1071-412X/97/$04.0010
Copyright © 1997, American Society for Microbiology
Western Blot Analysis of Antibody Response to Pneumococcal
Protein Antigens in a Murine Model of Pneumonia
HYAM MOUNEIMNE,1MANETTE JUVIN,1JEAN-LUC BERETTI,1ESTHER AZOULAY-DUPUIS,2
ERIC VALLEE,2PIERRE GESLIN,3PATRICK PETITPRETZ,4PATRICK BERCHE,1 ANDJEAN-LOUIS GAILLARD1*
Laboratoire de Microbiologie, INSERM U411, Faculte´ Necker-Enfants Malades,1and INSERM U13,
Hoˆpital Bichat,2Paris, Centre National de Re´fe´rence des Pneumocoques, Cre´teil,3and
Service de Pneumologie, Hoˆpital Andre´ Mignot, Versailles,4France
Received 12 May 1997/Returned for modification 5 June 1997/Accepted 23 July 1997
To detect new antigen candidates for serological tests, we studied the antibody response to pneumococcal protein antigens in mice infected intratracheally with various Streptococcus pneumoniae strains. Sera were tested by Western blotting against whole-cell protein extracts. Mice developed a detectable immunoglobulin G-type response against a small number of polypeptides. The antibody response was strain dependent: sera from individuals infected with the same strain gave similar banding patterns on immunoblots. The banding patterns varied with the strain used for infection. However, a band at 36 to 38 kDa was recognized by all reactive sera. This band appeared to correspond to a polypeptide that was antigenically well conserved among the differentS. pneumoniaeserotypes. An antibody response to this antigen developed in mice irrespective of the capsular type, the virulence, and the susceptibility to penicillin G of the infecting strain. Thus, this 36- to 38-kDa protein antigen may be of value for the development of a serological test for humans.
Streptococcus pneumoniae is a major etiological agent of
community-acquired pneumonia (14). An estimated 150,000 to 270,000 cases of pneumococcal pneumonia occur annually in the United States, with a fatality rate of approximately 5% (15). The emergence of multiple-antibiotic-resistant strains is a growing problem and complicates the therapeutic strategy for this disease (5).
Diagnosis of pneumococcal pneumonia is difficult. The iso-lation of pneumococci from blood is definitive proof of the disease’s presence (14), but it is estimated that only 20 to 25% of the cases of pneumococcal pneumonia are bacteremic (15, 17). The demonstration of pneumococci in sputum or in the nasopharynx is of little diagnostic value, as S. pneumoniae can be found as a commensal organism in the upper respiratory tracts of healthy individuals (17). Moreover, bronchial coloni-zation with S. pneumoniae is common in patients with chronic obstructive lung disease. Invasive diagnostic techniques, such as transtracheal aspiration, are both specific and sensitive. However, these techniques are unpleasant and not suitable for nonhospitalized patients. The detection of pneumococcal an-tigens in body fluids is rapid and specific but lacks sensitivity (12). Serodiagnosis of pneumococcal pneumonia could be a use-ful approach for establishing diagnosis retrospectively and for large-scale epidemiological studies. To date, interest has mainly been focused on the antibody response to pneumococ-cal capsular polysaccharides, which are major virulence factors (10). Other nonprotein pneumococcal components, such as C polysaccharide and phosphorylcholine, have also been evalu-ated as antigens in serological tests (4, 7). Among pneumococ-cal proteins, only pneumolysin has been the subject of exten-sive studies (4, 9). The sensitivity of serological tests based on this protein does not exceed 50% for patients with bacterio-logically proven pneumococcal pneumonia.
Using a Western blot technique, Renneberg et al. recently detected serum antibodies against pneumococcal polypeptides in apparently healthy individuals (18). The numbers of differ-ent polypeptides recognized by these sera were consistdiffer-ent with the levels of antibodies against type-specific polysaccharides and C polysaccharide, suggesting the possible value of this approach for serological analysis. We used a similar Western blot technique to study the antibody response to pneumococcal protein antigens in a murine model of pneumonia. This eximental model has the following advantages. Intratracheal per-oral inoculation is highly reproducible. Blood and lung cultures of samples collected 48 h after inoculation allow the degree of infection to be assessed (21). Comparable antigenic burdens can be obtained irrespective of the intrinsic virulence of the strains by adjusting the dose given to animals. Finally, experi-mental pneumococcal pneumonia in mice is a good model of primary infection, as mice do not naturally carry S.
pneu-moniae.
The S. pneumoniae strains used for infecting mice are listed in Table 1. The other S. pneumoniae strains used in this study, all obtained from the Centre National de Re´fe´rence des Pneu-mocoques (Cre´teil, France), were strains 40492 (serotype 4), 40336 (serotype 7), 40500 (serotype 9V), 40527 (serotype 14), and 40421 (serotype 18). Strains were stored at280°C in brain heart infusion (BHI) broth (BioMe´rieux, Marcy l’Etoile, France) supplemented with 5% filtered horse serum (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France). The MIC of penicillin G was determined by E-test (BMD, Marne la Valle´e, France). The intraperitoneal 50% lethal dose was es-timated by the probit method. Groups of eight mice were challenged intraperitoneally with various doses of bacteria, and the mortality rates were monitored for 3 weeks. For infection of mice, pneumococci were grown in BHI broth for 5 to 6 h at 37°C in a 5% CO2atmosphere until the optical density at 600
nm reached 0.4. Ten-milliliter aliquots were centrifuged, and the resulting pellets were resuspended in the same volume of phosphate-buffered saline (PBS; pH 7.2). For inoculations, this bacterial suspension was diluted 1:100 in PBS (virulent strains * Corresponding author. Mailing address: Laboratoire de
Microbi-ologie, INSERM U411, Faculte´ Necker-Enfants Malades, 156 rue de Vaugirard, 75730 Paris Cedex 15, France. Fax: 33 (1) 44 49 49 60.
778
on August 17, 2020 by guest
http://cvi.asm.org/
4241 and 6254) or used undiluted (all other strains). The titers of the inocula were determined by plating 0.1 ml each of serial 10-fold dilutions on Columbia agar containing 5% sheep blood (BioMe´rieux).
Female Swiss mice (Iffa-Credo Laboratories, St. Germain sur l’Arbresle, France), aged 7 weeks (20 to 22 g each), were infected by the intratracheal peroral route as described in detail elsewhere (6). Animals were anesthetized intraperitone-ally with 0.2 ml of 0.65% sodium pentobarbital (Dolethal; Vetoquinol, Lure, France). They were suspended vertically by placing the upper incisor teeth on a wire hook, with the ventral side of the mouse facing the experimenter. The trachea was then cannulated with a blunt metal needle (23 gauge) by the oral route. The bacterial inoculum was delivered in a volume of 0.05 ml via a Microliter syringe (Hamilton, Reno, Nev.). Mice were kept vertical for 5 min to allow distal alveolar migration of bacteria. Fifteen mice per group were inoculated. Amoxi-cillin (50 mg/kg of body weight) was administered intraperito-neally 48 and 72 h after pneumococcal inoculation, irrespective of the susceptibility of the infecting strain to penicillin G. Blood and lung cultures were initiated 48 h after bacterial inoculation, before amoxicillin injection. Five mice per group were sacrificed by cervical dislocation. The thorax was opened, and heart blood was collected and cultured for 18 h at 37°C in BHI broth. Cultures turbid to the naked eye were considered positive and were subcultured to confirm the presence of pneu-mococci. The lungs were dissociated from the trachea and other structures and homogenized in 1 ml of PBS. Bacterial titers were determined by plating 0.1 ml each of serial 10-fold dilutions of the homogenates on Columbia agar.
Western blots were prepared as follows. Bacteria were grown in BHI for 15 h at 37°C in a 5% CO2 atmosphere.
Ten-milliliter cultures were centrifuged, and the resulting pel-lets were washed once in PBS and resuspended in 500ml of sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) sample buffer (100 mM Tris-HCl [pH 6.8], 2% SDS, 5% 2-mercaptoethanol, 10% sucrose, 0.01% bromophe-nol blue). SDS-PAGE was carried out as described by Laemmli (11) in 11% polyacrylamide minigels (Mini Protean II; Bio-Rad, Ivry sur Seine, France). Protein concentrations were mea-sured by the method of Bradford (3): 10 to 15mg was loaded into each lane. The banding patterns obtained after staining with Coomassie brilliant blue were superimposable for all strains (not shown). The proteins were transferred to nitrocel-lulose membranes (BA 85; Schleicher & Schuell, Dassel, Ger-many) with a Mini TransBlot cell (Bio-Rad) in transfer buffer (25 mM Tris [pH 8.5], 0.2 M glycine, 20% [vol/vol] isopropa-nol). Transfer of the proteins was verified by staining with Ponceau S (Sigma). The membranes were stored at 220°C until use.
Blood samples to be analyzed were obtained by retro-orbital puncture (individual kinetics of antibody response) or heart puncture. After centrifugation, serum samples were stored at
280°C until use. Antipneumococcal antibodies were detected by probing the Western blots with the sera. Immunoglobulin G (IgG) was removed from the sera prior to IgM detection with protein G columns (HITRAP columns; Pharmacia Biotech, Or-say, France). The nitrocellulose membranes were first blocked by incubation with washing buffer (0.15% Tween 20 in PBS [pH 7.2]) containing 5% skim milk for 1 h at room tempera-ture. The membranes were then incubated for 45 min at room temperature with murine serum samples diluted 1:100 (IgG) or 1:10 (IgM). After being washed, the membranes were incubat-ed with peroxidase-conjugatincubat-ed goat anti-mouse IgG or IgM (Organon Teknika, West Chester, Pa.) for 45 min at room temperature. All sera and labeled antibody dilutions were pre-pared in washing buffer containing 5% skim milk. Antibody binding was revealed by adding 0.05% diaminobenzidine-tetra-hydrochloride (Sigma) and 0.03% hydrogen peroxide. No anti-pneumococcal response was detected in serum samples ob-tained from noninfected control mice.
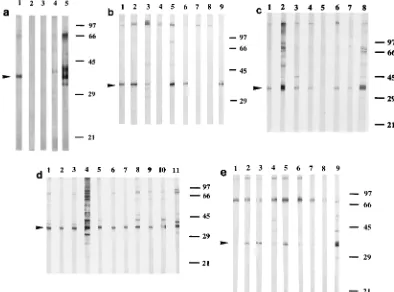
FIG. 1. Kinetics of the antipneumococcal IgG-type response in a mouse challenged with 53105CFU of strain 4241. Western blot strips of S. pneumoniae
4241 whole-cell extracts were probed with sera collected from an individual mouse on days 0 (lane 1), 3 (lane 2), 10 (lane 3), 16 (lane 4), and 30 (lane 5). Arrowheads indicate the main labeled bands, at 36 to 38 and 75 kDa. Numbers on the right are molecular masses (in kilodaltons).
FIG. 2. Antipneumococcal IgG-type antibodies 30 days after challenging mice with strain 4241 at doses of 53103(a), 53104(b), and 53105(c) CFU
per animal. Ten mice per group were infected for Western blot analysis. Western blot strips of S. pneumoniae 4241 whole-cell extracts were probed with sera collected on day 30 from surviving mice. Arrowheads indicate the 36- to 38-kDa polypeptide. Numbers on the right are molecular masses (in kilodaltons).
TABLE 1. S. pneumoniae strains used for infection
Strain Serotype Source
MIC of penicillin G
(mg/liter)
Intraperitoneal 50% lethal dose (CFU/mouse)
6254 1 Blood 0.008 13103
4241 3 Blood 0.008 ,23103
26772 6 Blood 0.032 ,2.83104
60600 6 Ear 1 ,3.43104
15986 19 Ear 4 6.33106
54988 23 Sinus 4 1.83106
TABLE 2. Results of blood and lung cultures 48 h after infection with various doses of strain 4241
Inoculum (CFU/mouse)
No. of bacteremic mice (n55)
Bacterial count in the lungs (log10CFU)a
53105 5 6.661.0
53104 2 5.162.0
53103 1 2.263.3
53102 1 1.762.9
aValues are means6standard deviations.
on August 17, 2020 by guest
http://cvi.asm.org/
Antibody response to inoculation with strain 4241 at a dose of 53105
CFU/mouse.Strain 4241 (serotype 3) was tested first because its behavior in the mouse pneumonia model is well documented (1, 21). Mice were inoculated by the intratracheal route with 53105CFU per animal, a dose at which strain 4241
has been reproducibly shown to cause pneumonia with bacte-remia in Swiss mice (21). Blood was collected by retro-orbital puncture on days 0, 3, 10, 16, and 30, and sera were tested by Western blotting for the presence of IgM and IgG antibodies against crude protein extracts prepared from strain 4241.
A group of 15 mice was challenged. The five mice sacrificed on day 2 were all bacteremic, and the bacterial count in their lungs (mean log10CFU6standard deviation) was 6.961.1.
Of the 10 mice kept for analysis of the antibody response, 5 survived until completion of the study protocol. The IgG-type responses were similar in all five. No response was observed
until day 10, when the sera recognized two bands at 37 and 75 kDa (Fig. 1). On days 16 and 30, these bands became much stronger, while several fainter bands appeared at 40, 46, 48, and 66 kDa. Overall, the sera recognized very few polypep-tides. No IgM-type response was detected in any of the mice tested.
Dose-response effect in mice challenged with strain 4241.
Groups of 15 mice were challenged with various doses of strain 4241 (53 102 to 5 3 105 CFU per animal). Five mice per
group were killed on day 2. The results of the blood and lung cultures for these animals are shown in Table 2.
Blood was collected on days 10 and 16 from five animals per group and on day 30 from all the animals still living. Only the IgG-type response was studied. No response to the smallest inoculum (53102CFU per mouse) was detected at any time.
Mice inoculated with 53104or 53103CFU did not develop
detectable responses until day 30. The reactivities of sera col-lected at this time from mice inoculated with 53103, 53104,
or 53105CFU are shown in Fig. 2. Three of four, two of four,
and four of four serum samples obtained from mice inoculated with, respectively, doses of 53103, 53104, and 53105CFU
gave positive reactions. Interestingly, the patterns of response were similar with the three doses. As previously found, the strongest response was directed against a 37-kDa polypeptide. Fainter bands were detected at 40, 48, 75, and.90 kDa.
Antibody response after infection with various S. pneu-moniaestrains.Using the same murine model of pneumonia, we studied the IgG-type responses induced by S. pneumoniae strains of various serotypes and with various susceptibilities to
FIG. 3. Antipneumococcal IgG-type antibodies 30 days after challenging mice with S. pneumoniae 6254 (a), 26772 (b), 60600 (c), 15986 (d), and 54988 (e). Ten mice per group were infected for Western blot analysis. Sera collected on day 30 from surviving mice were tested at a dilution of 1:20 (strain 6254) or 1:100 (other strains) against the whole-cell protein extracts from the corresponding infecting strains. Lanes 5, 9, 8, 11, and 9 in panels a to e, respectively, are Western blot strips probed with a serum sample obtained 30 days after inoculation of strain 4241. Arrowheads indicate the 36- to 38-kDa polypeptide. Numbers on the right are molecular masses (in kilodaltons).
TABLE 3. Results of blood and lung cultures 48 h after infection with various S. pneumoniae strains
Strain (serotype)
Inoculum (CFU/mouse)
No. of bacteremic mice/no. studied
Bacterial count in the lungs (log10CFU)a
6254 (1) 3.53105 0/3 4.560.2
26772 (6) 6.53105 3/5 5.461.2
60600 (6) 3.03105 2/5 4.860.5
15986 (19) 2.03107 0/5 4.760.5
54988 (23) 2.93107 1/5 5.561.4
a
Values are means6standard deviations.
on August 17, 2020 by guest
http://cvi.asm.org/
penicillin G and intrinsic virulences (Table 1). Groups of 15 mice were each challenged with a different strain. The infecting dose was adjusted according to the virulence of the strain so that similar bacterial burdens were obtained: on day 2, the mean bacterial counts in the lungs (for five mice per group) were between 4.5 and 5.5 log10CFU for all strains (Table 3).
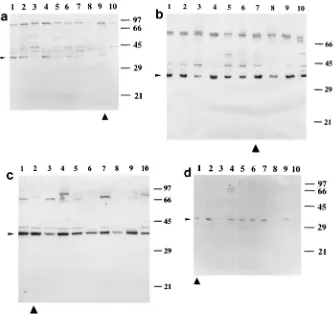
Sera collected on day 30 were analyzed by Western blotting with the infecting strain as the source of protein antigens (Fig. 3). Serum samples diluted 1:100 from surviving animals (4 to 10 mice) were tested for each strain. Strains 26772, 60600, 15986, and 54988 elicited detectable IgG-type responses in most animals. Sera from mice challenged with the same strain gave similar staining patterns. The response was strain depen-dent: the banding patterns varied according to the infecting strain. However, for all infecting strains, sera consistently gave positive signals at 36 to 38 kDa. In most cases, the band detected at this position was the most pronounced. Serum samples from mice challenged with strain 6254 were negative at a dilution of 1:100 (not shown). At a dilution of 1:20, anti-pneumococcal IgGs were detected in one of four mice (Fig. 3). Again, the strongest response was directed against a 36- to 38-kDa polypeptide.
Recognition of conserved antigens by sera. We studied whether the antigens recognized by the sera, especially the 36-to 38-kDa antigen, were type specific. For this purpose, immu-noblots of protein extracts prepared from a panel of S.
pneu-moniae strains belonging to the most common serotypes were
probed with sera obtained on day 30 of infection with strains
4241, 26772, 60600, 6254, and 15986. Each serum gave similar banding patterns with all antigenic preparations (Fig. 4). In particular, the 36- to 38-kDa antigen was recognized in all cases. This suggests that the antibodies elicited in the animals reacted with antigens found in all of the serotypes tested.
In the model of pneumonia used in this study, mice devel-oped detectable antibody responses against a small number of pneumococcal polypeptides. Sera collected 30 days postinfec-tion gave a maximum of four or five strong bands on immu-noblots, irrespective of the infecting strain. This contrasted with the large number of polypeptides stained by Coomassie brilliant blue after separation of the pneumococcal extracts by SDS-PAGE. Although outbred mice were used in this study, sera from animals challenged with a given S. pneumoniae strain gave very similar banding patterns on immunoblots. The band-ing patterns varied substantially among the strains. Thus, in the murine model of pneumonia, the antibody response to pneu-mococcal proteins appears to be strain dependent. However, sera from mice infected with one S. pneumoniae strain showed strong cross-reactivity with extracts of S. pneumoniae strains of other serotypes. This suggests that most antipneumococcal an-tibodies elicited during murine infection recognize well-served protein antigens. Rennenberg et al. also found a con-siderable degree of similarity in the banding patterns when using a given human serum to probe protein extracts from S.
pneumoniae strains of different serotypes (18). This is
consis-tent with previous studies showing that most S. pneumoniae
FIG. 4. IgG-type reactivities of sera obtained 30 days after inoculation of strains 4241 (a), 26772 (b), 15986 (c), and 54988 (d) against protein extracts from S. pneumoniae strains of the most common serotypes. Extracts were from strains 54988, serotype 23 (lanes 1); 15986, serotype 19 (lanes 2); 40421, serotype 18 (lanes 3); 40527, serotype 14 (lanes 4); 40500, serotype 9V (lanes 5); 40336, serotype 7 (lanes 6); 26772, serotype 6 (lanes 7); 40492, serotype 4 (lanes 8); 4241, serotype 3 (lanes 9); and 6254, serotype 1 (lanes 10). Arrowheads indicate the 36- to 38-kDa antigen; triangles indicate the protein extracts from infecting strains. Numbers on the right are molecular masses (in kilodaltons).
on August 17, 2020 by guest
http://cvi.asm.org/
protein antigens are common to the different capsular sero-types (16).
Among the polypeptides recognized by the sera, a polypep-tide with an apparent mobility of 36 to 38 kDa might be particularly useful as a diagnostic antigen. An antibody re-sponse to this polypeptide developed in all mice, irrespective of the capsular type, the virulence, and the susceptibility to pen-icillin G of the infecting strain. This response was the earliest and, for most strains, the strongest that was observed. Anti-bodies elicited after infection with one strain recognized a 36-to 38-kDa polypeptide in the antigenic extracts prepared from
S. pneumoniae strains of the most common serotypes. Thus,
this 36- to 38-kDa polypeptide appears to be both strongly immunogenic and highly conserved among the different S.
pneumoniae serotypes. Two other findings suggest that this
polypeptide may be suitable for serological tests with humans. First, the antibody response to the 36- to 38-kDa polypeptide was not abrogated by early antibiotic treatment. Second, the response was detectable with small bacterial burdens in the lungs. The 36- to 38-kDa polypeptide remains to be identified. However, it may be the pneumococcal protein PsaA, whose gene has been recently cloned and sequenced (20). PsaA is a 37-kDa adhesin present in virtually all S. pneumoniae strains (19). It is probably exposed on the pneumococcal surface (19) and therefore may be a preferred target for the humoral im-mune response in infected hosts. Alternatively, the 36- to 38-kDa polypeptide may be the pneumococcal autolysin LytA, whose molecular mass is 35 to 36 kDa (8), or a previously undescribed protein.
There are some limitations to this study. First, we used crude bacterial extracts as antigenic preparations and consequently may not have detected antibodies against pneumococcal pro-teins present in small amounts in these extracts. This is prob-ably why we did not find an antibody response against a 53-kDa antigen that could correspond to the pneumococcal toxin pneumolysin (2). Secondly, we detected IgG- but not IgM-type antipneumococcal antibodies. This is unlikely to be due to the inability of mice to mount an IgM-type antibody response to pneumococcal antigens. A recent study reported that mice as young as 2 weeks old produced antipneumococcal IgM anti-bodies (13). Possibly, the Western blot technique may fail to reveal low levels of IgM-type antibodies. Other techniques, for example, immunocapture assays, are much better suited to the detection of IgMs. Third, the local immune response is poten-tially important in pneumococcal pneumonia. This fact should be considered in future studies. Finally, the antibody response to pneumococcal infection may be different in humans. We are currently assessing the relevance of our findings for humans.
Financial support was received from Institut Beecham, Nanterre, France.
REFERENCES
1. Azoulay-Dupuis, E., J. P. Be´dos, E. Valle´e, D. J. Hardy, R. N. Swanson, and
J. J. Pocidalo.1991. Antipneumococcal activity of ciprofloxacin, ofloxacin,
and temafloxacin in an experimental mouse pneumonia model at various stages of the disease. J. Infect. Dis. 163:319–324.
2. Boulnois, G. J., J. C. Paton, T. J. Mitchell, and P. W. Andrew. 1991. Struc-ture and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol. Microbiol. 5:2611–2616.
3. Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye bind-ing. Anal. Biochem. 72:248–254.
4. Burman, L. A., B. Trollfors, B. Andersson, J. Henrichsen, P. Juto, I.
Kall-ings, T. Lagergard, R. Mo¨llby, and R. Norrby.1991. Diagnosis of pneumonia by culture, bacterial and viral antigen detection tests, and serology with special reference to antibodies against pneumococcal antigens. J. Infect. Dis.
163:1087–1093.
5. Doern, G. V., A. Brueggemann, H. P. Holley, Jr., and A. M. Rauch. 1996. Antimicrobial resistance of Streptococcus pneumoniae recovered from out-patients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob. Agents Che-mother. 40:1208–1213.
6. Esposito, A. L., and J. E. Pennington. 1983. Effects of aging on antibacterial mechanisms in experimental pneumonia. Am. Rev. Respir. Dis. 128:662– 667.
7. Holmberg, H., A. Krook, and A.-M. Sjo¨gren.1985. Determination of anti-bodies to pneumococcal C polysaccharide in patients with community-ac-quired pneumonia. J. Clin. Microbiol. 22:808–814.
8. Holtje, J. V., and A. Tomasz. 1976. Purification of the pneumococcal N-acetylmuramoyl-L-alanine amidase to biochemical homogeneity. J. Biol. Chem. 251:4199–4207.
9. Kanclerski, K., S. Blomquist, M. Granstro¨m, and R. Mo¨llby.1988. Serum antibodies to pneumolysin in patients with pneumonia. J. Clin. Microbiol.
26:96–100.
10. Kelly, T., J. P. Dillard, and J. Yother. 1994. Effect of genetic switching of capsular type on virulence of Streptococcus pneumoniae. Infect. Immun.
62:1813–1819.
11. Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685.
12. Lenthe-Eboa, S., G. Brighouse, R. Auckenthaler, D. Lew, A. Zwahlen, P. H.
Lambert, and F. A. Waldvogel.1987. Comparison of immunological methods for diagnosis of pneumococcal pneumonia in biological fluids. Eur. J. Clin. Microbiol. Infect. Dis. 6:28–34.
13. Lu, C.-H., C.-J. Lee, and P. Kind. 1994. Immune responses of young mice to pneumococcal type 9V polysaccharide-tetanus toxoid conjugate. Infect. Im-mun. 62:2754–2760.
14. Marrie, T. J., H. Durant, and L. Yates. 1989. Community-acquired pneu-monia requiring hospitalization: 5-year prospective study. Rev. Infect. Dis.
2:586–599.
15. Mufson, M. A. 1990. Streptococcus pneumoniae, p. 1539–1550. In G. L. Mandell, R. G. Douglas, Jr., and J. E. Bennett (ed.), Principles and practice of infectious diseases, 3rd ed. Churchill Livingstone, New York, N.Y. 16. Mun˜oz, R., C. Martin-Bourgon, and J. Casal.1988. Analysis of protein
antigens as a potential marker for Streptococcus pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 7:296–300.
17. Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity and treatment. Clin. Infect. Dis. 14:801– 809.
18. Renneberg, J., M. Svinhufvud, K. Prellner, and P. Christensen. 1991. West-ern-blot analysis of immunoglobulin G antibodies to pneumococcal protein antigens in healthy adults. Eur. J. Clin. Microbiol. Infect. Dis. 10:73–76. 19. Russell, H., J. A. Tharpe, D. E. Wells, E. H. White, and J. E. Johnson. 1990.
Monoclonal antibody recognizing a species-specific protein from Streptococ-cus pneumoniae. J. Clin. Microbiol. 28:2191–2195.
20. Sampson, J. S., S. P. O’Connor, A. R. Stinson, J. A. Tharpe, and H. Russell. 1994. Cloning and nucleotide sequence analysis of psaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect. Immun. 62:319–324.
21. Valle´e, E., E. Azoulay-Dupuis, J. P. Be´dos, B. Veber, and J. J. Pocidalo. 1993. Apport des mode`les expe´rimentaux de pneumopathie, p. 33–42. In C. Car-bon, C. Chastang, and J. M. Decazes (ed.), Infections a` pneumocoques de sensibilite´ diminue´e aux beˆta-lactamines. Springer-Verlag, Paris, France.