake trout population trends, biology, and current status. Searches were conducted using primary research papers, agency reports, on-line
resources, fish stocking databases, and journals relating to lake trout refuge issues in the Great Lakes. The review focused on the history of lake trout ecology, population
dynamics, and management in Lake Huron, and included: the decision process for refuge placement and scale; analysis on lake trout movements in and around refuges; and the population bottlenecks to reproductive success. Emphasis was placed on understanding the behaviors, successes, and failures of lake trout populations in Lake Huron with reference to species ecology. Specific subtopics included, but were not limited to, reproductive success, competition, habitat, predation, behavior, movement, and food availability for lake trout in the Great Lakes.
Another section of the literature review focused on the Six Fathom Bank refuge in Lake Huron. Factors considered were present physical size of the refuge, lake trout population status and size, and specific areas of the refuge that have been successful or those areas that have shown little improvement. By studying the relationship between the refuge and lake trout population within it, conclusions and recommendations were formed to help increase the success and effectiveness of stocking lake trout in the Great Lakes and the potential for alternative strategies.
2.2. Case Study of Six Fathom Bank Refuge
To gain additional detail, an in-depth case study was conducted on the Six Fathom Bank refuge, located in central Lake Huron (Appendix A). This refuge was selected due to its history, location, and crucial management decisions made within the refuge. Furthermore, Six Fathom Bank is the only international refuge within the Great Lakes, sharing its borders between the United States and Canada. This is a particularly interesting factor that played a role in choosing this location for the case study. Specific habitat
characteristics for each section of the protected area were documented, stocking history for lake trout in the area was recorded, history of management decisions leading to creation and development of the refuge was documented, and success and extent of enforcement for implementing the regulations was assessed.
2.3. Stocking Data Analysis
Lake trout stocking data were collected from the entire Lake Huron basin from the time stocking began in 1972. This information was collected from the Great Lakes Fishery Commission’s (GLFC) Great Lakes fish stocking database. The database was searched using lake trout as the target species, the years from 1972 to the most recent (2005), and focused on Lake Huron as the location. Stocking data on various genetic strains were gathered for the Six Fathom Bank refuge where stocking began in 1985. Results were analyzed from a coded wire tag study of lake trout survival rates studying movement inside and outside the Six Fathom Bank refuge.
2.4. Lake Trout Life Stage Analysis
A literature review was conducted of factors affecting survival rates of lake trout life stages to determine the factor(s) and stage(s) most likely to contain bottlenecks to
successful lake trout reproduction. Lake trout life stages were separated into four distinct categories; egg stage from deposition to hatching, fry stage from time of hatching to swim-up period, juvenile stage from one to five years of age, and adult stage greater than six years old.
2.5. Calculation of Minimum Viable Population
I performed an analysis to determine the number of mature females needed to create a self-sustaining lake trout population in the Six Fathom Bank refuge given existing mortality rates. To determine the number of eggs needed to produce a successful
spawning population, I used data on stocking rates of yearling lake trout, the survival rate from yearling to mature adult, size frequencies of mature adults, size-specific fecundities, and survival rates through egg, fry, and juvenile stages. The Madenjian et al. (2004) study was used to determine the age, number, and mean length of female lake trout captured in the Six Fathom Bank refuge from 1992 to 2000. A length-fecundity relationship of lake trout in Lake Superior (Peck 1986) was applied to length-frequency distributions to calculate the average fecundity for each female at a certain age class in Six Fathom Bank refuge. Egg to predator density ratios for locations where lake trout spawning is
successful (Jonas et al. 2006) were applied to the estimated population fecundities. An equation was then created to determine the total number of females needed in the Six Fathom Bank refuge.
2.6. Comparative Study of Refuge Network
An analysis of the lake trout refuge network was conducted through an extensive literature review to investigate the success of the location and arrangement of protected areas. Additional research was conducted to compare the lake trout refuge network to other successful Marine Protected Area networks. Although the target species differ in other MPA examples, this provided further insight into the positive and negative aspects of the refuge network as it is currently arranged, and suggested areas for improvement. Lake trout refuges that have not reached self-sustaining levels were compared with other self-sufficient populations in other areas of the Great Lakes.
3. Results
3.1. Lake Huron Lake Trout Stocking Strains
Lake trout stocking began in 1973 to overcome high mortality rates from sea lamprey predation and overfishing. Five different strains of lake trout have been stocked in Lake Huron to attempt to increase survivorship and determine which strain may be most successful. These strains include Marquette – Superior (MS), Lewis Lake (LL), Jenny Lake (JL), Seneca Lake (SEN), and Ontario (ONT) strains (Madenjian et al. 2006a).
Lake Trout Stocking History in Lake Huron
The MS strain originates from the deep waters of Lake Superior (Madenjian et al. 2006a) and was the initial strain introduced into Lake Huron in 1973. The MS strain was the only strain used for stocking in Lake Huron from 1973 to 1985, and is the single strain stocked in Lake Huron consistently every year since 1973 (Madenjian et al. 2006a). As a result of low reproductive success rates in 1985, other lake trout strains were stocked in hopes of producing a self-sustaining population.
The LL and JL strains originate from Lewis Lake in Yellowstone Park and Jenny Lake outside of Jackson, WY, respectively. These strains originally were native to northern Lake Michigan but were stocked in high mountain lakes in the late 1880s. This is one reason that this stock had the potential to be successful because the genetic strain originated from the Great Lakes. The JL strain is currently no longer used due to a genetic bottleneck that the population faced in the early 1990s and a bacteria disease that significantly decreased the population size (Madenjian et al. 2006a). The genetic
bottleneck reduced populations to insufficient levels and population diversity was no longer maintained. The LL strain has been stocked in Lake Huron since 1990 and has been stocked for nine years.
The SEN strain originates from Seneca Lake in New York. This strain of lake trout began in an area where lake trout have coexisted with sea lamprey for centuries (Madenjian et al. 2006a). This strain type was introduced in Lake Huron in 1985 in hopes of creating a lake trout population resistant to sea lamprey predation. Variations of this stock have been created to overcome disease that developed within this strain type.
The ONT strain is considered to be a hybrid lake trout strain between wild Lake Ontario lake trout and the Seneca Lake strain (McClain personal communication 2007). This strain was introduced into Lake Huron in 1989. This strain type was stocked in Lake Huron for 3 years and was created to overcome a shortage in the SEN stock (Madenjian et al. 2006a). Similar to the SEN stock, the ONT strain is somewhat resistant to sea lamprey but was not used for a significant period of time.
3.2. Stocking Trends in Lake Huron
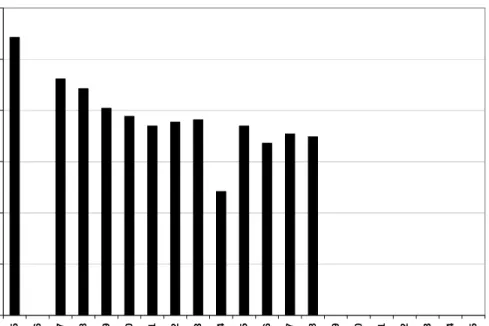
Lake trout stocking in Lake Huron began in 1973 in an attempt to combat the severe decline in lake trout populations over the past century (Figure 3.1). The maximum stocking level was reached during 1998 but has since declined. Even though stocking efforts have decreased in the past 8 years, the number of lake trout recaptured in Lake Huron has increased (Figure 3.2). There were approximately 60, 866 lake trout harvested
from ten different locations in Lake Huron in 2004, where numbers represent total lake trout harvested by commercial fishing operations (Bence, 2005).
0 1,000,000 2,000,000 3,000,000 4,000,000 5,000,000 6,000,000 19 72 19 74 19 76 19 78 19 80 19 82 19 84 19 86 19 88 19 90 19 92 19 94 19 96 19 98 20 00 20 02 20 04 Year N u m b er L ake T ro u t S to cke d
Figure 3.1. Total number of lake trout stocked in waters of Lake Huron since 1973
0 10,000 20,000 30,000 40,000 50,000 60,000 70,000 19 86 19 88 19 90 19 92 19 94 19 96 19 98 20 00 20 02 20 04 Year N u m b er o f L ake T ro u t C o ll ect ed
Figure 3.2. Number of lake trout harvested from ten locations within Lake Huron (Bence, 2005)
In contrast, the number of coded wire tagged (CWT) lake trout recaptured in Lake Huron has recently declined (Figure 3.3). Coded wire tags have been recaptured by Chippewa Ottawa Resource Authority, Ontario Ministry of Natural Resources, U.S. Fish and Wildlife Services, U.S. Geological Survey, Michigan Department of Natural Resources, and Michigan sport fisherman (Madenjian et al. 2006a). The maximum recapture
numbers occurred in 1999 with 1928 lake trout caught with coded wire tags, but the most recent numbers of lake trout recovered with coded wire tags were only 664 in 2005. Recapture rates declined as CWT numbers stocked declined in 1998.
0 500 1000 1500 2000 2500 1997 1998 1999 2000 2001 2002 2003 2004 2005 Year N umb er of I nd ivi d ual L ake Tro ut Reco vered
Figure 3.3. Total lake trout recovered in Lake Huron with coded-wire tags annually since 1997
3.3. Six Fathom Bank Case Study
The Six Fathom Bank lake trout refuge is located in central basin of Lake Huron. The refuge was officially designated in April 1997 to protect and improve the reproductive success of lake trout in Lake Huron (Ebener 1998). The refuge location was based on areas that have the largest historical and potential spawning habitat (Ebener 1998). Six Fathom Bank reef provided a superior site to protect lake trout because of the habitat and fish retention rates (Madenjian et al. 2003).
The current regulations within the Six Fathom Bank refuge promote the rehabilitation of lake trout by prohibiting the “taking, keeping, and sport fishing of lake trout” within the refuge boundaries (Fishing Orders 2005). This recently amended regulation took effect on April 1, 2006 and will continue through March 31, 2011 (Fishing Orders 2005). No commercial fishing is permitted within the refuge boundaries. It is important to note that these regulations are only targeted towards lake trout and do not apply to any other species of fish.
The current enforcement of the above regulations is conducted by the Michigan Department of Natural Resources Conservation Officers. Enforcement efforts are performed by periodic aerial and water surveillance efforts (McClain personal communication 2007). Public education is necessary to delineate and inform various stakeholders that come into contact with the refuge. Refuge regulations can be found in the statewide harvest regulation booklets, entitled “State Trout, Salmon, Whitefish and Lake Herring Regulations”. Participants in fishing tournaments throughout the years are
also informed of the regulations surrounding lake trout capture within the boundaries of the Six Fathom Bank refuge (McClain personal communication 2007).
All five strains of lake trout used in rehabilitation have been stocked in the Six Fathom Bank lake trout refuge since 1985 (Figure 3.4). The Jenny Lake strain was stocked from 1985 to 1990, the Marquette – Superior strain from 1985 to 1998, the Lewis Lake strain from 1990 to 1998, the Seneca Lake strain from 1985 to 1998 (spanning the entire stocking period targeted in the Six Fathom Bank refuge), and the Ontario strain from 1990 to 1993. All stocking efforts declined steadily since 1985 and ceased in 1998 (Figure 3.5). 0 10,000 20,000 30,000 40,000 50,000 60,000 70,000 80,000 90,000 100,000 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 Year Num ber La ke Trou t S toc k e d Ontario Jenny Lake Marquette-Superior Seneca Lake Lewis Lake
0 50,000 100,000 150,000 200,000 250,000 300,000 198 5 198 6 198 7 198 8 198 9 199 0 199 1 199 2 199 3 199 4 199 5 199 6 199 7 199 8 199 9 200 0 200 1 200 2 200 3 200 4 200 5 Year Number Lake Trout S tockeded
Figure 3.5. Total number of lake trout stocked in Six Fathom Bank refuge since 1985 (Madenjian et al. 2006a)
Following lake wide trends, recapture rates of coded wire tags have recently declined in the Six Fathom Bank refuge (Figure 3.6). The maximum number of recaptured CWT lake trout took place in 2000 with 589 fish. In 2005, there were only 27 lake trout recaptured (Madenjian et al. 2006a). Stocking efforts ceased in the SFB refuge after 1998 because it is thought that the amount of hatchery-reared fry may inhibit the potential growth of wild-reared lake trout and cause negative impacts to naturally reproducing populations (McClain personal communication 2007).
0 100 200 300 400 500 600 700 1997 1998 1999 2000 2001 2002 2003 2004 2005 Year N u m b er of I ndi vi du a l L a ke Tr ou t R e cover ed
Figure 3.6. Total number of lake trout recovered with coded wire tags within the Six Fathom Bank refuge (Madenjian et al. 2006a)
3.4. Lake Trout Early Life History
The age and size at first spawning of female lake trout varies with location and
environmental conditions but is generally between 4 and 6 years old or 2 to 4 lbs (Bence et al. 2003). Spawning generally occurs at older ages during fall when lake temperatures are generally lower (Bence et al. 2003). Lake trout are known to spawn in various habitats within the Great Lakes, including offshore reefs, nearshore environments, and mouths of rivers (Claramunt et al. 2005). Egg deposition by lake trout can occur at many different depths. A study conducted by Claramunt et al. (2005) observed natural egg deposition at 1, 3, and 9 meters. Lake trout generally spawn in areas with abundant loose
rocks during the fall season. Released eggs incubate during the winter and emerge from aquatic substrates on the lake bottom in the early spring (Eschenroder et al. 1995).
Goodyear et al. (1982) concluded that lake trout spawn at varying depths in Lake Huron. Spawning was observed at depths from 6 to 120 feet on reefs that were generally 15 to 20 miles from shore (Goodyear et al. 1982). Spawning commonly took place over
honeycomb shaped bedrock or masses of rock. Eggs are deposited in the crevasses of bedrock and provide natural protection against predators. In general, eggs are most vulnerable in the first month after deposition (Jonas et al. 2005b). Historically, spawning has been observed in Lake Huron from October 15 – December 1, but the exact date varies with the specific strain of lake trout and area within the lake (Goodyear et al. 1982). Lake trout fry generally hatch from mid-April to late-May and are approximately 15 mm in length. The fry stage lasts from time of hatching to time of swim-up, when juveniles are approximately 24 mm in length and begin to feed (Jones et al. 1995).
A typical female lake trout originating from a hatchery can produce an average of 1,748 eggs per kilogram of body weight (Peck 1986). Moreover, a large, mature female lake trout can produce more than 10,000 eggs during one spawning event (Peck 1986). The deposited eggs are generally large, measuring up to 6 mm in size.
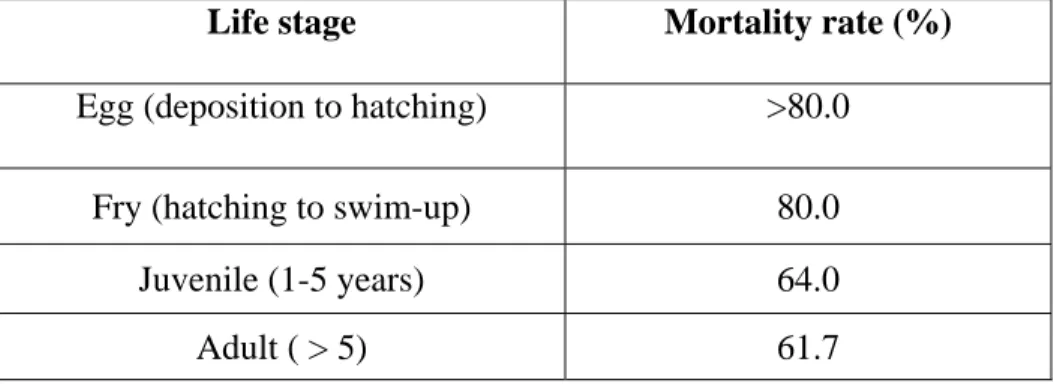
3.5. Current Threats to Lake Trout Populations in Six Fathom Bank Refuge
The major threat was found to occur at the fry stage when lake trout are most vulnerable to predation (Jonas et al. 2005b). Threats to other stages include predation of deposited
eggs, predation by lake trout and other major Great Lakes predators during the juvenile stage, and sea lamprey mortality during the juvenile and adult phases (Table 3.1) (Wilberg et al. 2002).
Table 3.1 Approximate mortality rates reported during four lake trout life stages (% dying during each life stage) (Claramunt et al. 2005; Sitar et al. 1999; Madenjian et al 2004)
Life stage Mortality rate (%)
Egg (deposition to hatching) >80.0
Fry (hatching to swim-up) 80.0
Juvenile (1-5 years) 64.0
Adult ( > 5) 61.7
The Six Fathom Bank (SFB) area was chosen for a lake trout refuge because of its unique habitat structure and location (Appendix B). This area was originally selected because it contains suitable spawning habitat and has historically provided an environment for lake trout reproduction (Desorcie and Bowen 2003). The Six Fathom and Ipperwash scarps, both located within the SFB refuge, offer bedrocks ridges and masses of cobble that supply optimal spawning habitat and territory for fry to mature (Desorcie and Bowen 2003). Much of the bedrock and loose rock contained within the boundaries of the SFB refuge are pitted and grooved, providing essential habitat for lake trout. Historical spawning accounts document this area as being the most important lake trout spawning ground in all of Lake Huron (Edsall et al. 1992). Edsall et al. (1992) discussed the suitability of the SFB refuge habitat for lake trout and explained that the strains of stocked fish within the refuge require habitat that is similar to the actual SFB ecosystem.
The SFB refuge should be sufficient in size and habitat type to protect the early life history stage of lake trout. The water depth in the SFB refuge generally varies from 20 to 50 meters (Edsall et al. 1992).
Food availability and quality may be limiting factors to successful recruitment of lake trout populations in the Six Fathom Bank refuge. Madenjian et al. (2006b) studied the spring and summer diet of lake trout in the SFB refuge and found that all sizes of lake trout prefer to consume alewives (Madenjian et al. 2006b). Of the largest lake trout caught and examined (n=5), the diet was comprised of 100% alewife (Madenjian et al. 2006b). Other lake trout prey found in the study included bloaters, smelt, invertebrates, sculpins, and miscellaneous organisms (Madenjian et al. 2006b). Alewives comprised a higher percentage (80% by wet weight) of lake trout diets on offshore reefs than in diets (65% by wet weight) of fish from Lake Huron’s nearshore waters (Madenjian et al. 2006b).
The quality of diet can affect that success or mortality in lake trout populations. With the majority of the lake trout diet comprised of alewives, early mortality syndrome (EMS) can result in the lack of success in lake trout rehabilitation (Bronte et al. 2003). EMS is a thiamine deficiency problem that occurs when females lay eggs that have a thiamine level lower than 1.0 nmol/g (Fitzsimons et al. 2005). EMS results in the early mortality of lake trout fry by causing behavioral and physiological changes in the swim-up events at early life stages (Fitzsimons et al. 2005). Fitzsimons et al. (2005) concluded that the variation in thiamine levels in alewives has the potential to affect lake trout predators and the
success of survival for fry. Alewives contain the enzyme thiaminase, responsible for destroying thiamine (Honeyfield et al. 2005). Honeyfield et al. (2005) found a positive correlation between a higher diet percentage of alewives and a higher thiamine deficiency in female lake trout, leading to EMS. Spawning females with a diet comprised of more than 35% alewives will produce eggs with significantly lower thiamine levels and
females lake trout with a diet of 100% alewives will produce eggs with very low levels of thiamine and swim-up fry with EMS (Honeyfield et al 2005). EMS is a contributing factor to the lack of success in fry survival, but is not the solitary cause.
Egg predation is the largest threat that could limit the recruitment success of lake trout (Table 3.1). In a study conducted by Jonas et al. (2005b) in three locations of the Great Lakes, eight noteworthy egg predator species were identified. In the Lake Huron location, these predators include 5 native fish species and 3 native crayfish species (Jonas et al. 2005b). Locations with high densities of lake trout eggs coincided with low egg predator densities, allowing for more successful lake trout populations. In Parry Sound Ontario, where lake trout reproduce successfully, the ratio of lake trout eggs to predators per m2 averaged 82.1, with predator densities at 5.5 ± 0.5 predators per m2 and egg densities at 454.7 ± 36.3 eggs per m2(Jonas et al. 2005b). Claramunt et al. (2005) showed that more than 80% of the eggs deposited were lost to predators 2 weeks after a spawning event. The greatest mortality rates occurred shortly after spawning during the egg life stage and declined thereafter (Claramunt et al 2005). This sharp decline in mortality rates is thought to be due to decreased predator activity as water temperatures declined during late fall and early winter (Claramunt et al. 2005). Low density of lake trout egg predators and a
high density of egg production was highly correlated with size and success of lake trout populations (Jonas et al. 2005b). Invasive species predation was highest on inshore reefs, while offshore reefs were dominated by native crayfish predators (Jonas et al. 2005b).
Another major potential population bottleneck for successful natural reproduction of lake trout seems to occur during the fry stage (Jonas et al. 2005b). Lake trout fry generally hatch and appear in the water column from early April to the end of May (Krueger et al. 1995). At this time, fry are most vulnerable because their behavior makes it simple for them to be captured by predators. Additionally, lake trout fry are generally most active at night and alewives often feed during this time period, making them more vulnerable (Krueger et al. 1995). Lake trout fry often swim towards the water surface to inflate their swim bladders during nighttime hours (Krueger et al. 1995). Alewives generally prey on large zooplankton but shift their diets to include more lake trout fry when zooplankton levels are relatively low (Krueger et al. 1995). High fry survival has been documented when alewife population levels are lower than average (Krueger et al. 1995). Lake trout fry mortality by alewife consumption has been observed at highest levels near inshore reefs, where alewives congregate most frequently (Krueger et al. 1995).
3.6. Number of Reproducing Females Needed
The number of hatchery-reared fry and naturally produced lake trout eggs must be large enough to overcome predation of eggs by various predators and mortality of fry by alewives. In the Jonas et al. (2005b) study, the lowest ratio of egg to predators per square meter that resulted in successfully produced lake trout fry was 24.3. The average ratio of
egg to predator per square meter was 190 for successful spawning locations that produced lake trout fry (Jonas et al. 2005b). I applied the results from the Jonas et al. (2005b) study to estimate low (3.6 predators per meter2) and high (7.6 predators per meter2) predator densities for successful spawning areas using data collected from Parry Sound in Lake Huron.
The total reef size of the Six Fathom Bank refuge is 168,000 hectares (1.68*109 m2). Edsall et al. (1992) surveyed 700 ha of reef habitat and determined that more 43.6% (305 ha) was suitable spawning habitat. I multiplied the habitat area of the entire Six Fathom Bank refuge by the fraction suitable to estimate the total available spawning habitat, approximately 7.32 *104 ha. I estimated the numbers of females needed to reproduce successfully for a fraction (50 and 300 ha, or 0.07% and 0.4%, respectively) of total available habitat as it is unnecessary to have adult females occupy the entire spawning habitat in the Six Fathom Bank refuge.
Number of eggs needed = 190. eggs * predator density = X eggs * Area of potential spawning habitat predator m2
Lower predator density and higher spawning habitat:
190. eggs * 3.60 predators = 684 eggs * 3.0 *106 m2 = 2.05 * 109 eggs needed predator m2 m2
Higher predator density and higher spawning habitat:
190. eggs * 7.60 predators = 1444 eggs * 3.0 *106 m2 = 4.33 * 109 eggs needed predator m2 m2
190. eggs * 3.60 predators = 684 eggs * 5.0 *105 m2 = 3.42 * 108 eggs needed predator m2 m2
Higher predator density and lower spawning habitat:
190. eggs * 7.60 predators = 1444 eggs * 5.0 *105 m2 = 7.22 * 108 eggs needed predator m2 m2
For 300 ha of suitable spawning habitat, the number of eggs needed to produce a successful population is 2,050,000,000 lake trout eggs at low predator densities and 4,330,000,000 eggs at high predator densities. For 50 ha of suitable spawning habitat, 342,000,000 eggs are needed to produce a successful population at low predator densities and 722,000,000 eggs at high predator densities.
Using results from Madenjian et al. (2004) and Peck (1986), I calculated the number of mature female lake trout needed to achieve natural reproduction in the Six Fathom Bank refuge. The ages of first spawning for lake trout in the Six Fathom Bank Refuge ranged from 6 to 8 years old (Madenjian et al. 2004). Using data regarding age, number, and mean length of lake trout captured in gillnets on the Six Fathom Bank reef from 1992 to 2000 (Madenjian et al 2004), I determined the percentage of females for each length category (Table 3.2) and applied length-specific fecundities for each spawning age class using the fecundity equation generated by Peck (1986).
Table 3.2. Number of eggs produced by each age class, calculated using the mean length of lake trout captured on Six Fathom Bank reef from 1992 to 2000, fecundity calculated using Peck (1986) fecundity calculations
Age N Percentage of population Avg. length (mm) Fecundity (eggs/female) # eggs produced 6 156 56.3 615 2,051 319,940 7 101 36.5 660 3,593 362,853 8 20 7.2 693 4,723 94,464
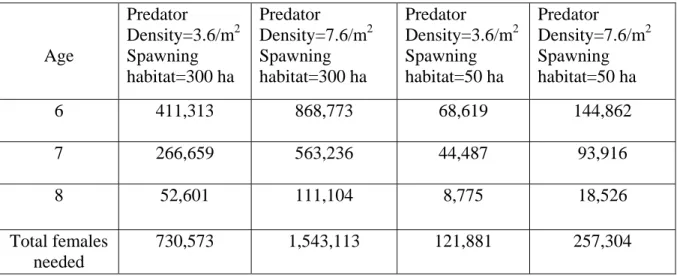
From the number of eggs produced by each age class, the number of females needed to produce a successful population was calculated for low and high predator densities and two sizes of spawning habitat used (Table 3.3):
Number of needed eggs = (# eggs produced at age 6* % of population) + (# eggs produced at age 7* % of population)+ (# eggs produced at age 8* % of population)
Lower predator density and higher spawning habitat:
2.05 * 109 eggs = (2050.9 * (0.563X)) + (3592.6 * (0.365X)) + (4723 * (0.072X)) X = 730,573 mature females needed
Higher predator density and higher spawning habitat:
4.33 * 109 eggs= (2050.9 * (0.563X)) + (3592.6 * (0.365X)) + (4723 * (0.072X)) X = 1,543,113 mature females needed
Lower predator density and lower spawning habitat:
3.42 * 108 eggs = (2050.9 * (0.563X)) + (3592.6 * (0.365X)) + (4723 * (0.072X)) X = 121,881 mature females needed
Higher predator density and lower spawning habitat:
7.22 * 108 eggs = (2050.9 * (0.563X)) + (3592.6 * (0.365X)) + (4723 * (0.072X)) X = 257,304 mature females needed
Table 3.3 Summary of number of spawning female lake trout by age class, needed in Six Fathom Bank Refuge to produce a self-sustaining population, per area of spawning habitat used and egg predator density
Age Predator Density=3.6/m2 Spawning habitat=300 ha Predator Density=7.6/m2 Spawning habitat=300 ha Predator Density=3.6/m2 Spawning habitat=50 ha Predator Density=7.6/m2 Spawning habitat=50 ha 6 411,313 868,773 68,619 144,862 7 266,659 563,236 44,487 93,916 8 52,601 111,104 8,775 18,526 Total females needed 730,573 1,543,113 121,881 257,304
The estimated minimum number of females needed to produce a successful population in Six Fathom Bank ranges from approximately 122,000 lake trout at low egg predator densities (3.6/m2) and small amount of spawning habitat (50 ha) to 1.5 million lake trout at high predator densities (7.6/m2) and a larger amount of spawning habitat (300 ha).
The exact number of lake trout currently in the Six Fathom Bank refuge is not known. I calculated the current number of mature spawning females based on data from the Great Lakes Fish Stocking Database. Since stocking ceased in 1998 within the Six Fathom Bank refuge, the youngest hatchery stocked lake trout would be 9 years of age. Since 1985, 1,254,509 female lake trout have been stocked in the Six Fathom Bank refuge. Assuming an average survival rate from yearling to age 5 (36%), a retention rate of approximately 43% (Adlerstein et al. 2007), and an average annual mortality rate for lake trout older than age 5 (61.7%) (Madenjian et al. 2004), the maximum number of females in the refuge currently would be 74,378 and would range in age from 9 to 22. However, these numbers could differ due to higher mortality rates at older ages and naturally produced wild lake trout that may reside in the refuge that were not taken into account.
3.7. Lake Trout Movement Study
Beginning in 1985, lake trout have been stocked in Lake Huron with coded wire tags (CWT) and marked by removing the adipose fin (Madenjian et al. 2005). CWT are implanted in the snout of lake trout with clipped adipose fins to help track movements, growth, and reproductive success (Adlerstein et al. 2007). Prior studies concluded that lake trout movement was localized in the Great Lakes, but a study by Adlerstein et al. (2007) showed that lake trout movement was less localized than formerly believed. More than one third (42.7%) of all lake trout stocked in the Six Fathom Bank refuge were recaptured in this location (Madenjian et al. 2003; McClain et al. 1998). Larger, mature lake trout tended to travel longer distances from where they were stocked as yearlings.
However, 2-year old lake trout have been found as far as 113 kilometers away from their original stocking site (Appendix C) (Madenjian et al. 2003).
3.8. Comparative Reserve Network
A literature review was conducted with the intention of finding and comparing marine protected area to the lake trout refuges in the Great Lakes. Due to the unique nature of the lake trout refuges, no currently-designated, aquatic protected areas were similar enough to directly compare management techniques and overall reserve success. The majority of aquatic protected areas have been designated in marine habitats and exist to protect the entire ecosystem (Agardy 1994). As a result, information was collected about general reserve networks and effective Marine Protected Areas.
The size of a designated aquatic protected area greatly affects the success of the reserve. Generally stated, the advantage that a reserve can provide is directly correlated with its size; larger reserves offer greater conservation benefits by providing more protected habitat (Airame et al. 2003; Walters 2000). The size of reserves included within the network ultimately depends upon various demographic factors for the species under protection, including dispersal potential, home range size, population growth rate, and extraneous pressures that are potentially suppressing the populations (Airame et al. 2003). The reserve size must adequately balance economic gains versus ecological benefits and attempt to maximize both sectors.
Effective marine protected area networks focus on reserves with diverse biogeographical assemblages and habitats (Airame et al. 2003). It is important to represent various aquatic habitats to account for the diverse needs of different fish populations. Numerous types of reserves must encompass critical habitat for the intended species protected. Additionally, reserves must contain the necessary elements for species to survive and reproduce, including food and habitat variability (Gray 1997).
A reserve network must contain an element of connectivity to reach its full protection potential. Multiple reserves provide for protection of various habitats and help conserve a variety of species (Sala et al. 2002). Additionally, reserves must be close enough to each other to allow species to travel between each protected area. They must not be too far away to prohibit the migration of species amid multiple refuges (Sala et al. 2002). The number of reserves within the network is dependent on the total amount of critical habitat existing, the available regulation enforcement officials, and the movement of the target species. From a case study conducted in California’s Channel Islands, recommendations were made to protect approximately 30-50% of the 2200 km2 biogeographical region, resulting in two to three reserves ranging from 200 to 550 km2 in size (Airame et al. 2003). Several smaller reserves may be more beneficial than a single large protected area because they provide a more diverse habitat, less species density, and allow for more movement to other desired areas.
4. Discussion
The refuges created in the Great Lakes to protect and improve lake trout populations have been ineffective in promoting successful reproduction of lake trout populations. Despite aggressive stocking levels, managers have tried unsuccessfully to increase naturally reproducing population numbers. Furthermore, stocking practices have ceased completely in the Six Fathom Bank refuge for nearly ten years, decreasing the probability that natural populations will emerge. Based on simple calculations of spawner abundance needed to overcome high egg predation rates, population levels are currently too low to create a healthy lake trout population in the Six Fathom Bank and the lower four Great Lakes.
Five major environmental variables were analyzed within the Six Fathom Bank refuge. Physical habitat quality and available spawning areas were not considered to be limiting factors in creating a self-sustaining lake trout population. As a historic spawning ground, the habitat in the Six Fathom Bank refuge seems adequate to support lake trout
reproduction (Goodyear et al. 1982, Edsall et al. 1992). The bedrock and habitat characteristics provide a region for lake trout eggs to be naturally protected within the crevasses and honeycomb features of rocks within the refuge.
On the other hand, food quality, egg predation, and predation of fry by alewives are considered limiting factors to lake trout reproductive and recruitment success. Alewives account for more than 80 percent of the lake trout diet in the Six Fathom Bank refuge (Madenjian et al. 2006b). Alewives are invasive species to the Great Lakes and do not provide the same quality of nutrients and energy source to lake trout as native species
would. The majority of the Great Lakes ecosystem has been disrupted by invasive
species, which greatly affect all native species to some extent. Related to food quality, the availability of food for lake trout is not a limiting factor for success. There is an adequate food source for lake trout; however, the quality of this food is deficient to support
populations. Together, predation on eggs and fry also are problems that affect the success of lake trout populations in the Great Lakes. Low predator densities may greatly improve the success of survival for eggs and fry in the area. The predation of fry by alewives is a limiting factor that creates a population bottleneck and reduces the success of creating self-sustaining populations.
The number of spawning lake trout females needed to overcome the population
bottleneck within Six Fathom Bank ranges from approximately 122,000 at low predator densities and small spawning habitat area to 1.5 million at high predator densities and larger spawning habitat area. To put this number in perspective, the number of
reproducing females increased by approximately 90% from 1999 to 2004 in all of Lake Huron from 509,000 to 953,000 lake trout (Bence, 2005). I estimated that current numbers of females are lower than what is needed to achieve successful lake trout reproduction. My calculations are based on a fecundity equation created from lake trout in Lake Superior. Fecundity may be lower in Lake Superior due to colder water
temperatures and different environmental variable. The calculation of mature female lake trout needed in the Six Fathom Bank refuge should be used as an approximation of the minimal viable population needed to produce self-sustaining populations.
In addition to limiting environmental variables, the lake trout refuges are not large enough to accommodate lake trout movement. Almost half of the lake trout within the Six Fathom Bank refuge stay within in the boundaries of the reserve. However, the movement of lake trout is not as localized as once thought and many mature lake trout move outside of the refuge where they are subjected to fishing pressures.
“No-take” refuges may provide large paybacks to the complete ecosystem of the Great Lakes. A no-take reserve can be used as an effective management tool to provide a more sustainable fishery. A study conducted by Ward and Hegerd (2003) determined that major benefits exist from the implementation of marine protected areas in Australia; improving the success of stock management and fishery stability, providing a location that can serve for “ecological offsets”, and improving socio-economic returns for communities based on the fishing industry. The most applicable benefit for the Great Lakes is related to stock management and increased population size from protected areas. This can include protecting critical life stages, protection of habitat and essential survival functions, the “spillover effect” of possible increases in population sizes within the refuge, and serving as a center for offspring recruitment (Ward and Hegerd 2003). Many valuable conservation benefits can occur from the implementation of reserves, but it is important to note that the majority of these benefits are a result of “no-take” refuges (Ward and Hegerd 2003).
Stocked lake trout are more likely to spawn in shallower habitats than naturally produced (wild) lake trout (Edsall et al. 1992). Hatchery-reared lake trout in Lake Huron tend to
spawn at depths averaging 5-9 meters, however stocked lake trout also spawn at varying depths (Goodyear et al 1982; Edsall et al. 1992). Taking this into account, many historic lake trout spawning grounds are located nearshore. These sites are generally more susceptible to heavier fishing pressures because of close proximity to the shoreline. Protecting nearshore historic spawning reefs could improve survival rates for lake trout that migrate to these areas. Additionally, a refuge network could be created, incorporating nearshore areas, to maximize the protection of lake trout via refuges. Fishing pressures are generally greatest at locations that are closer to land and these refuges would help alleviate demands.
Richards and Rago (1999) described the successful recovery of depressed striped bass (Morone saxatilis) populations in Chesapeake Bay through adaptive management practices. The Chesapeake Bay striped bass was thought to be the most productive stock on the Atlantic Coast (Richards and Rago 1999). During the late 19th century, populations were declining as a result of over-harvesting and habitat destruction. Populations
recovered in size during the mid 20th century, but then greatly declined as a result of high harvest rates. Consequently, the Atlantic States Marine Fisheries Commission developed strict regulations on size limits and focused on protecting female striped bass (Richards and Rago 1999). Some regional states created more stringent regulations, and two declared a complete moratorium on harvesting striped bass from the Chesapeake Bay (Richards and Rago 1999). The Atlantic Striped Bass Conservation Act was passed in 1984, putting federal pressure on states to comply with strict harvest regulations. Stringent fishing regulations and stocking efforts greatly increased striped bass
populations, creating a 400% increase in coastwide recreational catches from 1985 to 1988 (Richard and Rago, 1999). Stocks were confirmed to be fully recovered after only ten years of strict regulations (Richards and Rago, 1999).
Even if more lake trout refuges were created in the Great Lakes, hatcheries are already at maximum capacity for producing lake trout fry. It is not realistic to artificially create a lake trout population that consists of 1.5 million spawning females in the Six Fathom Bank refuge when current population numbers are well below this and extensive fishing pressures still exist in neighboring waters. Management regulations must be created to significantly decrease fishing pressures on lake trout in the Great Lakes. A complete suspension of lake trout harvesting would allow populations to increase in size as more mature, larger lake trout females survive and reproduce. Female lake trout fecundity increases greatly with size. These rigorous regulations would be lifted once the minimum viable population is met.
Lastly, gaps in prior research were identified to improve knowledge about the factors limiting reproductive success of lake trout populations in the Great Lakes. Only modeling and laboratory work has been conducted relating to predation of early life stages of lake trout (Jonas et al. 2005b). Limited field work has been conducted on this subject and more studies are needed on impacts of introduced invasive predators (i.e rusty crayfish and round gobies) on lake trout reproductive success. Other research is needed to determine the spawning habitat area and spawner densities needed to achieve successful reproduction. Research should be carried out to determine where further refuges could be
placed in the Great Lakes. Factors to consider would be habitat quality, density of possible predators, amount of fishing pressure, and lake trout movement to the area. Nearshore reefs should be evaluated to determine which locations would be most appropriate for a future lake trout refuge. With the addition of three to four smaller refuges created within Lake Michigan and Lake Huron to include nearshore reefs, lake trout populations would be subjected to lower fishing pressure levels and have a larger likelihood of creating self-sustaining populations.
Acknowledgements
I would like to greatly thank my two advisors, Dr. Edward Rutherford and Dr. Donald Scavia for offering invaluable comments and revisions for drafts, providing further insight into many topics related to my research, and supplying suggestions throughout the past year and a half. Thank you to Dr. Jim Diana for filling the position of my senior honors thesis reader. Gratitude goes to Dr. Chuck Madenjian, Dr. Randy Eschenroder, Ellen Brody, Jim Johnson, and Jerry McClain for taking the time to meet with me or provide information that contributed to the content of my thesis. I would also like to thank Dr. Rosina Bierbaum and Dr. Dean Bavington for advising all senior thesis environment students.
References
Adlerstein, S.A., Rutherford, E.S., Clevenger, J.A., Johnson, J.E., Clapp, D.F., and Woldt, A.P. 2007. Lake trout movements in US waters of Lake Huron
interpreted from coded wire tag recoveries in recreational fisheries. Journal of Great Lakes Research 33: 186-201.
Agardy, M.T. 1994. Advances in marine conservation: The role of marine protected areas. Trends in Ecology and Evolution 9: 267-270.
Airame, S., Dugan, J.E., Lafferty, K.D., Leslie, H., McArdle, D.A., and Warner, R.R. 2003. Applying ecological criteria to marine reserve design: A case study from the California Channel Islands. Ecological Applications 13 (Supplement): S170-S184.
Bence, J.R., Ebener, M.P., S.P. Sitar, S.P., and Woldt, A.P. 2003. Summary status of lake trout and lake whitefish populations in the 1836 treaty-ceded water of Lakes Superior, Huron and Michigan in 2001, with recommended yield and effort levels for 2002. Technical Fisheries Committee.
Bence, J.R., Johnson, J.E., He, J., Schaeffer, J., Riley, S., Young, R.J., Ebener, M., Reid, D., Mohr, L., Gonder, D., Cottrill, A., Woldt, A., Morse, T.J., Christie, G.C., and Ridgway, M. 2005. Offshore Predators and their Fish Community. unpublished.
Bronte, C.R., Jonas, J., Holey, M.E., Eshenroder, R.L., Toneys, M.L., McKee, P., Breidert, B., Claramunt, R.M., Ebener, M.P., Krueger, C.C., Wright, G., and Hess, R. 2003. Possible impediments to lake trout restoration in Lake Michigan. Lake Michigan Lake Trout Task Group. Great Lakes Fishery Commission. Claramunt, R.M., Jonas, J.L., Fitzsimons, J.D., and Marsden, J.E. 2005. Influences of
spawning habitat characteristics and interstitial predators on lake trout egg deposition and mortality. Transactions of the American Fisheries Society. 134: 1048-1057.
Coble, D.W., Bruesewitz, R.E., Fratt, T.W., and Scheirer, J.W. 1990. Lake trout, sea lampreys, and overfishing in the upper Great Lakes: A review and reanalysis. Transactions of the American Fisheries Society 19: 935-995.
Desorcie, T.J. and Bowen, C.A. 2003. Evidence of offshore lake trout reproduction in Lake Huron. North American Journal of Fisheries Management 23: 1253–1256 Ebener, M. P. [ED.]. 1998. A lake trout rehabilitation guide for Lake Huron. Great Lakes
Edsall T. A., Brown, C.L., Kennedy, G.W., and Poe, T.P. 1992. Lake trout spawning habitat in the Six Fathom Bank-Yankee Reef lake trout sanctuary, Lake Huron. Journal of Great Lakes Research 18: 70–90.
Eschenroder, R.L., Bronte, C.R., and Peck, J.W. 1995. Comparison of lake trout-egg survival at inshore and off shore and shallow-water and deepwater sites in Lake Superior. Journal of Great Lakes Research 21 (Supplement 1): 313-322. Eschenroder, R.L., Payne, N.R., Johnson, J.J., Bowen II, C., and Ebener, M.P. 1995.
Lake trout rehabilitation in Lake Huron. Journal of Great Lakes Research 21 (Supplement 1): 108-127.
Fitzsimons, J.D., Williston, B., Zajicek, J.L., Tillitt, D.E., Brown, S.B., Brown, L.R., Honeyfield, D.C., Warner, D.M., Rudstam, L.G., and Pearsal, W. 2005. Thiamine content and thiaminase activity of ten freshwater stocks and one marine stock of alewives. Journal of Aquatic Animal Health 17: 26–35.
Fisheries Orders - Statewide trout, salmon, whitefish and lake herring regulations. 2005. Michigan Department of Natural Resources. 200.06
Goodyear, C.D., Edsall, T.A., Ormsby-Dempsy, D.M., Moss, G.D., and Polanski, P.E. 1982. Atlas of the spawning and nursery areas of Great Lakes fishes. U.S. Fish and Wildlife Service. 5: 1-114.
Gray, J.S. 1997. Marine biodiversity: patterns, threats and conservation needs. Biodiversity and Conservation 6: 153-175.
Great Lakes Fish Stocking Database. 2007. Great Lakes Fishery Commission.
http://www.glfc.org/fishstocking/exactsearch.htm
Great Lakes Fishery Commission. 2007. Success of sea lampreys control.
http://www.glfc.org/sealamp/success.php
Hansen, M. J. [ED.]. 1996. A lake trout restoration plan for Lake Superior. Great Lakes Fishery Commission.
Hansen, M.J. and Peck, J.W. 2006. Lake trout in the Great Lakes.
http://biology.usgs.gov/s+t/noframe/m2130.htm
Honeyfield, D.C., Hinterkopf, J.P., Fitzsimons, J.D., Tillitt, D.E., Zajicek, J.L., and Brown, S.B. 2005. Development of thiamine deficiencies and early mortality syndrome in lake trout by feeding experiemental and feral fish diets
containing thiaminase. Journal of Aquatic Animal Health. 17: 4-12.
Horns, W.H., Bronte, C.R., Busiahn, T.R., Ebener, M.P., Eshenroder, R.L., Gorenflo, T., Kmiecik, N., Mattes, W., Peck, J.W., Petzold, M., and Schreiner, D.R. 2003.
Fish-community objectives for Lake Superior. Great Lakes Fishery Commission. Special publication 03-01.
Jonas, J.L., Clapp, D.F., Bence, J.R., and Holey, M.E. 2005a. Salmonine community. In
The state of Lake Michigan in 2000. Great Lakes Fishery Commission. Special publication 05-01.
Jonas, J.L., Claramunt, R.M., Fitzsimons, J.D., Marsden, J.E., and Ellrott, B.J. 2005b. Estimates of egg deposition and effects of lake trout (Salvelinus namaycush) egg predators in three regions of the Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 62: 2254-2264.
Jones, M.L., Eck, G.W., Evans, D.O., Fabrizio, M.C., Hoff, M.H., Hudson, P.L., Janssen, J., Jude, D., O’Gorman, R., and Savino, J.F. 1995. Limitations to lake trout (Salvelinus namaycush) rehabilitation in the Great Lakes imposed by biotic interactions occurring at early life stages. Journal of Great Lakes Research 21 (Supplement 1): 505-517.
Kernen, L.T. 1995. Lake trout: Valuable native fauna or just grease? Journal of Great Lakes Research 21(Supplement 1): 198-201.
Krueger, C.C., Perkins, D.L., Mills, E.L., and Marsden, J.E. 1995. Predation by alewives on lake trout fry in Lake Ontario: Role of an exotic species in preventing restoration of a native species. Journal of Great Lakes Research
21(Supplement 1): 458-469.
Madenjian, C.P, Desorcie, T.J., and McClain, J.R.. 1999. Lake trout rehabilitation in Lake Huron - 1998 progress report on coded-wire tag returns. Lake Huron Committee. Presented at Great Lakes Fishery Commission, Lake Huron Committee Meeting, Milwaukee, Wisconsin, March 22-23.
Madenjian, C.P, Desorcie, T.J., McClain, J.R., and Woldt, A. 2006a. Lake trout
rehabilitation in Lake Huron - 2005 progress report on coded-wire tag returns. Lake Huron Committee. Presented at Great Lakes Fishery Commission, Lake Huron Committee Meeting, Windsor, Ontario, March 21.
Madenjian, C.P, Desorcie, T.J., McClain, J.R., and Woldt, A. 2003. Lake trout
rehabilitation in Lake Huron - 2002 progress report on coded-wire tag returns. Lake Huron Committee. Presented at Great Lakes Fishery Commission, Lake Huron Committee Meeting, Milwaukee, Wisconsin, March 17.
Madenjian, C.P., Desorcie, T.J., McClain, J.R., Woldt, A.P., Holuszko, J.D., and Bowen, C.A. 2004. Status of lake trout rehabilitation on Six Fathom Bank and Yankee Reef in Lake Huron. North American Journal of Fisheries Management 24: 1003-1016.
Madenjian, C.P., Fahnenstiel, G.L., Johengen, T.H., Nalepa, T.F., Vanderploeg, H.A., Fleischer, G.W., Schneeberger, P.J., Benjamin, D.M., Smith, E.B., Bence, J.R., Rutherford, E.S., Lavis, D.S., Robertson, D.M., Jude, D.J., and Ebener, M.P. 2002. Dynamics of the Lake Michigan food web, 1970–2000. Canadian Journal of Fisheries and Aquatic Sciences 59: 736-753.
Madenjian, C.P., Holuszko, J.D., and Desorcie, T.J. 2006b. Spring-summer diet of lake trout on Six Fathom Bank and Yankee Reef in Lake Huron. Journal of Great Lakes Research 32: 200-208.
Marsden, J.E., Ellrott, B.J., Claramunt, R.M., Jonas, J.L., and Fitzsimons, J.D. 2005. A comparison of lake trout spawning, fry emergence, and habitat use in Lakes Michigan, Huron, and Champlain. Journal of Great Lakes Research 31: 492-508.
McClain, J.R., Bowen, C. and Savino, J. 1998. Lake trout rehabilitation in Lake Huron- 1997 progress report on coded wire tag returns. Presented at Great Lakes Fishery Commission, Lake Huron Committee Meeting, Thunder Bay, Ontario, March 18-19, 1998.
McClain, J. R. 2007. Personal communication. January 23, 2007 and March 22, 2007. Mills, E.L., Casselman, J.M., Dermott, R., Fitzsimons, J.D., Gal, G., Holeck, K.T.,
Hoyle, J.A., Johannsson, O.E., Lantry, B.F., Makarewicz, J.C., Millard, E.S., Munawar, I.F., Munawar, M., O’Gorman, R., Owens, R.W., Rudstam, L.G., Schaner, T., and Stewart, T.J. 2005. A synthesis of ecological and fish community changes in Lake Ontario, 1970-2000. Great Lakes Fishery Commission. Technical report 67.
National Research Council. 2001. Marine protected areas: Tools for sustaining ocean ecosystems. National Academies Press.
Peck, J.W. 1986. Fecundity of hatchery-origin and wild lake trout in Lake Superior. Michigan Department of Natural Resources Fisheries Division. Fisheries Research Report 1942.
Richards, R.A., and Rago, P.J. 1999. A case history of effective management: Chesapeake Bay striped bass. North American Journal of Fisheries Management 19: 356-375.
Ryan, P.A., Knight, R. MacGregor, R., Towns, G., Hoopes, R., and Culligan, W. 2003. Fish-community goals and objectives for Lake Erie. Great Lakes Fishery Commision. Special publication 56.
Sala, E., Aburto-Oropeza, O., Paredes, G., Parra, I., Barrera, J.C., and Dayton, P.K. 2002. A general model for designing networks of marine reserves. Science. 298: 1991-1993.
Sitar, S.P., Bence, J.R., Johnson, J.E., Ebener, M.P., and Taylor, W.W. 1999. Lake trout mortality and abundance in southern Lake Huron. North American Journal of Fisheries Management. 19: 881-900.
Stanley, J.G., Eshenroder, R.L. and Hartman, W.L. 1987. Sanctuaries for lake trout in the Great Lakes. Coastal Zone 3141-3152.
Walters, C. 2000. Impacts of dispersal, ecological interactions, and fishing effort dynamics on efficacy of marine protected areas: how large should protected areas be? Bulletin of Marine Science 66: 745-757.
Ward, T. and Hegerd, E. 2003. Marine protected areas in ecosystem-based management of fisheries. Department of Environment and Heritage – Australia. 1-73 Wilberg, M.J., Bence, J.R., and Johnson, J.E. 2002. Survival of juvenile lake trout
stocked in western Lake Huron during 1974–1992. North American Journal of Fisheries Management 22: 213-218.
Appendix
Appendix A Lake trout management units and refuges