HOST RESISTANCE AND INTERACTION BETWEEN ROOT KNOT NEMATODES AND FUSARIUM WILT OF TOMATO
KARIUKI PAULINE M (B.Ed Science.)
I56/CE/22259/2010
A thesis submitted in partial fulfillment of the requirements for the award of the degree of Master of Science (Plant Pathology), in the School of Pure and Applied Sciences of
Kenyatta University.
DEDICATION
ACKNOWLEDGEMENTS
TABLE OF CONTENTS
DECLARATION………. Error! Bookmark not defined.
DEDICATION……… i
ACKNOWLEDGEMENTS……… iv
TABLE OF CONTENTS……… v
LIST OF FIGURES……… ix
LIST OF TABLES………. xi
LIST OF PLATES………. xii
ABBREVIATIONS AND ACRONYMS……… xiii
ABSTRACT………... xv
CHAPTER ONE;………. 1
INTRODUCTION………. 1
1.1 Background to the study………... ... 1
1.2 Problem statement and justification ... 3
1.3 Research questions ... 4
1.4 Objectives ... 4
1.4.1 General Objective ... 4
1.4.2 Specific objectives ... 4
1.5 Hypotheses ... 5
CHAPTER TWO………. 6
LITERATURE REVIEW…... 6
2.1 Tomato production in Kenya………... 6
2.2 Ecological requirements of tomato ... 7
2.3 Tomato nutrient composition and benefits ... 7
2.5 Meloidogyne species ... 9
2.5.1 Economic importance of Meloidogyne species ... 9
2.5.2 Biology, life cycle and symptoms of Meloidogyne species ... 12
2.5.3 Nematode survival and dispersal... 13
2.5.4 Identification of Meloidogyne spp ... 14
2.5.5 Symptoms of Meloidogyne spp. infection in host crop ... 15
2.6 Fusarium oxysporum ... 16
2.6.1 General characteristics of Fusarium spp. ... 16
2.6.2 Economic importance of F. oxysporum ... 17
2.6.3 Fusarium wilt of tomatoes ... 18
2.7 Biology, life cycle and symptoms of Fusarium wilt pathogen ... 19
2.7.1 Fusarium oxysporum Taxonomy ... 19
2.7.2 Identification of Fusarium species ... 21
2.7.3 Symptoms of Fusarium wilt of tomatoes ... 22
2.7.4 Resistance to Fusariumwilt by host plants ... 23
2.8 Interaction between plant parasitic nematodes and Fusarium wilt pathogen ... 23
2.9 Management of Meloidogyne spp. and Fusarium spp. ... 27
2.10Interaction of nematodes with tomato wilt inducing fungus ... 29
2.11Screening for resistance ... 31
CHAPTER THREE:……….. 33
MATERIALS AND METHODS………... 33
3.1 Identification of root knot nematodes and Fusarium with pathogen infecting tomato in the Coastal region of Kenya ... 33
3.1.1 Description of the study sites ... 33
3.1.2 Sampling of Meloidogyne spp. and Fusarium spp. infected plants and infested soil .... 34
3.2 Determination of interaction between Meloidogyne spp. and Fusarium spp. on
tomato ... 36
3.2.1 Preparation of nematode inocula ... 36
3.2.2 Identification of Meloidogyne spp. isolated from soils ... 37
3.2.3 Isolation and identification of Fusarium spp. from the infected tomato plants ... 37
3.2.4 Preparation of F. oxysporum inoculum ... 38
3.2.5 Determination of pathogenicity of the F. oxysporum isolates ... 39
3.2.6 Molecular characterization of Fusarium spp. ... 40
3.3 Evaluation of tomato cultivars for resistance to root knot nematodes and Fusarium wilt ... 42
3.3.1 Effect of disease complex on the tomato cultivars ... 42
3.3.2 Effect of disease complex on tomato growth in the screen house ... 42
3.4 Field experiment... 46
3.5 Statistical analysis of data ... 47
CHAPTER FOUR……….. 48
RESULTS……… 48
4.1 Meloidogyne spp. isolated from the Coastal region ... 48
4.1.1 Molecular identity of Meloidogyne spp. ... 48
4.2 Fusarium species isolated from tomato in Coastal region ... 49
4.3 Effect of Meloidogyne spp. and Fusarium oxysporum f. sp. Lycopersici infection on tomato growth ... 52
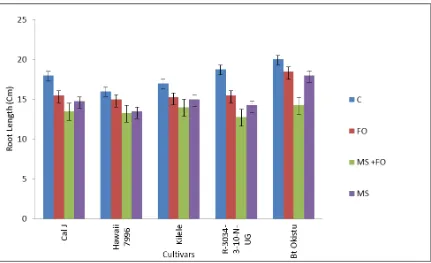
4.3.1 Effects of F. oxysporum and Meloidogyne spp. on root length on the five tomato cultivars ... 52
4.3.2 Effects of F. oxysporum and Meloidogyne spp. on root dry weight on the five tomato cultivars ... 52
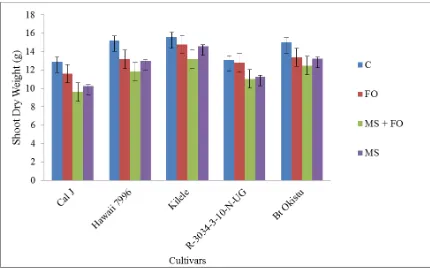
4.3.4 Effects of F. oxysporum and Meloidogyne spp. on shoot dry weight on the five
tomato cultivars ... 55
4.4 Nematodes infection and Fusarium wilt severity on five tomato cultivars with dual inoculation with F. oxysporum and Meloidogyne spp. ... 56
4.4.1 Screen house experiment ... 56
4.4.2 Field experiment ... 57
4.4.3 Effects of five cultivars on galling index of Meloidogyne spp. ... 59
4.4.4 Effects of five cultivars on egg mass index of Meloidogyne spp. ... 62
4.4.5 Effects of five cultivars on second stage juveniles of Meloidogyne spp. ... 64
4.4.6 Effects of five cultivars on reproductive index of Meloidogyne spp... 66
4.4.7 Effects of five cultivars on wilting index ... 68
4.5 Effect of dual inoculation of tomato with Meloidogyne spp. and F. oxysporum on fruit yield ... 71
4.5.1 Effects of Meloidogyne spp. and F. oxysporum on the yields of thefive cultivars ... 71
CHAPTER FIVE……… 74
DISCUSSION……… 74
CHAPTER SIX……….. 83
CONCLUSIONS AND RECOMMENDATIONS……… 83
6.1 CONCLUSIONS ... 83
6.2 Recommendations ... 84
LIST OF FIGURES
Figure 3.1: A map showing different counties that were surveyed in the Coastal region. ... 34 Figure 4.1: Mean root length of tomato cultivars when subjected to F. oxysporum and
Meloidogyne spp…….. ... 52 Figure 4.2: Mean root dry weigh of tomato cultivars when subjected to F. oxysporum and
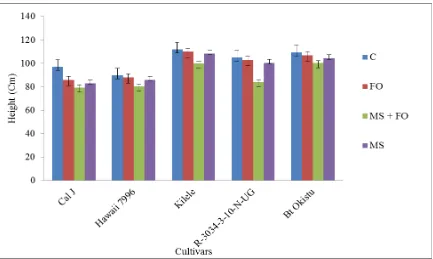
Meloidogyne spp. ... 53 Figure 4.3: Mean height of tomato cultivars when subjected to F. oxysporum and
Meloidogyne spp. ... 54 Figure 4.4: Mean shoot dry weight of tomato cultivars when subjected to F. oxysporum
and Meloidogyne spp... 55 Figure 4.5: Mean galling index of tomato cultivars when subjected to F. oxysporum and
Meloidogyne spp under screen condition. ... 60 Figure 4.6: Mean galling index of tomato cultivars when subjected to F. oxysporum and
Meloidogyne spp. under field condition ... 61 Figure 4.7: Mean egg mas index of tomato cultivars when subjected to F. oxysporum and
Meloidogyne spp. under screen house condition ... 63 Figure 4.8: Mean egg mass index of tomato cultivars when subjected to F. oxysporum
and Meloidogyne spp. under field condition ... 64 Figure 4.9: Mean second stage juveniles of tomato cultivars when subjected to F.
oxysporum and Meloidogyne spp. under screen house condition... 65 Figure 4.10: Effects of five cultivars on second stage juveniles of Meloidogyne spp. under
field condition ... 66 Figure 4.11:Mean reproductive index of tomato cultivars when subjected to F. oxysporum
and Meloidogyne spp. under screen house condition ... 67 Figure 4.12:Mean reproductive index of tomato cultivars when subjected to F. oxysporum
and Meloidogyne spp. under field condition ... 68 Figure 4.13: Mean wilting index of tomato cultivars when subjected to F. oxysporum and
Figure 4.14: Mean wilting index of tomato cultivars when subjected to F. oxysporum and Meloidogyne spp. under field conditions ... 71 Figure 4.15: Mean yields (fruit number) of tomato cultivars when subjected to F.
oxysporum and Meloidogyne spp. ... 72 Figure 4.16: Mean yields (in grams) of tomato cultivars when subjected to F. oxysporum
LIST OF TABLES
Table 3.1: Fusarium disease severity rating scale ... 40
Table 3.2: Virulence scale applied in categorization of pathogenic Fusarium spp. ... 40
Table 3.3: Galling index scale ... 44
Table 3.4: Egg mass rating index ... 45
Table 4.1: Root-knot nematode species identified from different counties in Coast region ... 49
Table 4.2: Pathogenicity levels of F. oxysporum isolates ... 50
Table 4.3: Characterization of F. oxysporum isolates ... 51
Table 4.4: Effect of five tomato cultivars on Meloidogyne spp reproduction parameters and wilting ... 57
LIST OF PLATES
Plate 3.1: Screen house tomato seedlings being tested for pathogenicity with Fusarium
spp. isolates ... 39 Plate 3.2: (A) Seedling tray setup per cultivar (B) Screen house ... 43 Plate 4.1: F. oxysporum plates (A) Front side (B) F.oxysporum spores (×40) ... 50 Plate 4.2: Tomato cultivar Cal J plant (A) In Meloidogyne spp. and F. oxysporum infested
field (B) In F. oxysporum infested field... 59 Plate 4.3: (A) Galling in Cal J non inoculated (B) inoculated with Meloidogyne spp. ... 60 Plate 4.4: Varied root galling damage on tomato cultivars root ... 62 Plate 4.5: Wilting in Cal J inoculated with; (A) Meloidogyne spp. and F. oxysporum (B)
ABBREVIATIONS AND ACRONYMS
AFLP Amplified Fragment Length Polymorphisms ANOVA Analysis of Variance
AVRDC Asian Vegetable Research and Development Centre CAN Calcium Ammonium Nitrate
CFUs Colony Forming Units DAP Diamonium Phosphate DNA Deoxyribonucleic Acid DSI Disease Severity Index EMI Egg Mass Index
FAOSTAT Food and Agriculture Organization of the United Nations FI Fusarium Index
GI Galling Index
IGS Intergenic Spacer Region
IITA International Institute of Tropical Agriculture ITS Internal Transcribed Spacer
J2 Second Stage Juveniles
KARLO Kenya Agricultural and Livestock Research Organization LCD Least Significant Difference
MtDNA Mitochondrial DNA NaCL Sodium Chloride
TFWRKN Tomato Fusarium Wilt Root-knot Nematodes Disease Complex NaOCL Sodium hypochlorite
PCR Polymerase Chain Reaction PDA Potato Dextrose Agar PF Final Nematode Population Pi initial population
PPN Plant parasitic Nematodes PPN Plant Parasitic Nematodes
RCBD Randomized Complete Block Design RDW Root Dry Weight
RF Reproductive Index
RFLP Restriction Fragment Length Polymorphism RKN Root Knot Nematodes
RL Root Length
rDNA ribosomal DNA
SCAR Sequence Characterized Amplified Region Markers SDA Sabouraud Dextrose Agar
SDH Shoot Dry Weight
SH Shoot Weight
USA United State of America
µl Microliter
ABSTRACT
CHAPTER ONE;
INTRODUCTION 1.1 Background to the study
Tomato (Solanum lycopersicum L.) (Peralta and Spooner, 2001) is cultivated as an annual crop in most regions of the world (Vossen van der et al., 2004). It belongs to the Solanaceae family together with other economically important crops such as pepper (Capsicum annum L.), tobacco (Nicotiana tabacum L.) and potato (Solanum tuberosum L.). It originated from South America and was introduced in Europe in the 16th century and later in East Africa by the colonial settlers in the early 1900’s. It is an important local market vegetable in Kenya (Da Silva et al., 2008). Tomato fruits are used either fresh in salads, cooked as a vegetable, or in processed form as tomato paste (puree), tomato sauce, ketchup, juice and can also be dried. Besides being tasty, tomatoes contribute to a healthy diet being a source of vitamins A, B and C and it also contains some good amounts of potassium, iron, and phosphorus (Vossen van der et al., 2004).
Family: Pleosporaceae), anthracnose (Colletotrichum spp.) (Order: Glomerellales, Family: Glomerellaceae), bacterial wilt (Ralstonia solanacearum) (Order: Pseadomonadeles, Family: Pseudomonadaceae), bacterial cancers (Clavibacter michiganensis subs. michiganensis) (Order: Actinomycetales, Family: Microbacteriaceae), Verticillium wilt (Verticillium albo-atrum) (Order: Hypocreales, Family: Incertae sedis), late blight (Phytophthora infestans) (Order: Peronosporales, Family: Pythiaceae) and Fusarium wilt (F. oxysporum) (Order: Hypocreales, Family: Nectriceae) are the most common diseases of tomato (Winwand and William, 1999; Agrios, 2005). Damage and loss of yield associated with plant pathogens and plant parasitic nematodes are on average greater in the tropics than in the temperate regions. This is mainly due to the favorable environmental conditions for the pathogen colonization, development, reproduction and dispersal, lack of human activity as well as limited financial resources to combat infections (De Waele and Elsen, 2007).
Fusarium wilt is of particular importance because of the losses that are associated with it (Altınok, 2005). Fusarium oxysporum f. sp. lycopersici is the most common pathogen
1.2 Problem statement and justification
Tomato production in the coast region is becoming an important business venture and a major source of livelihood. Tomato is one of the main horticultural crops in Coastal region especially in Taita Taveta as well as the main income generator to the farmers (Omari, 2004). However, production has not picked up as expected because of various constraints, among them pests and diseases (Masinde et al., 2011).
One of the key factors to successfully manage diseases is the use of cultivars that suppress disease causing pathogen population and consequently reduce yield losses of tomato (Molinari, 2011). In Kenya, the Meloidogyne spp. problem is a serious problem as the country is located in the tropical region where the climate is suitable throughout the year for nematode reproduction and survival (Kariuki et al., 2010).
The Fusarium wilt caused by F. oxysporum is reported to form a disease complex with root-knot nematodes (Abuzar and Haseeb, 2006). This disease complex is of particular importance because of combined damage caused by the two pathogens (Abuzar and Haseeb, 2006). In Kenya, there is no study that has documented this disease complex. The disease complex symptoms are usually manifested as above-ground includes: poor growth, yellow foliage, wilting during hot weather. Farmers tend to apply fungicides or fertilizer believing that the cause is either fungal or bacterial or nutrient deficiency, but without success. In addition, the use of susceptible and inappropriate cultivars fails to alleviate the problem leading to low production of tomato in the region (Mtei, 2013).
their association with crop damage is mostly associated with sandy soils (Van Gundy, 1985; Bertrand et al., 2001) hence the need of managing them. Successful production of tomato depends on the choice of cultivars for particular location (Chaerani, 2006). The aim of this research was to evaluate the potential of different tomato cultivars in their effectiveness against the disease complex with regard to disease tolerance and yield superiority. It was hypothesized that the new cultivars are superior to the local tomato checks cv. Cal-J and cv. Kilele.
1.3 Research questions
i. Which Meloidogyne spp. species and F. oxysporum infect tomato in the coastal region?
ii. What is the combined effect of the interaction between Meloidogyne spp. and F. oxysporum on tomato plants?
iii. Do we have tomato cultivars that are resistant to Meloidogyne spp. and F.
oxysporum under field condition and do their yields differ?
1.4 Objectives
1.4.1 General Objective
To identify Meloidogyne spp. and F. oxysporum pathogen affecting tomatoes and evaluate new and existing tomato cultivar(s) for resistance to F. oxysporum and Meloidogyne spp. individually and as a complex in the coastal regions of Kenya
1.4.2 Specific objectives
ii. To study the interaction between Meloidogyne spp. and F. oxysporum in tomato plants.
iii. To evaluate tomato cultivars for resistance to root knot nematodes and F. oxysporum.
1.5 Hypotheses
i. There are no Meloidogyne spp. and F. oxysporum associated with tomato cultivated in the coastal region of Kenya.
ii. Meloidogyne spp. and F. oxysporum pathogens do not form disease complex on tomato plants.
CHAPTER TWO
LITERATURE REVIEW 2.1 Tomato production in Kenya
Kenya has had a vibrant horticultural industry for several years in the production of vegetables, fruits and cut flowers for both domestic and export markets (Research Solutions Africa Ltd, 2015). Tomato is among the key crops in the horticultural industry in the country and it is one of the leading processed vegetable crops in Kenya. Tomato is among the most widely cultivated, popular and the most important vegetable food crop, not only in Kenya but also worldwide (Maerere et al., 2006). The leading vegetable in production and value are irish potatoes, tomatoes, kales and cabbage (HCDA, 2014). They are generally grown for domestic use as well as export but there is also increasing demand for processing (Mungai et al., 2000).
The edible part of the vegetable is the juicy berry fruit. In spite of the country being able to produce enough tomato to satisfy the needs and also supply a strong export demand, there remains a seasonal scarcity. Tomatoes contribute to a healthy diet as they contain high amounts of iron, potassium and phosphorous (Wener, 2000). Tomato plants are severely affected by over 200 diseases caused by pathogenic nematodes, fungi, bacteria and viruses (Moriones and Navas-Castillo, 2000), posing a major challenge to the modern tomato breeder in developing cultivars with improved resistance to a range of biotic constraints, which vary by locality and agro-ecological conditions.
2.2 Ecological requirements of tomato
The tomato crop grows well in warm sub-tropical conditions. Optimal temperatures of 20 – 27°C day time and about 15 – 17°C at night are required. Very high temperatures
(above 28°C) cause pollen sterility (Rice et al., 1993), while temperatures below 21°C can cause flower abortion. They do well in soils that are well drained with high organic matter content and a pH of 5 – 7 (Tilahun, 2013).
2.3 Tomato nutrient composition and benefits
common health problems (Collins, 2007) and it also acts as an antioxidant (Clinton, 2005).
A 100 g weight of vegetable typically contains 93.8 g water, 4.8 g carbohydrates, 1.2 g protein, 7 mg calcium, 0.6 mg carotene, 0.06 mg thiamine, 0.6 mg niacin, 0.04 mg riboflavin and 23 mg vitamin C (De Lannoy, 2001). In addition, according to the National Cancer Institute (NHB Data Base, 1999) there is sufficient data to prove that people that consume large amounts of tomato products have significantly lower risks of lung, prostate and stomach cancer. Ripe tomatoes contain high quantities of carotenoids, of which carotenes makes up to 90 to 95 % (Guil-Guerrero and Rebolloso-Fuentes, 2009). They are known to contain vitamin E, trace elements, phytosterols and flavonoids (Beecher, 1998).
2.4 Plant parasitic nematodes
Plant parasitic nematodes have a stylet in their mouth region that they use to penetrate the plant cells and absorb their contents. Apart from the direct effect they impact on plant health, nematodes play different roles in disease complexes such as: vectors, wounding agents, host modifiers, resistance breakers, and rhizosphere modifiers (Abad, et al., 2003). Nematodes also cause increased root exudation hence affecting microbial communities as well as activity in the rhizosphere (Desaeger et al., 2004). Plant parasitic nematodes cause losses of about 80% on vegetable impacting high constrains on production (Nchore et al., 2010).
In most cases crop damage is caused by a small number of nematode genera which include; root-knot nematodes (Meloidogyne spp.), migratory nematodes (Pratylenchus and Radopholus spp.) and cyst nematodes (Globodera and Heterodera spp.) (Bird and Kaloshian, 2003). The most destructive nematode is the root-knot nematodes that belong to the genus Meloidogyne (Jones et al., 2013). Nematodes have been identified as among the major pest that affect tomato production in the world, mostly in the tropical and sub-tropical regions (Berlinger, 1986).
2.5 Meloidogyne species
2.5.1 Economic importance of Meloidogyne species
2011). Sandy soil favours nematode infestation ( Bertrand et al., 2001; Tariq, 2008). The presence of Meloidogyne spp. was first noted by Berkerly in 1855 when he correlated galls on roots of cucumber with nematodes (Hartman and Sasser, 1985). In Kenya, Meloidogyne spp. is the most devastating pathogen and they cause considerable yield loss in vegetable production (Kariuki et al., 2010). Meloidogyne spp. produce conspicuous galls on infected root and they are among the top five major plant pathogens (Bharadwaj and Sharma, 2007).
There are over 90 described spp. in the genus Meloidogyne but M. incognita, M. arenaria, M. javanica are considered to be the most damaging (Sasser et al., 1983; Hunt et al., 2005). Meloidogyne incognita, M. hapla, and M. javanica account for about 99% of all the population of Meloidogyne spp. found in agricultural soils globally (Mai and Abawi, 1987). In warm climates M. arenaria, M. incognita and M. javanica are ubiquitous while in temperate climates M. chitwoodi, M. hapla, and M. fallax are widespread (Taylor and Sasser, 1978). Among the Meloidogyne spp., M. incognita and M. javanica are the most wide spread and economically important (Anwar and Mckenry, 2010).
rendered unacceptable for export (Nchore et al., 2011). In Kenya, nematodes have been found to constrain the production of different vegetables (Waceke, 2007). In addition, farmers knowledge on the presence and the management of the nematodes remains quite low (Maina et al., 2010).
Market values of produce whose crops have been infected by Meloidogyne spp. are reduced by depriving them of nutrients required for their growth. The losses are profound at the seedling stage and it usually results in devastation of the crop (Huma et al., 2011). Root-knot nematodes are the most economically significant PPN (Ferraz and Brown, 2002). Tomato losses are high due to its vulnerability to various diseases that includes fungal, viral, bacterial and nematode diseases (Sasser et al., 1983). Unlike many other pathogens, nematodes are more challenging to manage because they live in the soil and they cannot be noticed easily by farmers (Mai, 1977). In particular Meloidogyne spp. are a limiting factor in vegetable production across the world, and Kenya is no exception, a situation that is over-looked and goes unnoticed by the majority of local farmers (Zarina, 1996; Kariuki et al., 2010). Meloidogyne spp. are difficult to control mainly because of their high reproductive potential (Huma et al., 2011) and a wide host range that restricts the effective use of crop rotation.
scale farmers (Onkendi et al., 2014). The impact of Meloidogyne spp. is underestimated, especially in Africa (Onkendi et al., 2014). In a number of agricultural regions in Africa there has been no comprehensive reports that focuses specifically on the economic importance of Meloidogyne spp. (Coyne et al., 2006). The damage caused by Meloidogyne spp. to the root system facilitates invasion by other plant pathogenic bacteria and fungi (Akhtar et al., 2007). The disease complexes formed by the two pathogens can increase damage relative to when compared to single pathogen attack (Sikora and Fernandez, 2005).
2.5.2 Biology, life cycle and symptoms of Meloidogyne species
Root tissue surrounding the nematode feeding site undergoes hyperplasia or hypertrophy, resulting in galls or root-knots, which vary depending on the nematode species, the host plant species and the host crop cultivar (Moens et al., 2009). Once the J2 has penetrated the root and established a suitable feeding site, it successively develops into a third, then fourth stage juvenile and finally into a male or female adult (Moens et al., 2009). Meloidogyne spp. females can reproduce using mitotic parthenogenesis, depositing hundreds of eggs into a gelatinous matrix, known as an egg mass, on the outer surface of the galled roots (Moens et al., 2009). A gelatinous matrix can consist of about 1000 or more eggs which can be observed attached to the protruding posterior of the female on the root surface (Mai and Abawi, 1987). The gelatinous martrix protects the eggs from dehydration (Pattison, 2007). The optimum temperature for Meloidogyne spp. survival as well as for reproduction varies, depending on the plant species and cultivar (Wang and McSorley, 2008). Meloidogyne spp. are easily recognized by characteristic knots or galls where they develop and feed (Caillaud et al., 2008). In addition, the affected roots are destroyed hence the affected plant will present symptoms of water stress and wilt, have stunted growth and turn chlorotic resulting, ultimately, in poor yields (Abad et al., 2003). All vegetables are heavily damaged by Meloidogyne spp. in the tropics and under greenhouse conditions in the temperate regions (Sikora and Fernandez, 2005).
2.5.3 Nematode survival and dispersal
vegetative plant parts as well as juveniles that adhere to farm implements, and animals (Mai and Abawi, 1987).
2.5.4 Identification of Meloidogyne spp
Meloidogyne spp. identification is an important tool for effective design of nematode management practices, such as plant resistance and crop rotation which requires precise species identification and also for quarantine purposes (Zijlstra and Van Hoof, 2006). Traditionally, morphological techniques were mainly used for the Meloidogyne spp. identification (Eisenback, 1985). However, the use of morphological identification is difficult and time consuming mainly because of many overlapping characters between species (Jepson, 1987; Karssen, 2002). The use of molecular tools is a significant complement to morphological identification in order to identify individuals precisely to the species level whereby several molecular techniques have been developed (Blok and Powers, 2009).
Recently, molecular methods that are based on deoxyribonucleic acid (DNA) and polymerase chain reaction (PCR) such as random amplified polymorphic DNA (RAPD), sequence characterized amplified region markers (SCAR), restriction fragment length polymorphisms RFLP), amplified fragment length polymorphisms (AFLP) and Multiplex PCR have been developed and used successfully to aid in the identification of a number of Meloidogyne species (Adam et al., 2007). The use of sequence based diagnostic techniques is becoming a common technique in the identification of Meloidogyne spp. which relies on obtaining specific gene regions and comparing them with a reference sequence deposited in a public data base (Onkendi et al., 2014). This technique uses mitochondrial DNA (mtDNA) and ribosomal DNA (rDNA) (Blok and Powers, 2009).
2.5.5 Symptoms of Meloidogyne spp. infection in host crop
The above ground symptoms can be confused with nutritional deficiency or water stress (Nicol and van Heeswijck, 1997). The symptoms can include wilting during the day, stunting and general off-coloured appearance (yellowing or chlorosis) on the affected plants (Pattison, 2007). Dwarfing and leaf chlorosis is a manifestation of heavily infested plants (Nicol and van Heeswijck, 1997). The damage inflicted by Meloidogyne spp. to the plant roots results in the impairment of the water and nutrient absorption mechanism thus restricting growth and increasing the opportunity for other pathogen infections (Nicol and van Heeswijck, 1997). This leads to reduced yields, which may be of low quality (Pattison, 2007).
1983) the external symptoms are pronounced and diagnostic. Tomato roots that have been attacked by Meloidogyne spp. show varying degrees of galling depending on a number of factors that includes: nematode species, plant species, cultivar, inoculum level (Mai and Abawi, 1987).
Infected plants also show excessive branching of secondary roots, injured root tip, rough root surfaces with clubbed appearance and the roots may be small and present areas of necrosis (Pandey and Kalra, 2003). The damaged root growth is reduced hence reduced root volume and surface area. The capacity of the root to absorb water, mineral salts and synthesis of cytokinins, gibberellins and other growth regulators and metabolites is reduced (Hussey, 1985). Severe galling reduces root efficiency and results in mature wilting, premature defoliation and eventually the death of the plant (Mai and Abawi, 1987).
2.6 Fusarium oxysporum
2.6.1 General characteristics of Fusarium spp.
be recovered from any part of the plant from the deepest root to the highest flower (Summeral et al., 2003).
Fusarium spp. are usually saprophytic, filamentous and widely distributed on plants as well as in the soil. They grow rapidly on Sabouraud Dextrose Agar (SDA) at 25°C producing woolly to cottony, flat spreading colonies. The front colour on the colony may range from white, cream, cinnamon, yellow, red, violet, pink to purple while the reverse (underneath) may be colourless, tan, red, dark brown to brown (de Hook et al., 2000).
2.6.2 Economic importance of F. oxysporum
Fusarium oxysporum causes diseases to a wide range of economically important food crops (Booth, 1971). They are known to cause economic losses in many plant species (Rosa-Marque and Guillermo, 2004). Fusarium wilt is a temperate to tropical disease (DeVay et al., 1997a). The main diseases caused by the pathogen are the wilt and root rots (Hillocks, 1992) and the F. oxysporum is the most common fungi causing these diseases. F. oxysporum from agricultural crops have been studied with respect to their mycotoxin production as well as genetic diversity (Leslie et al., 2005). F. oxysporum are known to produce a wide range of known mycotoxins and secondary metabolites that include: gibberellic acid, fusaric acid, beauvericin, moniliformin, fusaproliferin and fumonisins (Leslie et al., 2005).
associated disease (Leslie and Summeral, 2006). The disease that forms in the host plants are varied and may include: wilts, cankers, stem or root rot, fruit or seed rots and leaf diseases while the severity of the diseases can also vary (Leslie et al., 2005). Fusarium oxysporum is a soil borne fungal pathogen that infects plants through the roots at all the stages of growth which then extends to the vascular bundles producing mycelia and spores. It causes major economic losses by inducing wilting and necrosis symptoms leading to the death of the plant (El- Khallal, 2007).
Some strains cause root rot or vascular diseases on specific host plant species (Olivain et al., 2003). The fungus has highly specialized strains that are classified into about 120 formae speciales and races based on the plant species and cultivars they infect (Alabouvette et al., 2001). Vascular wilt is a major limiting factor in the production of a number of horticultural as well as agricultural crops, for example tomato (F. oxysporum f. sp. lycopersici), cabbage – Brasicca Oleracea (F. oxysporum f. sp. conglutinana), banana - Musaceae (F. oxysporum f. sp. cubense), cotton – Gossypium (F. oxysporum f. sp. vasinfectum) among others (Armstrong and Armstrong, 1981).
2.6.3 Fusarium wilt of tomatoes
2008). Production of tomato is constrained in tropical countries due to diseases mainly Fusariumwilt contributing to huge loss (Jarvis, 1998) thus management of the disease is important.
Tomato Fusarium wilt causes immense loss, particularly on the susceptible cultivars and especially when the soil and air temperatures are high during the warm season (Mandal et al., 2009). It is known to cause major losses in tomato production in the field as well as in the greenhouse (Schwarz and Grosch, 2003). Fusarium wilt is a soil borne disease that is widely distributed and difficult to control (Fawzi et al., 2009). Management of Fusarium wilt is complex mainly because it lives near or in the dynamic environment of the rhizosphere and they can survive for as long as 30 years through the formation of certain resistant structures of the pathogen (Blum and Rodriques, 2004). The challenge now for tomato production is finding suitable and efficient methods of controlling Fusarium wilt (Muthomi et al., 2002). There are several ways that have been used to manage Fusarium wilt and they include: biological control, crop rotation, cultural techniques, resistant cultivars as well as the use of chemicals (Kamal and Hashem, 2009). Among the methods that are used in management of the disease the most effective is the use of resistant cultivars (Amini, 2009).
2.7 Biology, life cycle and symptoms of Fusarium wilt pathogen 2.7.1 Fusarium oxysporum Taxonomy
1971) and it produces three types of asexual spores: microconidia, macroconidia and chlamydospores (Nelson et al., 1983).
Conidia are produced on the monophialides and in the sporodochia, and they are generally scattered loosely over the surface of the mycelium (Griffin, 1994). Microconidia are the most abundantly produced spores, they are oval, kidney shaped and they are produced on aerial mycelia. They are also uninucleate and they germinate poorly and variably (Ebbole and Sachs, 1990). Macroconidia have three to five cells that have curved edges. They are sub cylindrical, equally and gradually tapering in both ends with a pointed apical cell (Nelson et al., 1981) they are produced abundantly and are commonly found on the surfaces of plants that have been infected by Fusarium wilt.
Chlamydospores are formed either singly or in pairs but can also be in clusters or in chains. They are abundant in hyphae and conidia; they are smooth or rough walled, globuse to sub globuse. They are thick walled spores and they can survive for long periods. They are mostly found in old cultures and in senescent host tissues. They are viable, asexually produced spores resulting from structural modification of a vegetative hyphal segment (Agrios, 1997). They remain dormant in the soil but they germinate more frequently and grow vigorously (Smith and Snyder, 1972).
microconidia germinate. The spores and the mycelia block the vascular vessels preventing the flow of vascular fluids, reducing water and nutrient up-take. Generally, the fungus causes vascular wilt by infecting and growing internally through the cortex to the stele (Bowers and Locke, 2000). The infected plant transpires to a greater extent than the water it is able to transport and the stomata close, the leaves wilt and eventually the plant dies. After the death of the plant the fungus invades all the tissues, sporulates and infects neighbouring plants (Kutama et al., 2016).
2.7.2 Identification of Fusarium species
Fusarium pathogen differentiation is not simple. Traditionally, the use of morphological and physiological features was used as a basic identification method. Though Fusarium species can be identified morphologically by the use of selective media, the pathogenic types as well as the races cannot be differentiated through this method (Nelson et al., 1983; Burgess et al., 1994). Plant pathogenic forms of F. oxysporum have been divided in to formae speciales based on the host plant that they infect (Armstrong and Armstrong, 1981). Further sub-divisions of formae speciales into races are based on their virulence to a set of differential host cultivars (Correll, 1991).
oxysporum, the use of molecular techniques for rapid and reliable identification have come to the fore in recent years (Woo et al., 1996).
Molecular methods are universally applied for fungal identification as a practical and reliable method. The use of molecular markers has gradually become simpler to use and now readily yield informative, as well as conclusive, results in identification of the species and fungus sub-species. Basically, there are two techniques for DNA marking. There is the use of restriction fragment length polymorphism (RFLP) that is based on DNA hybridization, while polymerase chain reaction (PCR) (O’Donnell et al., 2009) includes the use of random amplified polymorphic DNA, simple sequence repeats, amplified fragment length polymorphism, and sequence related amplified polymorphism (O’Donnell et al., 2009; Irzykowska et al., 2012). The rDNA is utilized by many
researchers for species determination in a wide range of fungi (Farr et al., 2002). The use of rDNA from the same species maintains the same sequence, which is due to the conserved nature of the DNA, whereas phylogenetically different species have greater difference in the rDNA sequence (Irzykowska et al., 2012).
2.7.3 Symptoms of Fusarium wilt of tomatoes
green on the outer side, but exhibit a narrow band of brown discoloration (streaking) in the vascular tissue. Disease development is effective in the high air and soil temperatures of 28°C, optimum soil moisture for plant growth, low soil pH and weak plants (Megan Kennelly, 2007).
2.7.4 Resistance to Fusarium wilt by host plants
Fungal wilt disease resistance depends on the inoculum concentration as well as the genetic potential for the virulence of the pathogen (Bergman, 1966). Host resistance mechanisms to Fusarium wilt disease may be due to inhibition of any of the stages of pathogenesis which includes; germination of the propagule and its establishment on the host plant, penetration of the fungus through the root or the stem in to xylem vessel. The spores growth and proliferation happens within the xylem into the surrounding stem or leaf tissue (Bell and Mace, 1980).
2.8 Interaction between plant parasitic nematodes and Fusarium wilt pathogen
In the soil ecosystem, both pathogenic and non-pathogenic micro-organisms are in continuous competition with each other on a microscopic scale, with “survival of the fittest” the rule of law (Sikora and Reimann, 2004). There are many microbial groups and
The main significant role of nematodes in the development of diseases complexes caused by soil borne pathogens has been demonstrated in a number of host crops throughout the world. In many cases, nematode–fungus disease complexes involve root-knot nematodes (Meloidogyne spp.), although several other endoparasitic (Globodera spp., Heterodera spp., Rotylenchulus spp., Pratylenchus spp.) and ectoparasitic (Xiphinema spp., Longidorus spp.) nematodes have been associated with diseases caused by soil borne fungal pathogens (Sharma and McDonald, 1990). Many reports on the interactions of soil borne plant pathogens with plant parasitic nematodes (PPN) maintain the observation that the damage caused by nematodes increases damage due to secondary infecting pathogens (Wardle et al., 2004). It has been noted that certain microorganisms are destructive only when they occur in combination with other biological agents (Powell, 1971). Plant damage caused by PPN is difficult to assess because they facilitate the invasion of plants by other plant pathogens, for example: fungi, bacteria, insects and viruses (Manzanilla-lopez et al., 2004).
soil pathogens such as Fusarium spp., Verticillium spp., Phytophthora spp. and Rhizoctonia spp. (Mclean and Lawrence, 1993).
Plants that are otherwise known to possess resistance to bacterial or fungal pathogens are then shown to become susceptible when additionally infected by Meloidogyne spp. (Hirano, 1975). Meloidogyne spp. causes severe annual yields losses and at times total crop failure due to complexes that involve soil borne pathogens (Back et al., 2002). The incidences as well as the severity of Fusarium wilt increases in Meloidogyne spp. infected plants, compared to nematode-free plants due to the wounds they create as they enter the plant root (Scott, 2012).
Disease complexes involving Meloidogyne spp. and fungi cause more losses than either occurring individually (Hussey and McGuire, 1987). Such interactions are considered to be synergistic when the combined effects of the two pathogens on the host plant results in more extensive damage than the sum of the effects of two pathogens acting independently (Armstrong et al., 1976). Wilt resistant or tolerant crop cultivars not only exhibit severe wilting but also present advanced expression of wilting in the presence of PPN (Nagesh et al., 1998). It is important to note that even with a low density of the fungus or nematodes, the disease complex formed is of significant importance (Bowers et al., 1996).
1977). Meloidogyne incognita race 1 was shown to increase wilt caused by Ralstonia solanacearum and F. oxysporum f. sp. lycopersici on resistant tomato cultivars when inoculated simultaneously (Chindo et al., 1991).
On four tomato cultivars the Fusarium wilt infection by F. oxysporum f. sp. lycopersici was greatly increased when Meloidogyne spp. were present, while the galling reduced (Price et al., 1980). In chickpea (Cicer arietinum), the interaction of F. oxysporum f. sp. ciceris and M. incognita or M. javanica led to the breakdown of resistance to the F. oxysporum f. sp. ciceris (Uma et al., 1997). The entry of F. oxysporum f. sp. lycopersici on the host plant however, is not fully understood (Walker, 1971) but it has been noticed that it is enhanced by the presence of other organisms, such as nematodes (Bergesson et al., 1970).
2.9 Management of Meloidogyne spp. and Fusarium spp.
Different strategies of nematodes management are used with varying degree of success. The most effective and simplest means of nematodes control is prevention of field contamination with Meloidogyne spp. However, fields are already infested, and farmers must choose a management option (Trudgill and Blok, 2001).
Management options may include chemical, cultural or biological methods, although the use of chemical nematicides is harmful for the environment when used improperly (Sikora and Fernandez, 2005; Kamal and Hashem, 2009). The long term use of nematicides has led to underestimated effect of the Meloidogyne spp. (Onkendi et al., 2014). Most of the nematicides however have been banned and removed from the market hence Meloidogyne spp. problems are steadily beginning to resurface (Onkendi et al., 2014) hence there is a rising demand for alternatives. Cultural methods may include flooding, fallow, crop rotation (based on fact some species are able to multiply on host crop), use of resistant cultivars, incorporation of organic amendments and soil solarization and steam treatment (Sikora and Fernandez, 2005). The use of host-plant resistance shows major advantages in the management of nematode because of the “self-protection” of the resistant crop, which provides an effective and economical method for
managing nematodes (Molinari, 2011).
that are resistant to different Meloidogyne spp. have been bred or selected in pepper and tomato (Netscher and Sikora, 1990).
Several types of resistance have been reported, which includes resistance to the invasion of the nematodes, resistance following penetration that results in the J2 being unable to complete their development as well as reproduction (Howarr, 1970). In addition, tolerance where the plant is able to sustain nematode infection and multiplication, but not suffer injury; such plants are of considerable value (Cook and Evans, 1987). It is emphasized that resistance is often quite selective against particular species and that cultivars of crops resistant to certain species of Meloidogyne do not necessarily protect against all species within the genus; there may also be races or pathotypes of individual species that can develop and break the resistance (Roberts et al., 1986). Resistant cultivars should be used with caution and should be tested for their durability of resistance or susceptibility in the field before commercially used (Roberts et al., 1986).
at 28°C whereas it was susceptible at 32°C (Dropkin et al., 1969). The reproduction of M. incognita at high temperatures were noted to be race dependent (Arujo et al., 1983) and that race 4 reproduces better on resistant tomato cultivars at 32.5°c than race 1.
The control of fungus is importance in the management of fungal diseases and they mainly depend on fungicides treatments (El-Mougy et al., 2004). However, the application of fungicides causes hazards to human health and also increases environmental pollution. Of all the methods available, the use of host resistance is the most effective measure in the control of Fusarium wilt (Amini, 2009). The use of resistant cultivars is the most effective and eco-friendly components of integrated pest management (Khan and Mukhopadhyay 2004). It is important to develop cultivars that have at least some ability to resist Fusarium wilt hence help in managing the disease (Hillocks, 1992). Fusarium spp. causes wilting of the infected plants that may lead to death. Infections of roots by root-knot nematodes predispose plants to infection by other soil-borne root-infecting fungi resulting in the development of root-rot and wilt diseases (Armstrong et al., 1976) hence the control of the two pathogens is crucial.
2.10 Interaction of nematodes with tomato wilt inducing fungus
both pathogens, than plants grown in soil infested by F. oxysporum f. sp. ciceri alone. More than 56% of the plants wilted with the combined infection of both nematode and fungus compared with 32% of the plants with fungus alone treatments (Ramnath and Dwivedi, 1981). Reduction of shoot and root weight in chickpea was noted when there was simultaneous inoculation of M. javanica and F. oxysporum f. sp. ciceri than by inoculation of either of the pathogens alone (Sharma and Cerauskas, 1985). The study on the effect of combined inoculation of M. incognita, F. oxysporum and Fusarium solani on growth of chickpea cv. JG-62 revealed that the occurrence of M. incognita in combination with both the fungi not only increased the severity of the damage but also shortened the incubation period by 6 – 11 days for disease expression when the nematode preceded F. solani (Mani and Sethi, 1987).
It was observed that the interaction of F. oxysporum f. sp. ciceri with M. incognita on chickpea cv. Dahood yellow revealed that these organisms either individually or in combination reduced plant height as well as root or shoot fresh weights. In addition the reduction made by M. incognita was greater than that made by F. oxysporum f. sp. ciceri. The galling index and nematode multiplication on the plants were higher when nematodes were inoculated alone but it was reduced in the presence of fungus. Severity of disease greatly increased when M. incognita was present together with the fungus (Patel et al., 2000). Maximum wilting of plants were observed when the fungus and M. incognita were inoculated simultaneously when compared to individual inoculation of the pathogens (Patel et al., 2000).
uninoculated plants. The reproduction of Meloidogyne spp. and the level of root galling was lower following pre-inoculation of the fungus although the severity of infection of the fungus was higher in the presence of nematodes alone (Akhtar et al., 2007).
2.11 Screening for resistance
Resistance to nematodes is defined as the ability of the plant to inhibit nematode attack and prevent reproduction (Cortada et al., 2009). Resistance is a useful management strategy that improves both the quantity and quality of the crop in the presence of nematode population. It is cheap, safe and an eco-friendly option for nematode management.
Nematodes are known to break disease resistance of different crop cultivars to different pathogens, such as in resistance of tomato to F. solani (Abawi and Barker, 1984). Twenty tomato genotypes were inoculated with F. oxysporum and Meloidogyne spp. disease complex whereby it was observed that all the genotypes tested were susceptible to the disease complex including those that were resistant to F. oxysporum (Almeida, 1972). A study conducted on cotton cultivars (Gossypium hirsutum L.) on the disease complex caused by M. incognita and F. oxysporum f. sp. vasinfectum revealed that the tolerant cultivars gave good yields compared to the resistant and susceptible variety (Hyer et al., 1979). Also Meloidogyne spp. resistance in cotton can protect plants from Fusarium wilt (Hyer et al., 1979). However, susceptibility to Fusarium was increased in the presence of Meloidogyne spp. (Garber et al., 1979).
Avrodhi were affected least by nematode and fungus individually or in combination (Krishna Rao and Krishnappa, 1994). In addition the highest reduction in plant growth was recorded in cv. JG-62. Minimal percentage wilt incidence was recorded in plants inoculated with the fungus alone (28.7%) while maximum wilting (45.1%) was recorded in presence of nematodes (Krishna Rao and Krishnappa, 1994).
Studies were carried out on the effect of combined inoculation of M. javanica and F. udum in ten wilt resistant/tolerant pigeon pea genotypes (Bansa et al., 2004). It was noticed that in the combined presence of both M. javanica and F. udum wilting increased from 8 to 33% in cv. KPL-44, 15-50% in cv. ICP 12745 and 15 to 50% in cv. AWR-74/15, 25-50 % in cv. ICP 8859 and cv. ICPL 89049. In the other five genotypes wilting did not increase much in the presence of nematodes. Various plant growth parameters were significantly lower in combined inoculations of pathogens when compared to nematode or fungus alone. The galling index varied from 4.5 to 3. The lowest root-knot index was recorded in cv. KPL 43 (1.5) and cv. GPS 33 (1.75) (Bansa et al., 2004).
CHAPTER THREE:
MATERIALS AND METHODS
3.1 Identification of root knot nematodes and Fusarium with pathogen infecting tomato in the Coastal region of Kenya
3.1.1 Description of the study sites
Figure 3.1: A map showing different counties that were surveyed in the Coastal region. Source: http://www.mapzones.
3.1.2 Sampling of Meloidogyne spp. and Fusarium spp. infected plants and infested soil
(Young, 1990). Areas infested with weeds or other plant species were avoided to prevent sampling nematodes from non-target hosts (Knight, 2001).
The composite soil sample was mixed thoroughly but gently before withdrawing one kilogram sub-sample of composite soil. The samples were then placed in clean polythene bags and the date, location and the sample number written using a permanent ink pen. The roots and the plants were also collected and labeled appropriately. Concurrently, the same records were also written in the field notebook with the farmer’s name and crop
type. Before moving to the next farm the hand shovel was cleaned after sampling to avoid contamination of soil samples and prevent spread of nematodes or pathogens between farms. A total of 125 composite field samples in the five counties (Kilifi, Taita Taveta, Kwale, Lamu and Mombasa counties – 25 samples per county) were collected and taken to the laboratory. The root and soil samples were transported in a cool box to the laboratory for processing and storage at 10oC.
3.1.3 Extraction of Meloidogyne spp. egg masses from infected tomato roots
Soil used in the screening experiment was sterilized in an autoclave at 121°C for 1 hour and blended with sterilized sand at the ratio of (1: 2 v/v). Root and soil samples collected from each region were mixed with autoclaved sterilized soil, potted into one kilogram kg pot (with 15 cm diameter and 10 cm depth) and three week old tomato cv. Cal J (that had been grown in sterilized soil) transplanted to the sleeves to culture the nematode populations (as mixed population) in the screen houses Pwani University (April 2013).
rinsed with tap water to remove rhizosphere-attached soil but taking care not to overly disturb the roots and dislodge the egg mass. Sections of the galled roots were then removed, placed in a petri dish with two milliliters distilled water and observed under a dissection microscope. To obtain pure populations, single egg masses were removed using fine forceps and placed individually into separate ink wells in distilled water. Second stage juveniles were allowed to hatch for one week from the egg masses. Once hatched, the J2s from each egg mass were inoculated, using a pipette, onto a three week old tomato cv. Cal-J plant that had been transplanted into 20 cm diameter plastic pots with sterilized soil. After three months, the tomato roots were harvested and used to obtain egg masses for molecular identification and for single species inoculum thereafter. To obtain the egg masses, roots samples were stained with Phloxine B (0.15 g/litre water) for 15 minutes and extracted using a dissecting microscope (Holbrook et al., 1983). From each single egg mass population, ten eggs mass were removed and five placed in two percent NaCl and stored at 10°C and used for mitochondrial haplotypes identification.
3.2 Determination of interaction between Meloidogyne spp. and Fusarium spp. on tomato
3.2.1 Preparation of nematode inocula
through nested sieves: 250 μm, 100 μm, 45 μm and 25 μm. Eggs were collected on the 25 μm aperture sieve and then rinsed with tap water to remove excess NaOCl. Egg inoculum
was transferred into a beaker and left to stand for one week at room temperature to hatch. The active J2s were separated from the un-hatched eggs and non-active second J2s using a modified Baermann technique (Whitehead and Hemming, 1965). The J2s were counted by means of a specially made counting chamber under an inverted microscope (Nikon Escipse Ts 100) at x 100 magnification to establish egg density.
3.2.2 Identification of Meloidogyne spp. isolated from soils
The egg masses of pure Meloidogyne spp. populations derived from single egg masses in ethanol were sent to the University of California, Davies, USA for molecular identification using mitochondrial haplotype protocol (Pagan et al., 2014). The mitochondrial genome is used due to its uniparental inheritance hence making it a useful tool for comparing and identifying closely related hybrid species. Using previously developed primer pairs that amplify two mitochondrial DNA sequences that span a non-coding spacer and part of the adjacent large subunit rDNA is used to amplify DNA from samples of Meloidogyne spp. (Pagan et al., 2014).
3.2.3 Isolation and identification of Fusarium spp. from the infected tomato plants
hood by dipping them in 0.5% sodium hypochlorite for three minutes followed by three rinses in sterile distilled water and dried using sterile blotting paper (Dababat et al., 2008). The fungi were isolated from tomato plants and grown in potato dextrose agar (PDA) (at 39 g of Difco PDA powder, in 1000 ml water) previously autoclaved for 15 minutes at 121ºC.
After drying the stems were cut into sections of about 1 cm then plated onto freshly prepared potato dextrose agar (PDA) amended with 50 mg/l chloramphenicol to suppress bacteria growth in plastic petri dishes measuring 90 mm followed by incubation at room temperature until the mycelium emerged. The fungal isolates were sub-cultured onto fresh PDA media by cutting at the margin of mycelia with a flame-sterilized 5 cm cork borer for purposes of purification until pure F. oxysporum cultures were obtained. Seven pure isolates were successfully isolated and maintained.
3.2.4 Preparation of F. oxysporum inoculum
3.2.5 Determination of pathogenicity of the F. oxysporum isolates
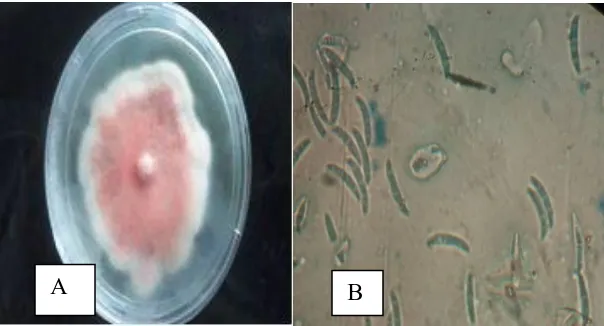
All the seven F. oxysporum isolates were inoculated individually onto tomato cv. Cal J seedlings to assess for pathogenicity (Plate 3.1). Three week old tomato seedlings were rinsed in sterilized water and cut at several times on the root tips before dipping the roots into a spore suspension for 15 min. A control was performed using sterile distilled water. At 30 days after inoculation, disease symptoms were recorded and the most pathogenic was used for the screen house and field experiment.
Plate 3.1: Screen house tomato seedlings being tested for pathogenicity with Fusarium spp. isolates
Table 3.1: Fusarium disease severity rating scale
Table 3.2: Virulence scale applied in categorization of pathogenic Fusarium spp.
3.2.6 Molecular characterization of Fusarium spp.
To confirm the reliability of morphological identification, seven (7) of the isolates were selected and subjected to molecular identification based on rDNA ITS and IGS sequence analysis. The molecular characterization was carried out on seven isolates at Bonn University, Germany to confirm the spp. of the genera (Bogner, et al., 2016).
Scale Yellowing and wilting
percentage
1 0 %
2 1 – 20 %
3 20 – 40 %
4 5 6
41 – 60 % 61 – 80 % 81 – 100 %
Virulence Level Disease severity index (DSI)
Avirulent (A) =1
Low virulence (LV) ≤ 3.50
Moderate virulence (MD) 3.50 to 4.50
The mycelia were harvested by filtration through miracloth, frozen, and lyophilized for 24 hours before grinding them to fine powder. Total DNA was extracted from 20 mg of lyophilized mycelia using the Wizard ® Magnetic DNA Purification System for Food (Promega) according to the manufacturer’s instructions. The total reaction volume of all polymerase chain reaction (PCR) mixtures was 50 ul. The reaction mixture consisted of distilled water, 25 mM dNTP, 1.5 units of GoTaq® polymerase, 1× of 5× GoTaq® Green reaction buffer, 0.2 μM of each primer and 5 ng/ul of total genomic DNA template.
Depending on the isolate, either the nuclear ribosomal internal transcribed spacer region (ITS) or the intergenic spacer region (IGS) was amplified. All the Fusarium isolates were amplified using the IGS primer set PNFo (CCCGCCTGGCTGCGTCCGACTC) and PN22 (CAAGCATATGACTACTGGC) (Edel et al., 2001). The ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC) primer set was used for the non‐Fusarium morphotypes (White et al., 1990). The amplification was conducted in a Biometra T‐gradient thermal cycler (Göttingen, Germany) using the following program: an initial denaturation step at 95 °C for 4 min, followed by 34 cycles of 95 °C for 1 min, 50 °C for 2 min. Finally, an extension step was executed at 72 °C for 3 min. The PCR products were purified using the illustra TM GFX TM PCR, DNA and Gel Band Purification kit (GE Healthcare UK Limited, Buckinghamshire, UK). Prior to purification, the PCR amplicons were run on a 1% (w/v) agarose Tris‐acetate‐EDTA (TAE) gel supplemented with 10 μg/ml ethidium bromide. The bands containing the
Germany). Sequencing was carried out on both strands. Sequences were edited and assembled using the CLC Main Workbench (version 6.9.1) software (CLC bio-Qiagen, Denmark) and blasted against nucleotide databases (Bogner, et al., 2016).
3.3 Evaluation of tomato cultivars for resistance to root knot nematodes and Fusarium wilt
3.3.1 Effect of Fusarium – rook knot nematodes disease complex on the tomato cultivars
The tomato cultivars assessed in the experiment were the locally sourced and cultivated cultivars (Cal J and Kilele) in addition to the experimental cultivars from AVRDC (Bt Okistu 101, R-3034-3-10-N-UG, and Hawaii 7996). The locally less preferred cultivated cv. Cal J was used as the known susceptible cultivar and low yielding while Kilele was used as the known resistant cultivar with high yield (Mtei, 2013).
3.3.2 Effect of Fusarium – rook knot nematodes disease complex disease complex on tomato growth in the screen house
Plate 3.2: (A) Seedling tray setup per cultivar (B) Screen house
Polythene sleeves (15 cm diameter and 10 cm depth) were filled with 1 kg sterilized sand – soil mixture (1: 2) and 5 g of Diamonium Phosphate (DAP)was used as basal fertilizer
at transplanting and mixed thoroughly with the soil. The polythene sleeves had drainage holes to prevent water logging and stagnation. Each polythene sleeve was transplanted with one seedling and grown under insect proof screen house in screen house located in Pwani University, Coast region as depicted in Plate 3.2.
The experiment consisted of four treatments: F. oxysporum (1 x 106) spores only, Meloidogyne spp. (500 J2s) only, Meloidogyne spp. (500 J2s)and F. oxysporum (1 x 106 spores) and sterile soil only (control). The evaluation in the screen house was carried out in a randomized complete block design replicated four times and repeated once to confirm the results. A total of 100 plants were planted. The screen house experiments were terminated at sixty days after inoculation.
At the end of the experimental period shoot heights were measured from the base of the stem
(soil line) to the newest apical node. The plants were then uprooted gently; roots gently
rinsed with tap water to remove adhering soil and then blotted dry with a serviette before
the tip of the tap root. Roots were then separated from shoots. Tomato roots were then blot
dried and visually examined for the galling index which was scored using diagrammatic root-knot scoring chart (Bridge and Page, 1980) (Table 3.3). All stems and root systems were then dried in an oven at 60oC until constant weight was obtained. Root and shoot dry
weights were determined separately. Data on number of fruits per plant and fruit weight per
plant were obtained in the field.
Table 3.3: Root knot galling index rating scale
Scale Galling index
0 1 2 3 4 5 6 7 8 9
Health root system Very few galls detected Smalls galls easily detected Numerous small galls
Numerous small and few big galls
25% of the root severely galled 50% of the root severely galled 75% of the root severely galled No healthy root, plant still green Plant and root dead
Table 2.4: Egg mass rating index
Scale Number of egg masses Reaction
1 0 Highly resistant
2 1 – 5 Resistant
3 6 -10 Resistant
4 11-15 Moderately resistant
5 16 -20 Moderately resistant
6 21 -30 Susceptible
7 31 – 40 Susceptible
8 41 -50 Susceptible
9 Above 50 Highly susceptible
factor (RF= Pf/Pi). The shoots and the roots were placed in khaki bags and dried in an oven at 60°C until a constant weight was recorded to establish dry weights.
3.4 Field experiment
Field experiments were conducted at selected locations in Pwani University farm Kilifi County. The initial population of Meloidogyne spp. and Fusarium spp. on the field was assessed by sampling soils from the field. The field was sub-divided into four micro-plot measuring 3 m by 4 m leaving a path of 1 m between the micro-plots. Two of the plots were treated with mocap (ethoprop) non-systemic nematicide (Bayer Crop Science) at the rate of 50kg/ha 1 week before transplanting for the control of Meloidogyne spp. of tomato under field conditions. Land preparation was carried out which included land clearing, ploughing and harrowing to obtain fine tilth.
In each micro-plot ten holes were excavated to 15 cm depth at a spacing of 50 cm between plants and 75 cm between rows. In each planting hole, five grams of Diammonium Phosphate (DAP) fertilizer was applied and mixed thoroughly with soil during transplanting. A total of 30 tomato seedlings per treatment were transplanted on each plot of the field experiments.
moisture, which causes fruit cracking. Plants were staked one month after transplanting. Fruit was harvested as the fruit matured so as to obtain fruit fresh weight. After each harvest, fruits were weighed in kilograms using balance scale for the determination of total yield. The total number of fruit per plant was also recorded. Data on production trends was taken from the first harvest to the last harvest for all the planted varieties. Ninety days after transplanting the experiments were terminated and data same to that of the greenhouse collected.
3.5 Statistical analysis of data
CHAPTER FOUR
RESULTS
4.1 Meloidogyne spp. isolated from the Coastal region 4.1.1 Molecular identity of Meloidogyne spp.
Table 4.1: Root-knot nematode species identified from different counties in Coast region Isolate origin Specific locality Meloidogyne species
Kwale Mwakabe M. javanica
Mombasa Likoni M. javanica
Mombasa Likoni M. incognita
Kwale Kazamwenye M. incognita
Kwale Msangatama M. incognita
Lamu Mpeketoni M. javanica
Lamu Mpeketoni M. javanica
Kilifi Mtwapa (Foretu) Novel
Kilifi Mtwapa (Foretu) M. arenaria
Kilifi Mtwapa (Foretu) M. incognita
Kilifi Mtwapa (KARI) M. javanica
Key: identification based on Mitochondrial haplotypes
4.2 Identification of Fusarium species isolated from tomato in Coastal region