ISSN 2348 – 7968
Distribution and ecological risk assessment of heavy metals in
the sediment cores from Manzala lagoon, Egypt
Atef M. Abu Khatita1, Ibrahim M. Shaker2, Mohamed K. El Zawahry1 and Said A. Shetaia1
1
Geology Department, Al Azhar University, Cairo, Egypt,
2 Limnology Department, Central Laboratory for Aquaculture Research, Sharkia, Egypt
Abstract
Five sediment cores were collected from Manzala lagoon- Egypt at stations close to wastewater discharge, center and coastal zones to study their textural characteristics, organic matter content and the concentration of heavy metals (Cd, Cu, Pb, Fe, Mn and Zn). Anthropogenic factor, local and regional reference values, sediment quality guidelines (SQGs) of US EPA, pollution load index and sum of toxic units were applied to evaluate the quality of sediments. The results demonstrate that the most of studied heavy metals are vertically increasing upward and spatially decreasing from southern and eastern parts to seaward with increasing the distance from the wastewater disposal. The main sources of heavy metal contamination and related potential ecological risk are associated with wastewater disposal at the southern and eastern parts. Therefore, Wastewater should be treated before disposal to protect Manzala lagoon from heavy metal contamination.
Keywords: Sediment cores, Manzala lagoon, heavy metal
pollution, sediment quality guidelines, Ecotoxicological sense
.
1. Introduction
The coastal environment represents an eventual depository of wastewater discharges. Although the dynamic nature of marine environment allows rapid assimilation of such wastes via different processes (e.g. dilution, dispersal, oxidation, degradation, or sequestration into sediments), the capacity of other aquatic environment like lagoons to assimilation is restricted (Natesan and Seshan, 2010). In spite of the fact that metals occur in the ecosystem naturally by geogenic and lithogenic processes, the heavy metals of anthropogenic origin tend to be bioavailable and then toxic pollutants (Kaasalainen and Yli-Halla, 2003; Wuana and Okieimen, 2011; Abu Khatita et al., 2016). When inter the aquatic environment, a significant amounts of such toxic element could become directly or indirectly in contact with humans through food webs. In specific concentration, certain heavy metals are nutritionally
essential for a healthy life (e.g. Fe, Cu, Mn, and Zn), since exist in higher than normal concentrations such metals could lead to threaten human health (Farmer, 1991; Förstner and Wittmann, 1981). On the other hand, other metals such as Pb and Cd are toxic even at low concentration. According to Smith (1986), once the concentration required to cause an acute response (usually death) or a sub-lethal response, the metal turned toxicant. The long-term intake of excessive concentration of heavy metals can lead to serious diseases, especially in young children like edema of eyelids, tumor, liver and kidney damage, congestion of nasal mucous membrane and pharynx, heart rhythm changes, stomach irritation, stuffiness of head, gastrointestinal, muscular, reproductive, neurological and genetic malfunctions (Reimann and De Caritat, 1998; Bradl et al., 2005; De Vos et al., 2006;
Duzgoren-Aydin et al., 2006). The geochemical
characteristics of toxic metals in sediments depend on their chemical form, nature of the particles with which they are associated and the physicochemical and biological processes of a system. In aquatic system, pH- Eh condition and organic matter are the most important parameter controlling heavy metal behavior in sediment. (Förstner and Wittmann, 1981). Vertical concentration gradients of heavy metals in sediment cores can provide temporal information about the perturbation in the aquatic environment (Zourarah et al., 2007; Badr et al., 2009; Tang et al., 2010). Numerous studies have been reported on the aquatic sediments of Egypt along the Mediterranean Sea concerning heavy metal pollution (Abdel-Moati, 1990; Siegel et al., 1994; Chen et al., 2010; Gu et al., 2013; Elkady et al., 2015). Manzala lagoon is located in the north Egypt (northeastern part of Nile delta) along the Mediterranean coast (Fig. 1). It is the most important national producer of fishing and aquaculture. About 2785 fishing boats and 15975 fishermen are licensed in the lake water (Abdel-Rasheed, 2011) and it is estimated to yield about 38.02 % of the northern Nile Delta Lakes and is considered as the second major source of fish after Lake El-Burollus (GAFRD, 2006). It has an area about 700 km2 and includes a large number of islands that reduce the area of open water to less than 500 kmP
2
P
ISSN 2348 – 7968
Ayache et al., 2009). Unfortunately, the lagoon like most of other Egyptian lagoon aggressively impacted by human-induced environmental change. During the last three decades, the percentage of loss in the surface area increased to reach about 26% of the total area (El Kafrawy, 2004) during this period by legal and/or illegal land reclamation. Moreover, the sediments of the lagoon have been subjected to the pressures of human (Abdel-Shafy and Aly, 2002; Chen et al., 2010). About 7500 million cubic meters of untreated domestic, industrial, and agricultural drainage waters are discharged annually (Abdel-Rasheed, 2011) in to the southeastern parts of lagoon through several drains without any treatments. Approximately 65% of Cairo's municipal and industrial wastes are discharged to Manzala via Bahr El-Bakar drain, explaining the drain’s nutrient rich effluent (El-Sherif and Gharib, 2001). Sewage from Port Said, Damietta, Matariya, Manzala and Gamaliya is also discharged into the lagoon (Khalil, 1990) without any treatments.
Therefore, the objectives of this paper are: (1) to investigate vertical and spatial distributions of heavy metals in the sediments of Manzala lagoon and assessing the potential contamination sources; (2) and the relation between heavy metals contents in the sediments and other sedimentological parameters such as grain size and organic matter; (3) to evaluate the ecological environmental risk by heavy metals in sediments using different sediment quality guidelines SQGs indices and reveal the characteristics of combined contamination using the toxic unit approach. The sampling stations were selected to represent zones that affected by wastewater discharging and zones at different distances from the potential sources of pollution.
2. Material and Method
El Manzala lagoon (latitude 31o: 00\ - 31o: 35\ N and longitude 31o: 45\ - 32o: 50\ E) is the largest lagoon in Egypt. It is situated in the extremity north-eastern sector of the Nile Delta along the Mediterranean Sea and bounded by Suez Canal to the east and Damietta Branch (the main eastern branch of Nile) to the west. The southern shores of the lagoon form the north boundary of Dakahliya and Sharkiya governorates. The lagoon has salt-water dominant northern zone graduated to brackish at the middle zone, then fresh water dominant southern and eastern parts. Manzala lagoon is shallow lagoon with an average depth of 1.3 m, and received both of fluvial, ground water and marine sea water (Reinhardt et al., 2001). It feds by sea water from Mediterranean Sea at the northern part and from the Red Sea via the Suez Canal at the northeastern part. Prior to High Dam construction (1964), the main freshwater sources supply the lagoon were the groundwater
and the annual flood of the Nile River, but the construction of the Dam leads to loss of the annul Nile flooding which compensated by an increase in drains and canals that inputs fresh water to the lagoon at the southeastern and eastern parts (Shaheen and Yousef, 1978). About 7500 million cubic meters of untreated domestic, industrial, and agricultural drainage waters are discharged annually (Abdel-Rasheed, 2011) to the lagoon through several drains include Bahr El-Bakar drain (domestic, industrial wastewater), Hadous, Ramsis, and El Tawil drains (mainly with agricultural domestic, industrial wastewater) at the south and El-Serw, Faraskor and El Inaniya drains (domestic and agriculture effluents) at southwest and western sides. This results in the accumulation and distribution of contaminants like heavy metals in the lagoon sediments. Manual push core (100- 130 cm length) sediment samples were collected from 5 sites (Fig. 1) by pushing a plastic tube (2-3m long and 9 cm diameter) through bottom sediment. Then, the core samples were raised with the help of a tight pneumatic seal at top of the plastic tube. The two ends of core were closed immediately after extraction by stopper and transferred to laboratory for analyses. The sampling sites include; core I represents outlet area (El-Gamil, New El-Gamil outlets) that connected lagoon to Mediterranean Sea; cores, II and IV represent the southern area closing the mouth of main drains that discharge the wastewater; core III at a distance from the mouth of drains and; core Vrepresents the main part of lagoon. In laboratory, the sediment cores have been splitted and the undisturbed sediments were described then every core has been sliced into samples of approximately 2-4 cm length. Grain size measurements were performed by hydrometer (152-H) method according to Bouyoucos (1962). For heavy metal analysis, the sediment samples were dried at 105°C, grinded, sieved and the most fine grains (1.0 gm) were digested with a mixture of HR2ROR2R(30 ml) and 2 N HC1 (10 ml) till evaporation, then with concentrated HCl (20 ml) and HNOR3R (10 ml). After dryness, the samples were dissolved in 6 N HC1 then filtered and kept in 2 N HCI after filtration prior to elemental measurements according to the method of Nelson & Sommers (1982). The amounts of heavy metals (Fe, Cu, Pb, Zn, Cd, Mn) were determined by the atomic absorption (Thermo Electron Corporation S-Series AA Spectrometer).
3. Results and Discussion
3.1 Grain size
ISSN 2348 – 7968
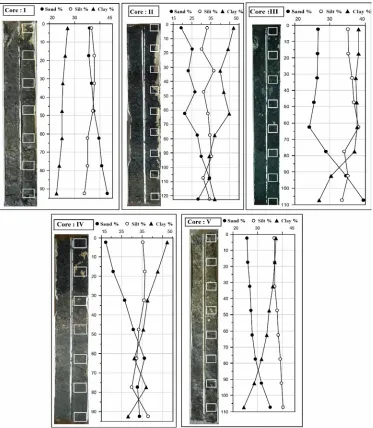
elements are not homogeneously distributed over the various grain size fractions (Abu Khatita, 2011). There is a strong relation between an increase in metal concentration and a decrease in grain size. Fine-grained particles, because of their large specific surface areas, are the main sites for the accumulation and transport of metals (Jenne et al., 1980; Abu Khatita, 2011).Since fine grain are more soluble than coarser ones the bioavailability of metals increase with decreasing the grains that metals are fixed with (Everaart and Fischer, 1992; Seshan et al., 2010). Grain size analysis was carried out for all sediment samples of collected cores. The result indicated that, the percentage of clay size increasing with decreasing the depth to reach the maximum values at the uppermost parts of all studied cores (Fig. 2). The highest of clay content
was registered at the uppermost parts of cores II and IV which display approximately the same value (47.2%). The average concentration of the clay size fraction can arrange as I < V < III < IV < II (Table 1). On the other hand, sand fraction increases with increasing the depth. The highest percentage of sand is 43 % at the lowermost part of core I. The average concentration of the sand size fraction can arrange as II < IV ≈ V < III < I. The silt contents fluctuate with narrow range (30- 40 %) along the studied downcores. The grain size distribution in the bottom sediments of lagoon may indicate that, sediment at the southern area transported in suspension with the material fluvial from agricultural, domestics and other anthropogenic activities.
ISSN 2348 – 7968
Fig. 2: The vertical distribution of sand-silt-clay fractions in downcore sediment samples.
3.2. Organic matter
Such as grain size, organic matter plays the key role as a heavy metal carrier. Dissolved organic matter can influences on the distribution of metals as follows: Dissolved organic substances are capable of (1) complexing metals and increasing metal solubility; (2) altering the distribution between oxidized and reduced formsof metals; (3) alleviating metals toxicityand altering metal availability to aquatic life; (4) influencing the extent to which metals are adsorbed on suspended matter, and (5) affecting the stabilityof metal-containing colloids (Singer, 1977; Förstner and Wittman, 1981). In this study, the vertical distributions of organic matter content illustrate
ISSN 2348 – 7968
Table 1: General features of sediment core samples (average values) of sampling sites.
Sediment
core O.M. % pH Sand
Grain Size%
Silt Clay
I 5.4 7.3 38.6 36.3 25.1
II 6.5 7.8 28.7 39.4 32.0
III 7.3 7.5 29.1 35.5 35.4
IV 9.3 7.4 39.4 35.8 24.8
V 6.0 7.5 28.7 38.3 33.0
Fig. 3: The vertical distribution of organic matter in downcore samples.
3.3. Heavy metals
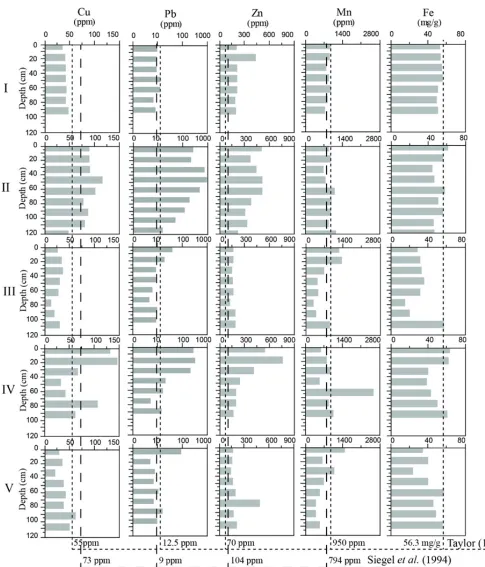
Average concentration of heavy metals in the core sediments are summarized in Table 2. The concentration of iron varied from 15486 mg/g (at lower parts of core II) to 63252 mg/g (at the surface layer of core IV) with an average concentration of 45923 mg/g. The vertical variation of iron (Fig. 4) indicates that the upper layers of cores II and IV are higher than crustal abundance (Taylor, 1964). Spatial variation of average concentration of iron is arranged in the order III < V < IV < II < I. The vertical and spatial concentration of iron confirm that, cores II and IV are affected by anthropogenic activates. The high concentration along core I may attributed to Fe- bearing minerals of black sand deposits that concentrated along the Mediterranean coast.
ISSN 2348 – 7968
The concentration of copper varies between 12.9 ppm (at the middle part of core II) to 146.3 ppm (at the subsurface layer of core IV) with an average concentration of 55.7 ppm. Excess Cu concentrations along cores II and IV than local and regional reference values of Siegel et al. (1994) and Taylor (1964) respectively comparing to other sites which confirm the anthropogenic sources. The average concentrations of cupper increase in the order III< V< I≤ IV < II.
Concentrations of Pb in the studied sediment cores varied from 4.7 ppm at 75-80 cm of core III to reach the highest concentration (954 ppm) at the upper half part (45-50 cm)
of core II with an average of 101 ppm in the study area in
Manzala sediment. Except core I, the concentration of Pb at the upper layers of all studied cores violates the
reference lines of Taylor (1964) Siegel et al. (1994) as
shown in Fig. 4, which indicates anthropogenic source of Pb. Through the spatial distribution, the highest average concentration of Pb throughout studied cores is observed at core II and decrease toward the coastal area. The average concentrations of Pb increase in the order I< III< V≤ IV < II.
Concentration of zinc varies from 123 ppm (at 75-80 cm of
core III) to 827.5 ppm (at 15-20 cm of core IV) with an
average of 273 ppm in the study area. Fig. 4 illustrates that, along all studied sediment cores, Zn values are exceeded than the values the reference values of Taylor (1964) and Siegel et al. (1994). Spatially, average concentrations of Zn exhibit the following order as III < V < I ≤ IV < II. It has observed however that, the vertical distribution of Zn in cores II and IV display similar distribution pattern of Pb which indicated the same anthropogenic source.
Through all studied samples, little amount of cadmium is present in some samples of Core II and IV. The low concentration (0.03 ppm) is observed at 60-65 cm of core II while the highest concentration at the upper most layer of Core IV (Fig. 5). The distribution pattern of Cd indicates localized contamination.
3.4. Anthropogenic factor (AF)
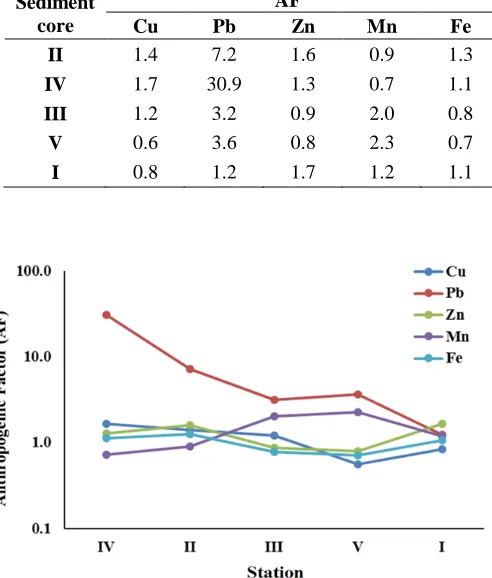
To identify and quantify the anthropogenic inputs of studied heavy metals in relation to a natural background, anthropogenic factors of elements in the core sediment samples were calculated according to the following algorithm AF = Cs/Cd (Siegel et al., 1994; Szefer et al., 1998). Where Cs and Cd are the concentration of elements in surface and depth samples respectively in the sediment core. In this study average element concentration in the upper 20 cm of a sediment core were selected to represent the surface concentration and samples deeper than 70 cm were established as the baseline concentrations. This depth was selected based on the calculation of sedimentation rate (0.5- 0.7 cm) in Manzala (Siegel, 1994) where samples deeper than this depth assumed to indicate pre industrialization baseline values. The calculated AF is listed in Table 3and graphically illustrated in Fig. 6. Core II and IV are influenced by all studied heavy metals particularly Pb which shows extremely high AF. As illustrated in Fig. 6, the AF is gradually decrease from the southern parts (close to the drain outfall) to rich the minimum value at the outlet.
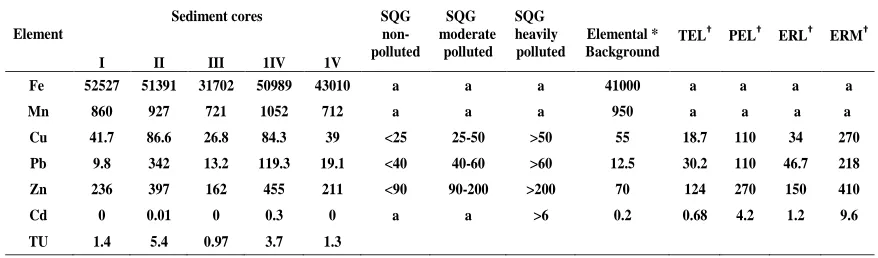
Table 2: Average concentration of heavy metals of sediment cores, its comparison with SQGs and sum of the toxic units (∑TU) of core sediments.
a; not available data. * Crustal average (Taylor, 1964). TEL: threshold effect level; PEL: probable effect level; ERL: effects range low; ERM: effects range median. † Threshold effect level or probable effect level for freshwater ecosystem (MacDonald et al., 2000).
Element
Sediment cores SQG
non- polluted
SQG moderate
polluted SQG heavily polluted
Elemental * Background
TEL† PEL† ERL† ERM†
I II III 1IV 1V
Fe 52527 51391 31702 50989 43010 a a a 41000 a a a a
Mn 860 927 721 1052 712 a a a 950 a a a a
Cu 41.7 86.6 26.8 84.3 39 <25 25-50 >50 55 18.7 110 34 270
Pb 9.8 342 13.2 119.3 19.1 <40 40-60 >60 12.5 30.2 110 46.7 218
Zn 236 397 162 455 211 <90 90-200 >200 70 124 270 150 410
Cd 0 0.01 0 0.3 0 a a >6 0.2 0.68 4.2 1.2 9.6
ISSN 2348 – 7968
ISSN 2348 – 7968
Fig. 5: the vertical distribution of Cd in core samples I and IV of Manzala lagoon. Dashed lines illustrate local and regional reference values of Siegel et al. (1994) and Taylor (1964) respectively.
3.5. Ecotoxicological sense of heavy metal
contamination
The sediment quality guidelines (SQGs) developed for aquatic ecosystem (Long and MacDonald, 1998; MacDonald et al., 2000; Pekey et al., 2004) were applied in this investigation to estimate the ecotoxicological sense of heavy metal contamination in the bottom sediments of Manzala lagoon. The SQGs including the following effects: effect range low (ERL) and effect range median (ERM), the threshold effect level (TEL) and probable effect level (PEL) values. Low-range values (i.e., ERLs or TELs) are concentrations less than which adverse effects upon sediment dwelling fauna would not be expected. In contrast, the ERMs and PELs represent chemical concentrations above which adverse effects are likely to occur (Long et al. 1998). From Table 2, it is evident that, sites I, III and V are at low to moderate toxicity according to TEL and PEL values. On the other hand, sites II and IV are most toxic because Pb and Zn violate PEL and ERM values. Exceedances of Cu than TEL value in all sediment samples indicates that, Manzala would be occasionally associated with biological effects regarding to this element. Moreover, the sediment samples of Manzala were estimated as the sum of the toxic units (∑TU) defined as the ratio of the determined concentration to PEL value (Pedersen et al. 1998). In Table 2 the ∑TUs values for each sites based on the average concentration of Cu, Pb, Zn and Cd are given and illustrated in Fig. 7. The stations can be ordered according to their pollution degree as II >IV >III ≈ V > I.
Table 3: Anthropogenic factor (AF) for heavy metals in sediment cores of Manzala lagoon. The lower parts of the downcore sections were taken as a background.
Sediment core
AF
Cu Pb Zn Mn Fe
II 1.4 7.2 1.6 0.9 1.3
IV 1.7 30.9 1.3 0.7 1.1
III 1.2 3.2 0.9 2.0 0.8
V 0.6 3.6 0.8 2.3 0.7
I 0.8 1.2 1.7 1.2 1.1
Fig. 6: Graphic representation of the anthropogenic factors (AF) of the heavy metals in the sediment cores.
Fig. 7: Estimated sum of the toxic units (∑TUs) of sediment cores in
Manzala lagoon.
ISSN 2348 – 7968
3.6. Assessment of sediment contamination
according to sediment quality guidelines
Depending on the SQG of US EPA, sediments were classified as: non-polluted, moderately polluted and heavily polluted (Perin et al., 1997). In general, average shale values (Turekian and Wedepohl, 1961) and average crustal abundance (Taylor, 1964) are frequently used as a global background concentrations of elements (Windom et al., 1989; Rubio et al., 2000; Pekey et al., 2004; Bakan and Özkoç, 2007). In this study, crustal abundance (Taylor, 1964) was used as background elemental concentrations. Average concentration of heavy metals in the core sediments that presented in Table 2 show that concentrations of Fe, Cu, Pb, and Zn in sites II and IV were greater than elemental background concentrations. Moreover, Mn exceeded the background concentration in core IV. What is interesting is the highest concentration (52527 ppm) of Fe is recorded in core I (at the outlet) close to the Mediterranean coast. The excessive of Fe at the Mediterranean coast most probably due to the concentration of black sand that contains high concentration Fe- bearing minerals (Wassef, 1981; Abdel-Karim and El-Shafey, 2012). Other sites - except Zn in site V- were equivalent or smaller than the reference baselines. Spatially, Manzala sediments can be categorized as moderately to heavily polluted with Cu, Fe, Pb, Mn, and Zn using SQGs. Cd in sediments was rated non-polluted element.
4. Conclusion
Along all collected core sediments, clay size fraction and organic matter are increased upward. The highest clay and organic matter contents were observed at the cores II and IV that collected from the southern and southeastern parts of the lagoon. Increasing the concentration of heavy metals (Cd, Cu, Pb, Zn and Fe) upward through sediment cores collected from the southern parts indicates deposition rate of heavy metals in the lake increases with time and violate both regional (Taylor, 1964) and local (Siegel et al., 1994) reference values. Sites I, III and V are at low to moderate toxicity according to TEL and PEL values, while sites II and IV are most toxic because Pb and Zn transcend PEL and ERM values. Spatially, Manzala sediments can be categorized as moderately to heavily polluted with Cu , Fe, Pb, Mn, and Zn using SQGs and based on toxic unit (∑TU), the stations can be ordered according to their pollution degree as II >IV >III ≈ V > I. The above results confirmed that, the lagoon is facing serious threat of metal pollution and the major source for the metal contamination is the wastewater effluents via drains at the southern and
southeastern parts of lagoon. Therefore, Wastewater should be treated before disposal to protect Manzala lagoon from heavy metal contamination.
References
Abdel-Karim, A. A. M., & El-Shafey, A. H. M. (2012). Mineralogy and chemical distribution study of placer cassiterite and some associated new recorded minerals, east Rosetta, Egypt. Arabian Journal of Geosciences, 5(4), 807-816.
Abdel-Moati, A. R. (1990). Speciation and behavior of arsenic in the Nile delta lakes. Water, Air, and Soil Pollution, 51(1-2), 117-132.
Abdel-Rasheed, M. E., (2011): Ecological studies on Lake El-Manzalah with special reference to their water quality and sediment productivity. M. Sc. Thesis, Fac. Sci., Al Azhar University. Egypt.
Abdel-Shafy, H., & Aly, R. (2002). Water issue in Egypt: Resources, pollution and protection endeavors.
Navigation, 49(3.1), 4-6.
Abu Khatita, A. M. (2011). Assessment of soil and sediment contamination in the Middle Nile Delta area (Egypt)-Geo-Environmental study using combined sedimentological, geophysical and geochemical methods (Doctoral dissertation, Doctoral thesis, 214p).
Abu Khatita, A. M. A., de Wall, H., & Koch, R. (2016). Anthropogenic particle dispersions in topsoils of the Middle Nile Delta: a preliminary study on the contamination around industrial and commercial areas in Egypt. Environmental Earth Sciences, 75(3), 1-19.
Ahmed, M. H., El Leithy, B. M., Thompson, J. R., Flower, R. J., Ramdani, M., Ayache, F., & Hassan, S. M. (2009). Application of remote sensing to site characterisation and environmental change analysis of North African coastal lagoons. Hydrobiologia,
622(1), 147-171.
Ayache, F., Thompson, J. R., Flower, R. J., Boujarra, A., Rouatbi, F., & Makina, H. (2009). Environmental characteristics, landscape history and pressures on three coastal lagoons in the Southern Mediterranean Region: Merja Zerga (Morocco), Ghar El Melh (Tunisia) and Lake Manzala (Egypt). Hydrobiologia,
622(1), 15-43.
Badr, N. B., El-Fiky, A. A., Mostafa, A. R., & Al-Mur, B. A. (2009). Metal pollution records in core sediments of some Red Sea coastal areas, Kingdom of Saudi Arabia. Environmental monitoring and assessment, 155(1-4), 509-526.
ISSN 2348 – 7968
sediments on biota from the mid‐Black Sea coast of Turkey. International Journal of Environmental Studies, 64(1), 45-57.
Bouyoucos, G. J. (1962). Hydrometer method improved for making particle size analyses of soils. Agronomy Journal, 54(5), 464-465.
Bradl, H., Kim, C., Kramar, U., and Stüben, D., (2005): Interactions of Heavy Metals, in Bradl, H. B. (Ed); Heavy Metals in the Environment: Origin, Interaction and Remediation. Elsevier Academic Press; Amsterdam, pp. 28-148.
Chen, Z., Salem, A., Xu, Z., & Zhang, W. (2010). Ecological implications of heavy metal concentrations in the sediments of Burullus Lagoon of Nile Delta, Egypt. Estuarine, Coastal and Shelf Science, 86(3), 491-498.
De Vos, W., and Tarvainen, T. (Chief-editors), Salminen R., Reeder S., De Vivo B. and Demetriades, A et al. (2006). Geochemical Atlas of Europe, part 2 - Interpretation of Geochemical Maps, Additional Tables, Figures, Maps, and Related Publications. Espoo: Geological Survey of Finland, pp. 690. Duzgoren-Aydin N. S., Wong C. S. C., Aydin A, Song Z.,
You M., Li X. D. (2006). Heavy metal contamination and distribution in the urban environment of Guangzhou, SE China. Environ Geochem Health 28,375–391.
Elkady, A. A., Sweet, S. T., Wade, T. L., & Klein, A. G. (2015). Distribution and assessment of heavy metals in the aquatic environment of Lake Manzala, Egypt.
Ecological Indicators, 58, 445-457.
El-Kafrawy, S. B. (2004). Monitoring of pollutants in marine environment using remote sensing and GIS system. M. Sc. Thesis, Fac. of Sci., Al-Azhar Univ., Egypt.
El-Sherif, Z. M., & Gharib, S. M. (2001). Spatial and temporal patterns of phytoplankton communities in Manzalah Lagoon. Bulletin of the National Institute of Oceanography and Fisheries(Egypt), 27, 217-239. Everaarts, J. M., & Fischer, C. V. (1992). The distribution
of heavy metals (Cu, Zn, Cd, Pb) in the fine fraction of surface sediments of the North Sea. Netherlands Journal of Sea Research, 29(4), 323-331.
Farmer, J. G. (1991). The perturbation of historical pollution records in aquatic sediments.
Environmental Geochemistry and Health, 13(2), 76-83.
Förstner, U., & Wittmann, G. T. W. (1981). Metal pollution in the Aquatic Environment. Springer Verlag, Berlin Heidelberg, pp. 486.
GAFRD, (2006). General Authority for Fishery Resources Development. Year-Book of fishery statistics in Egypt (1990-2005), Cairo.
Gu, J., Salem, A., & Chen, Z. (2013). Lagoons of the Nile delta, Egypt, heavy metal sink: with a special reference to the Yangtze estuary of China. Estuarine,
Coastal and Shelf Science, 117, 282-292.
Janaki-Raman, D., Jonathan, M. P., Srinivasalu, S., Armstrong-Altrin, J. S., Mohan, S. P., & Ram-Mohan, V. (2007). Trace metal enrichments in core sediments in Muthupet mangroves, SE coast of India: Application of acid leachable technique.
Environmental pollution, 145(1), 245-257.
Kaasalainen, M., & Yli-Halla, M. (2003). Use of sequential extraction to assess metal partitioning in soils. Environmental Pollution, 126(2), 225-233. Khalil, M. T. (1990). The physical and chemical
environment of Lake Manzala, Egypt. Hydrobiologia,
196(3), 193-199.
Long, E. R., & MacDonald, D. D. (1998). Recommended uses of empirically derived, sediment quality guidelines for marine and estuarine ecosystems.
Human and Ecological Risk Assessment, 4(5), 1019-1039.
Long, E. R., Field, L. J., & MacDonald, D. D., (1998). Predicting toxicity in marine sediments with numerical sediment quality guidelines. Environ. Toxicol. Chem. 17 (4), 714–727.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater
ecosystems. Archives of environmental
contamination and toxicology, 39(1), 20-31.
Natesan U, Seshan B R, (2010): Vertical profile of heavy metal concentration in core sediments of Buckingham canal, Ennore. Indian Journal of Geo-Marine Sciences, 40(1): 83-97.
Nelson, D. W., & Sommers, L. E. (1982). Methods of Soil Analysis Part 2, Chemical and Microbiological Properties. Amer. Soc. Agronomy, Madison.
Pedersen, F., Bjørnestad, E., Andersen, H. V., Kjølholt, J., & Poll, C. (1998). Characterization of sediments from Copenhagen Harbour by use of biotests. Water Science and Technology, 37(6), 233-240.
Pekey, H., Karakaş, D., Ayberk, S., Tolun, L., & Bakoǧlu, M. (2004). Ecological risk assessment using trace elements from surface sediments of Izmit Bay (Northeastern Marmara Sea) Turkey. Marine Pollution Bulletin, 48(9), 946-953.
ISSN 2348 – 7968
sediments (Rio de Janeiro, Brazil) and evaluation of the metal bioavailability by means of geochemical speciation. Water Research, 31(12), 3017-3028. Reimann, C. and de Caritat. P., (1998): Chemical Elements
in the Environment, Springer- Verlag, Berlin, Heidelberg, New York, pp. 398.
Reinhardt, E. G., Stanley, D. J., & Schwarcz, H. P. (2001). Human-induced desalinization of Manzala Lagoon, Nile Delta, Egypt: evidence from isotopic analysis of benthic invertebrates. Journal of Coastal Research,
17(2).431-442.
Rubio, B., Nombela, M. A., & Vilas, F. (2000). Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): an assessment of metal pollution. Marine Pollution Bulletin, 40(11), 968-980.
Seshan, B. R. R., Natesan, U., & Deepthi, K. (2010). Geochemical and statistical approach for evaluation of heavy metal pollution in core sediments in southeast coast of India. International Journal of Environmental Science & Technology, 7(2), 291-306. Shaheen, A. H., & Yosef, S. F. (1978). Effect of the
cessation of nile flood on the hydrographic features of lake manzala, egypt. Archiv fur hydrobiologie,
84(3), 339-367.
Siegel, F. R., Slaboda, M. L., & Stanley, D. J. (1994). Metal pollution loading, Manzalah lagoon, Nile Delta, Egypt: implications for aquaculture.
Environmental Geology, 23(2), 89-98.
Singer, P. C. (1977). Influence of dissolved organics on the distribution, transport, and fate of heavy metals in aquatic systems. In: Fate of Pollutants in the Air and Water Environment. Part. I. Suffet, I.H. (ed.). New York, pp. 155-182.
Smith, D.G. (1986): Heavy Metals in the New Zealand Aquatic Environment: A Review, Water Quality Centre, Ministry of Works and Development, Wellington, New Zealand.
Szefer, P., Glasby, G. P., Kusak, A., Szefer, K., Jankowska, H., Wolowicz, M., & Ali, A. A. (1998). Evaluation of the anthropogenic influx of metallic pollutants into Puck Bay, southern Baltic. Applied Geochemistry, 13(3), 293-304.
Tang, W., Shan, B., Zhang, H., & Mao, Z. (2010). Heavy metal sources and associated risk in response to agricultural intensification in the estuarine sediments of Chaohu Lake Valley, East China. Journal of Hazardous Materials, 176(1), 945-951.
Taylor, S. R. (1964). Abundance of chemical elements in the continental crust: a new table. Geochimica et cosmochimica acta, 28(8), 1273-1285.
Turekian, K. K. & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the earth‟s crust. Bulletin of Geological Society of America; 72, 175–192.
Wassef, S. N. (1981). Distribution and properties of placer ilmenite in East Rosetta beach sands, Egypt.
Mineralium Deposita, 16(2), 259-267.
Windom, H.L., Schropp, S.J., Calder, F.D., Ryan, J.D., Smith, R.G., Burney, L.C., Lewis, F.G., Rawlinson, C.H., (1989). Natural trace metal concentrations in estuarine and coastal marine sediments of the Southeastern United States. Environmental Science and Technology 23 (3), 314–320.
Wuana, R. A., & Okieimen, F. E. (2011). Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation.
ISRN Ecology, 2011.
Zourarah, B., Maanan, M., Carruesco, C., Aajjane, A., Mehdi, K., & Freitas, M. C. (2007). Fifty-year sedimentary record of heavy metal pollution in the lagoon of Oualidia (Moroccan Atlantic coast).