Vol. 4, No. 2 (2014): 507-511 Research Article
Open Access
I
ISSSSNN:: 22332200--66881100
Phytochemical Screening and Antimicrobial activity
of
Solanum erianthum
D. Don
Radhika Mahadev
1, Ramakrishnaiah Hanumanthaiah
1*, Krishna Venkatarangaiah
2,
Deepalakshmi Arjunan Paranthaman
11
Department of P. G. Studies and Research in Biotechnology, Government Science College, Bangalore -560001, India
2
Department of P. G. Studies and Research in Biotechnology and Bioinformatics, Kuvempu University, Shankaraghatta - 577451, India
* Corresponding author: R. Hanumanthaiah; e-mail: hramabt@yahoo.com
ABSTRACT
The present study was carried out for phytochemical screening of principle bioactive compounds in Solanum erianthum. Phytochemical analysis revealed the presence of tannin, saponin, terpenoid, steroid, flavonoid and alkaloid. The petroleum ether, chloroform, methanol and aqueous extracts were subjected for antimicrobial activity against twelve bacteria and three fungal strains using agar well diffusion method. Leaf extracts inhibited B. subtilis, P. aeruginosa, K. pneumoniae, S. typhi, C. albicans and C. tropicalis. Fruit extracts possessed antimicrobial activity against B. subtilis, P. aeruginosa, S. typhi, C. albicans and C. tropicalis. P. aeruginosa showed significant sensitivity to stem extract. The Minimal Inhibition Concentration (MIC) values ranged from 1.6 - 1.92 mgmL-1. Among the different solvent extracts used for the study, the extract prepared in methanol and water proved to have greater antimicrobial activity. The results suggested that methanolic and aqueous leaf, fruit and stem extracts were highly potent against P. aeruginosa and can be used in treatment of nosocomial infections such as pneumonia, urinary tract infections (UTIs) and bacteremia. The study revealed the high medicinal property of Solanum erianthum.
Keywords:
Phytochemical analysis, Solanum erianthum, antimicrobial activity, Minimal InhibitionConcentration.
INTRODUCTION
Higher plants constitute a major reservoir of raw materials used in pharmaceuticals, cosmetics and traditional Indian medicine. Indeed much of the pharmacopoeia of scientific medicine was derived from the herbal lore of native people and majority of the drugs commonly used today are derived from plants [1].
Solanum erianthum is known to be medicinally useful.
The flavanoid rich extract of S. erianthum leaves possesses antibacterial and antifungal activity. It is effective against Gram positive bacteria and fungi such
as Aspergillus flavus and some Candida species. Leaves
are considered to be laxative and diuretic, and are used in treating leprosy, sexually-transmitted diseases and malaria [2]. Leaves possess expectorant property and used to treat hemorrhoids, scrofula and leucorrhea. Heated leaves are applied to the forehead as an analgesic for headaches while the leaf decoction is used for vertigo. An extract of root is used to treat dysentery,
fever, diarrhea, digestive problems and body pains [3]. Further, root bark is used as an anti - inflammatory and used in the treatment of arthritis. Fruits are said to be poisonous due to the presence of glyco - alkaloids. Steroidal alkaloids from S. erianthum are useful in industries as steroidal precursors. Solasodine present in fruit is useful for the production of medicinal steroids like anti - inflammatory corticosteroids, contraceptive steroids and anabolic steroids [4, 2].
Literatures are available on the phytochemical analysis and antimicrobial activity of various Solanum species. Tannins, saponins, alkaloids, flavonoids, steroid and terpenoids were found to be present in extracts of S.
xanthocarpum, S. surattense, S. nigrum and S. trilobatum
[5-8]. The antibacterial property of S. torvum against B.
subtilis and P. aeruginosa is well established [9]. Similar
results were also reported in S. surattense, S.
xanthocarpum and S. trilobatum on P. aeruginosa, B.
subtilis and S. typhi [10-12].
The present study was undertaken to evaluate phytochemical analysis and antimicrobial activity of sequential extracts of S. erianthum.
MATERIALS AND METHODS
Plant material
The specimen was collected from outskirts of Bangalore city and was identified as Solanum
erianthum D. Don. (Syn S. verbascifolium non L).
Subsequent authentication was done at Regional Research Institute, Central Council for Research in Siddha and Ayurveda with the voucher specimen no RRCBI - 4865. Voucher specimen is also maintained in the herbarium of the Research Centre (VN 510).
Extraction of plant material
Fresh field grown plants were collected and washed with running tap water followed by distilled water to remove the adhered dust particles. After blotting, the sample was air dried in shade, ground to fine powder and stored in clean air tight containers. About 30 g of each sample was placed in the soxhlet extractor for 48 h in batches and the sample was sequentially extracted separately using the solvents petroleum ether, chloroform, methanol and water. All the extracts were dried in vacuum rotary evaporator at 40 ºC under reduced pressure, weighed and stored at 4 ºC in amber bottles.
Phytochemical Screening
Phytochemical analysis was carried out for identification of tannins, saponins, steroid, cardiac glycoside, alkaloids, terpenes, flavonoids, anthraquinone and phlobatannin according to standard methods [13, 14].
Antimicrobial activity Test microorganisms
Antibacterial assay was carried out on twelve bacteria listed in Table 2, were procured from American Type Culture Collection (ATCC), National Collection of Industrial Microorganisms (NCIM), National Collection of Type Culture (NCTC), and Microbial Type Culture Collection (MTCC) Institutes. All the organisms were sub cultured and maintained on nutrient media at 37 ºC.
Antifungal assay was conducted on Candida tropicalis MTCC 184, Candida albicans MTCC 183 and Candida
glabrata MTCC 3018. Fungal cultures were maintained
on Sabouraud dextrose agar at 30 ºC.
Antibacterial activity
Antibacterial activity of sequential extracts of S.
erianthum was carried out by agar well diffusion
method using the Clinical and Laboratory Standards Institute (CLSI) and National Committee for Clinical Laboratory Standards (NCCLS) [15, 16]. Pure culture from the plates were inoculated into Mueller-Hinton Agar (MHA) plate and sub cultured at 37 ˚C for 24 h.
Inoculum was prepared by aseptically adding the culture into fresh media and the cell density was adjusted to 0.5 McFarland turbidity standard to yield a bacterial suspension of 1.5×108 cfumL-1. Standardized inoculum was transferred and spread evenly on a MHA plate to yield a lawn culture. Wells of 6 mm diameter were cut on the agar plate and 100 µL of extracts were tested with a concentration of 10 mgmL-1 of DMSO. Extracts were allowed to diffuse for half an hour at 4 ˚C and incubated at 37 ˚C for 24 h. Well containing 5 % DMSO served as negative control and gentamicin (10 μg) was used as positive control. The plates were observed for inhibition of bacterial growth that was indicated by the clear zone around the well. The size of zone of inhibition (including well) was measured in millimeters. The absence of zone inhibition was interpreted as the absence of activity. All experiments were carried out in triplicates and repeated four times under strict aseptic conditions. The activities were expressed as resistant, if the zone of inhibition was less than 7 mm, intermediate (8 - 10 mm) and sensitive if more than 11 mm [15, 16].
Anti-fungal activity
Sequential extracts of S. erianthum were used for testing as described for antibacterial activity. Sabouraud Dextrose Agar (SDA) medium was taken as the assay medium and amphotericin B (100 µg) as standard antifungal agent [17].
Minimum Inhibitory Concentrations (MIC)
MIC test was carried out by agar dilution method [18]. Different concentrations of sequential extracts of S.
erianthum from 10 to 2 mg/5 mL were prepared in
DMSO and filtered using membrane filter. The extracts were mixed thoroughly with 5 mL of autoclaved MHA medium for bacterial and SDA medium for fungal strains and poured into sterile petriplates and were allowed to solidify. Inoculum suspensions (10 μL) of various microbial isolates were seeded on individual agar plates. Growth control was prepared by inoculating 10 μL of each culture suspension on respective medium without any extract or solvent (drug-free medium). Solvent control was prepared by pouring 0.5 mL of DMSO to 5 mL of MHA medium and SDA medium followed by seeding of cultures. The plates were allowed to diffuse for 1 h and incubated at 37 ˚C for 24 h for bacterial cultures and 30 ˚C for fungal cultures. Minimum Inhibitory Concentration was determined as the lowest concentration of extract that inhibited the visible growth of a microorganism after overnight incubation.
RESULTS AND DISCUSSION
Phytochemical analysis
Table 1. Phytochemical analysis of Solanum erianthum
Secondary Metabolites
Leaf Stem Fruit Root
PE CE ME AE PE CE ME AE PE CE ME AE PE CE ME AE
Tannin - + + + - - - -
Steroid - - + + - - + + - - + + - - - -
Flavonoid - - + + - - + + - - + + - - - -
Saponin + + + + + + + + + + + + + + + +
Phlobatanin - - - -
Cardiac glycoside - - - -
Anthraquinone - - - -
Terpenoid - - + + - - + + - - + + - - - -
Alkaloid
Dragendroff - + + + - + + + - - + + - + - +
Mayer - + + + - - + + - - + + - - - -
Hager + - + + - - + + - + + + - - + -
Wagner + + + + - + + + + - + + + + + -
Note: PE - petroleum extract, CE - chloroform extract, ME - Methanolic extract, AE - Aqueous extract, + - Present, - - Absent
Steroid, terpenoid, flavanoid, alkaloid, saponin and tannin were present in methanolic and aqueous leaf extracts. Stem and fruit aqueous and methanolic extracts showed the presence of steroid, terpenoid, saponin, alkaloid, flavonoid while tannin was absent. Root showed the presence of saponin and tannin in all the extracts. Considering all the four parts studied, methanolic and aqueous extracts revealed more number of compounds. This suggests that methanol and water were able to extract majority of the compounds. Studies conducted in S. xanthocarpum, S.
surattense, S. nigrum and S. trilobatum also exhibited
the presence of saponin, tannin, alkaloid, flavonoid and steroid [5-8].
Antimicrobial activity and Minimum Inhibitory Concentration
Antimicrobial activity of methanol, aqueous, petroleum ether and chloroform extracts of S. erianthum were analyzed against twelve bacterial and three fungal strains using agar diffusion method and the results are presented in Tables 2 and 3. MIC was determined by agar dilution method for the extracts which showed positive result with antimicrobial susceptibility test (Table 4). Of all the extracts tested only methanol and aqueous extract possessed antimicrobial activity.
Table 2. Antibacterial activity of Solanum erianthum
Microorganism Zone of inhibition in mm
Bacteria Gen Leaf Stem Fruit Root
PE CE ME AE PE CE ME AE PE CE ME AE PE CE ME AE
E. coli ATCC 25922 20±0.62 - - - -
E. coli NCIM 2931 22±0.22 - - - -
E. coli NCTC 12923 21±0.13 - - - -
S. aureus ATCC 25923 23±0.41 - - - -
S. aureus NCIM 5022 25±0.37 - - - -
S. aureus NCTC 10788 22±0.51 - - - -
P. aeruginosa ATCC
27853 25±0.08 - -
*20±
0.08
*16±
0.43 - -
*23±
0.86
*20±
0.74 - -
*22±
0.36
*19±
0.83 - - - -
B. subtilis NCIM 2480 28±0.29 - - *26 ± 0.40
*18
±0.65 - - - -
*20
±0.67
*19 ±
0.77 - - - -
B. subtilis NCTC
10400 25±0.36 - - - -
K. pneumoniae NCIM
2957 28±0.65 - -
*20 ±
0.58 - - - -
S. abony ACM 5080 19±0.31 - - - -
S. abony 21±0.23 - - - -
S. typhi 27±0.60 - - *20 ±
0.61
*16
±0.24 - - - -
*12 ±
0.09 - - - - -
n = 12, *P < 0.01. Gen - Gentamicin, PE - Petroleum ether Extract; CE - Chloroform Extract; ME - Methanol Extract; AE - Aqueous Extract. + - present, - - absent
Table 3. Antifungal activity of sequential extracts of Solanum erianthum
Fungi Amp
Leaf Stem Fruit Root
PE CE ME AE PE CE ME AE PE CE ME AE PE CE ME AE
C. albicans
MTCC 183 17±0.09 - -
*14±
0.35
*13±
0.17 - -
*15±
0.63
*14±
0.71 - -
*14±
0.08
*13±
0.76 - - - -
C. tropicalis
MTCC 184 16±0.54 - -
*15±
0.28
*14±
0.85 - -
*16±
0.04
*15±
0.18 - -
*15±
0.09
*14±
0.24 - - - -
C. glabrata
MTCC 3018 18±0.43 - - - -
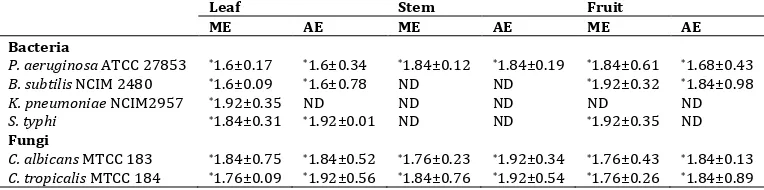
Table 4. Minimal Inhibition Concentration (MIC) of various extracts of Solanum erianthum
Leaf Stem Fruit
ME AE ME AE ME AE
Bacteria
P. aeruginosa ATCC 27853 *1.6±0.17 *1.6±0.34 *1.84±0.12 *1.84±0.19 *1.84±0.61 *1.68±0.43
B. subtilis NCIM 2480 *1.6±0.09 *1.6±0.78 ND ND *1.92±0.32 *1.84±0.98
K. pneumoniae NCIM2957 *1.92±0.35 ND ND ND ND ND
S. typhi *1.84±0.31 *1.92±0.01 ND ND *1.92±0.35 ND
Fungi
C. albicans MTCC 183 *1.84±0.75 *1.84±0.52 *1.76±0.23 *1.92±0.34 *1.76±0.43 *1.84±0.13
C. tropicalis MTCC 184 *1.76±0.09 *1.92±0.56 *1.84±0.76 *1.92±0.54 *1.76±0.26 *1.84±0.89
n = 12, *P < 0.01. ME - Methanol Extract; AE – Aqueous Extract, ND - not determined
Organisms such as B. subtilis NCIM 2480, P. aeruginosa ATCC 27853, K. pneumoniae NCIM 2957, Candida
albicans MTCC 183 and Candida tropicalis MTCC 184
were susceptible to methanolic leaf extract. The aqueous leaf extract showed inhibitory action against S.
typhi, P. aeruginosa ATCC 27853 and B. subtilis NCIM
2480. The results showed that the methanolic and aqueous extract of leaf have broad spectrum antibacterial activity against Gram positive and Gram negative bacteria. Amongst the test microorganisms, B.
subtilis was found to be most sensitive. Whilst E. coli
ATCC 25922, E. coli NCIM 2931, E. coli NCTC 12923, S.
aureus ATCC 25923, NCTC 10788 and NCIM 5022, S.
abony and Candida glabrata MTCC 3018 were
comparatively resistant. MIC values ranged from 1.92 - 1.6 mgmL-1 for methanol and aqueous leaf extracts. Methanolic leaf extract showed MIC of 1.6 mgmL-1 against P. aeruginosa and B.subtilis. Growth of K.
pneumoniae and S. typhi were inhibited at a MIC of 1.92
mgmL-1. MIC values against Candida albicans and
Candida tropicalis were 1.84 - 1.92 mgmL-1.
The methanolic fruit extract inhibited S. typhi, B. subtilis NCIM 2480 and P. aeruginosa ATCC 27853, while its aqueous extract was effective against B. subtilis NCIM 2480 and P. aeruginosa ATCC 27853. P. aeruginosa was highly susceptible. Rest of the organisms tested were found to be resistant to fruit extracts. P. aeruginosa inhibited growth at 1.84 mgmL-1 for methanolic extract and 1.68 mgmL-1 for aqueous extract. MIC values against Candida albicans and Candida tropicalis were 1.76 - 1.84 mgmL-1 for aqueous and methanolic extracts respectively.
Stem extracts were effective only against P. aeruginosa ATCC 27853, Candida albicans MTCC 183 and Candida
tropicalis MTCC 184. Other organisms tested showed
no inhibition and therefore did not show broad spectrum antimicrobial activity. Further MIC values were relatively low ranging between 1.76 mgmL-1 - 1.92
mgmL-1 for P. aeruginosa, Candida albicans and Candida
tropicalis. However, root extracts did not show
significant antimicrobial activity on the tested organisms.
Plants belonging to the Solanum genus have been reported to have remarkable pharmacological activity [19]. Wiart et al reported the antimicrobial activity of S.
torvum against B. subtilis and P. aeruginosa [9]. Similar
results were also reported in S. surattense, S.
xanthocarpum and S. trilobatum on P. aeruginosa, B.
subtilis and S. typhi [10-12].
The antimicrobial activity of methanol extracts in S.
erianthum was more pronounced than the aqueous
extract against all the organisms tested. The lower antimicrobial activities of the petroleum ether and chloroform extracts were noted against most microbial strains in the present study. Similar results were reported by Koduru et al in Solanum aculeastrum [20]. Since petroleum ether and chloroform can dissolve nonpolar compounds, due to the insolubility of the active compounds and denaturation of the active compounds during extraction process are regarded as reason for the lower activity as opined by Igbinosa et al [21].
It was noteworthy that abundance of phytochemicals such as terpenoid, alkaloid, flavonoid, steroid, saponin and tannin in S. erianthum constitutes the main antimicrobial principle as suggested by Reynolds and Dweck, Tambekar and Khante,Thambiraj and Paulsamy, Subashini et al [22-25].
In this study, the leaf and fruit extracts showed broad spectrum antimicrobial activity against Gram positive and Gram negative bacteria as well as fungal strains. The activity of the extracts against the Gram negative bacteria is significant as these bacteria are known to exhibit high degree of resistance to conventional antibiotics [26]. Similarly, fungal strains Candida
albicans MTCC 183 and Candida tropicalis MTCC 184
were inhibited by leaf, stem and fruit extracts which suggests the antifungal potential of S. erianthum. This was further corroborated by low MIC ranges for most of the organisms.
Inhibition of P. aeruginosa showed that S. erianthum can be effectively used for cure of urinary tract infection which corroborates the traditional claim of use of S. erianthum for the treatment of UTI and leucorrhoea [27]. The high inhibitory property of S.
erianthum on S. typhi and K. pneumoniae suggests that
it can be used in treatment of typhoid and pneumonia.
Candida infections of the mouth, skin, or vagina occur
for no apparent reason [28]. Since S. erianthum forbids
Candida species it can be used to reduce their
CONCLUSION
The plant S. erianthum exhibited the presence of many secondary metabolites and revealed broad spectrum antimicrobial activity on the tested microorganisms. This investigation strongly suggests the possibility of this plant as an important source of antimicrobial drug development.
REFERENCES
1. Margret C, Jayakar B. (2010). Formulation and evaluation of herbal tablets containing Ipomoea digitata Linn. extract. Int J of Pharm Sci Rev and Res 3:101-110.
2. Adam G, Huong HT, Khoi NH. (1979).The constituents of the Vietnamese drug plant Solanum verbascifolium L. Planta Med 36: 238–239.
3. Huang ST, Su YJ, Chien DK et al. (2009). Solanum erianthum intoxication mimicking an acute cerebrovascular disease. The Am J Emer Med 2: 249.
4. Blomqvist MM, Nguyentien B. (1999). Plant Resources of South-East Asia, Backhuys Publishers. Leiden, Netherlands; pp 453-460.
5. Udayakumar R, Velmurugan K, Raghuam K. (2004). Phytochemical and antimicrobial studies of extracts of Solanum xanthocarpum. Anc Sci of Life 24: 83-87.
6. Deepak SK, Sandipan D, Sunil Y. (2013). Anti-hyperglycemic activity of ethanolic extract of Solanum surattense root. Int J of Pharm Sci and Res 4: 2777-2781.
7. Zemali D, Ouahrani MR. (2014). Phytochemical study of selected medicinal plant, Solanum nigrum, the Algerian desert. Int Let of Chem, Phy and Ast 1: 25-30.
8. Doss A, Rangasamy D. (2008). Preliminary phytochemical screening and antibacterial studies of leaf extracts of Solanum trilobatum Linn. Ethno Leaflets 12: 638-642.
9. Wiart C, Mogana S, Khalifah S et al. (2004). Antimicrobial screening of plants used for traditional medicine in the state of Perak, Peninsular Malaysia. Fitoterapia 75: 68-73. 10. Sheeba E. (2010). Antibacterial activity of Solanum surattense
Burm. F. Kat Uni J of Sci, Eng and Tec 6: 1-4.
11. Raj SK, Suchitra. (2009). Evaluation of antimicrobial potential of different extracts of Solanum xanthocarpum Schard and Wendl. Afr J of Mic Res 3: 97-100.
12. Doss AH, Mohammed M, Dhanabalan R. (2009). Antibacterial activity of tannins from the leaves of Solanum trilobatum Linn. Ind J of Sci and Tec 2: 41-43.
13. Evans WC. (2008). Trease and Evans Pharmacognosy. 15th eds. Elsevier India Private Limited. Noida; pp 3-4.
14. Harborne JB. (1993). Phytochemistry. 4th eds. Academic Press. London; pp 89-131.
15. Clinical and Laboratory Standards Institute (CLSI). (2009). Method for dilution antimicrobial susceptibility tests for bacterial that grow aerobically: approved standard - 8th eds. CLSI document M07 - A8. Wayne. PA. USA.
16. National Committee for Clinical Laboratory Standards (NCCLS). (2003). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard - NCCLS document M7 - A6. Wayne. PA. USA.
17. Chee HY. (2002). In vitro evaluation of the antifungal activity of Propolis extract on Cryptococcus neoformans and Candida albicans. Mycobio 30: 93-95.
18. Mitscher LA, Lev R, Bathala MS et al. (1972). Antimicrobial agents from higher plants. Introduction, rational and methodology. Lioydi 35: 157-166.
19. Usubillago A.
(1988). Solanudine a steroid alkaloid from Solanum medum. Phy Chem. 27:30-31.
20. Koduru S, Grierson DS, Afolayan AJ. (2006). Antimicrobial activity of Solanum aculeastrum. Pharm Bio 44: 284-286. 21. Igbinosa OO, Igbinosa EO, Aiyegoro OA. (2009). Antimicrobial
activity and phytochemical screening of stem bark extracts from Jatropha curcas. Afr J of Pharm and Pharmaco 3: 58-62. 22. Reynolds T, Dweck AC. (1999). Aloe vera leaf gel; a review
update. J of Ethnopharma 68: 3-37.
23. Tambekar DH, Khante BS. (2010). Evaluation of antibacterial properties of ethnomedicinal herbs used by Korkus in Melghat of India against enteric pathogens. Int J Pharm Bio Sci 6: 31-34.
24. Thambiraj J, Paulsamy S. (2011). Evaluation of antimicrobial efficacy of the folklore medicinal plant, Acacia caesia. Asi J Pharm Clin Res 4: 103-105.
25. Subashini R, Mahesh V, Kavitha A et al. (2013) Comparative evaluation of antimicrobial activity of selected three herbal plants extract with digital image processing technique. Eur J of Bio Inf 9: 14-26.
26. Vlietinck AJ, Van Hoof L, Totte J. (1995). Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J of Ethnopharma 46: 31-47.
27. Burkill HM. (1995). The useful plants of West Tropical Africa. 2nd eds. Royal Botanic Gardens, Kew; pp 686.
28. Ahmed A, Tayela E, Wael F et al. (2013). Production of anticandidal cotton textiles treated with oak gall extract. Rev Arg Mic 45: 271-276
*****
© 2014; AIZEON Publishers; All Rights Reserved