POSSIBLE NONRANDOM UTILIZATION OF X - AND Y-BEARING SPERM IN DROSOPHILA MELANOGASTER
ARTHUR P. MANGE
Zoology Department, University of Massachusetts, Amherst, Mass. 01002 Received September 9, 1969
H E adult sex ratio in Drosophila melanogaster is quite variable even within single wild-type strains under ordinary and seemingly constant conditions. Such observations often go unreported even if the variation cannot reasonably be attributed to chance; or, if reported, the sex ratio is of incidental interest. Some of the published variations in normal strains involve differences between Ore- gon-R, Canton-S, and other stocks (HANKS 1965, 1969), differences over time within each of several inbred lines (LEVY 1965), larval crowding effects in Canton-S (MANGE 1969), paternal aging effects in Oregon-R
(YANDERS
1965), and maternal aging effects in Samarkand (HANNAH 1955). These responses in the sex ratio may be due to natural selection, or they may be physicochemical effects of no importance in evolution. In either event, it is misleading to specify a sex ratio for D . melanogaster unless one also describes in detail the prevailing conditions.The experiments described here were designed to confirm and extend the work of YANDERS (1965) who observed a large and progressive decrease in the propor- tion of sons from older fathers. The term “sex ratio” here will mean the percent- age of male offspring. Under my conditions a decreasing sex ratio from aging fathers (from 54% down to 46%) was observed, but it was limited to the first portion of eggs laid by equally aged mothers, and was absent, or reversed, as the successively brooded mothers continued to use up their stored sperm. In contrast to YANDERS’ results, all ages of fathers transferred, ouerall, approximately 50% each of X - and Y-bearing sperm as judged by the sex ratio among offspring of mothers who exhausted their sperm supply. The consequent difference in sex ratio between initial and later broods might mean a preferential utilization of Y-bearing sperm from younger males, and X-bearing sperm from older males.
MATERIALS A N D METHODS
All flies were Oregon-R obtained in 1965 from Dr. PHILIP IVES of Amherst College and main- tained by mass transfer. This stock and all experimental flies were kept in 25
x
95 mm shell vials containing 8.0 ml of a cornmeal-molasses-yeast-agar food with propionic acid as a mold in- hibitor and with a few specks of live yeast sprayed on the food surface. The food vials were either used immediately or refrigerated for up to three weeks in double plastic bags. The flies were kept in an incubator at 25-26°C on approximately a 14-hour light, IO-hour dark photocycle which was maintained for other purposes.In the two main experiments, labeled I and 11, newly eclosing males were collected 17, 13,
9, 5, and 1 day before the day of mating. Approximately 20 males were stored in each vial, with-
96 A R T H U R P. MANGE
out females, and transferred to fresh food every few days. Virgin females were collected 5 days before mating and stored, approximately 20 flies per vial. One day before mating, the two sexes were distributed separately to vials in the numbers which would then be combined a t the time of mating. The matings were thus made without etherization by appropriate transfers into fresh vials.
To avoid effects of larval crowding on sex ratio (MANGE 1969) a n attempt was made to equalize the numbers of developing larvae in any vial. This was done by varying the number of parents to offset the decrease in fertility of aging fathers. The number of parents per vial was:
Age of fathers (days) = 1 5 9 13 17
Experiment I, no. of fathers/no. of mothers = 3/2 3/2 3/2 4/3 4/3 Experiment 11, no. of fathers/no. of mothers=4/3 4/2 4/3 4/3 5/3 Twelve replicas of each age group were set up. The males were allowed to remain with the females for 21. hr, after which they were sucked out and discarded. The females were transferred successively to fresh food every day or two in order to exhaust their stored sperm. The length of these brood periods, in days, was:
Brood label: A B C D E F G
Experiment I: 1.8 2.1 2.4 2.7 3.0 3.1 - = 15.1 days total Experiment 11: 1.7 1.3 1.9 2.1 2.2 2.8 2.8 = 14.8 daystotal Brood A was the first brood, initiated at the time of mating. The variation in the timing of the broods was an additional device to obtain roughly equal numbers of offspring per vial. No micro- scopic check was made to see if all sperm were used, but Brood F of Experiment I produced only 8 flies (out of about 13,000 for all broods), and Brood G of Experiment I1 produced only 175 flies (out of about 15,000). It seems unlikely that the females retained any significant number of viable sperm.
Progeny counts were made daily for each vial from first eclosion until no flies emerged for three successive days. Practically all vials were exhausted by the 14th or 15th day after females started laying in them. Only a few percent of the roughly 550 vials produced over 90 offspring, and for only a handful of these was there a question of contamination by the next generation of flies.
In other experiments, labeled i and ii, which were done before the one described above, fathers older than 17 days were included and only a small proportion of the sperm stored by the mothers was sampled In Experiment i, the fathers were collected at two-day intervals starting 30 days before the day of mating. They were stored with females who were changed occasionally in order to continually deplete the males’ sperm supply (MANGE and ALEXANDER 1968). The males and females were allowed to mate for 48 hr, after which the fathers were sucked out and discarded. The females were transferred to fresh vials in which they were allowed to lay for 24 hrs (corresponding to a Brood B of the main experiments). The females were then discarded, as were the vials in which mating had occurred. In Experiment ii, the fathers were collected at four-day intervals starting 28 days before mating. The two sexes were allowed to mate for 42 h r in one set of vials (Brood A), and then transferred to a fresh set of vials (Brood B) for 24 hr, after which all parents were discarded.
In the regression analyses below, each data point is weighted by the number of flies on which the sex ratio is based. Since all sex ratios lie between about 44% and 56%, each weight is a very close approximation of the information, the reciprocal of the variance, represented by each point. Again since the sex ratios are relatively close to 50% no angular transformation is needed to pro- vide homoscedasticity. The term “significant” here refers to the 5% probability level as indicated by chi-square or Student’s t values.
RESULTS
TABLE 1
Sex ratio of offspring from increasingly older fathers mated to successively brooded equully aged mothers
A Broods Later Broods A l l Broods age of
father
Experiment (days) m l e s / t o t a l = $males malesltotal = %males malesltotal = %males
1 4Gola46 = 54.4 76711574 = 48.73 122712420 = 50.70 5 3841722 = 53.2 955/2006 = 47.61 imlzzs = 49.08
I 9 252/.5l8 = 48.6 57711191 = 48.45 a a m 0 9 = 48.51 13 4131924 = 44.7 1200/21105 = 49.90 161313329 = 48.45 17 4~81915 = 51.1 106512l43 = 49.70 1539130% = 50.13
TOTAL I 197713925 = 50.37 4 5 ~ 9 3 i 9 = 48.98 ~YQI~~ZU+ = 49.39
1 2011380 = 52.9 158613191 = 49.70 178719571 = 50.04 5 a51423 = 50.8 128912569 = 50.18 1504/2992 = 50.27
11 9 ZLzI430 = 49.1 1744/3407 = 51.19 ~ 5 1 3 8 3 7 = 50.95 13 U41447 = 47.9 120112480 = 48.43 1 4 1 5 l r s z = 48.34 17 1841371 = 49.6 9zoIi808 = 50.88 1104/2l79 = 50.67
TOTAL 11 1025/2051 = 49.98 6740113455 = 50.09 7765115506 = 50.08
1 66111226 = 53.92 a5314765 = 49.38 3014/5991 = 50.31
Weighted 5 59911145 = 52-91 zU+/4575 = 49.05 *3/5720 = 4.70
average 9 4631948 = 48.84 ~ z ~ 1 4 5 9 a = 50.48 278415% = 50.20 13 62711371 = 45.73 240111~8a5 = 49.15 3028/6256 = 48.40
17 652/1286 = 50.70 i9a51395i = 50.24 263715237 = 50.35
of I and I1
TOTAL
I and II 3002I5976 = 50.23 ll304I22774 = 49.636 14306/28750 = 49.760
of both experiments, but not in any other broods, the A Broods are shown separ- ately from the pooled results of the others. The overall sex ratio in Experiment I,
49.39%, is not significantly different from that in Experiment 11, 50.08%. Nor are there any significant sex ratio differences between the two experiments with respect to the
A
Brood totals, nor with respect to the Later Brood totals. Further- more, no significant sex ratio differences exist between the A Brood totals and the Later Brood totals within Experiment I, nor within Experiment 11. But for the paternal age effect within the first broods, and the consequent change in sex ratio between the first and later broods, the results are unexceptional.I n the regression analyses for Experiments I and 11, below, the data points for 17-day old fathers are omitted. The justification for doing so rests primarily on the results of the two prior experiments, i and ii, which indicated that the linearity of the sex ratio response did not extend beyond two-week old fathers.
98 ARTHUR P. MANGE
5 10 15 20 25 30
0
FA'IBER'S AGE AT START OF XATIAG (DAYS)
FIGURE 1.-Prior Experiment i: sex ratio of offspring from fathers of increasing age. Mothers' ages at start of mating were constant at four days. For each data point the average number of offspring per vial is indicated. In comparison with the main experiments, this one represents a
B Brood.
In Experiment ii, Brood A produced 6,441 progeny showing an overall sex ratio of 50.85%, and Brood B produced 6,921 progeny showing a n overall sex ratio of 50.07%. The sex ratios as functions of paternal age are indicated in Figure 2. It is seen that beyond age 16 the progeny sex ratio again stops decreas- ing, rises to a peak, and falls sharply. For days 0 through 16, the slopes of the regression lines are:
for Brood A, b = -0.396 [t = 14.1; df = 3; P = .001], and for Brood B, b = -0.199 [t = 4.60; df = 3; P = .02].
The slopes of these two regression lines are significantly different from each other [t = 3.8 for the hypothesis of no difference; df = 6; P = .01].
I I I I I
.I
--
b = -0.199
I I I I
0 5 10 15 20 25
FATHER'S AGE AT START OF MATING (DAYS)
0
FIGURE 2.-Prior experiment ii: sex ratio of offspring from fathers of increasing age. Mothers' ages at start of mating were constant at three' days. For each data point, the average number of
offspring per vial is indicated. The dashed lines show the significant linear regressions for days 0 through 16, the b-values being the slopes of these lines.
For the A Broods of the two experiments the slopes of the linear regression in Experiment I, b = -0.842 [t = 6.89; df = 2; P. = .02], and
in Experiment 11, b = -0.415 [t = 11.6; df = 2; P = .009]. 1' ines are:
While the two A Broods do not differ in overall sex ratio (see Table 1 j , the slopes of the regressions are just significantly different [t = 2.8 for the hypothesis of no difference; df = 4; P = .05]. In comparison with the A Brood of Experiment ii where b = -0.396, the sex ratio decrease is much steeper in Experiment I, but of comparable value in Experiment 11. Since no deliberate changes in the con- ditions between Experiment I and I1 were made, the reason for the slope differ- ence is unclear.
For the Later Broods of the two experiments the slopes of the regression lines are f0.133 for Experiment I, and -0.046 for Experiment 11, neither value being at all different from zero slope. Nor is there a slope which even approaches a non-zero value within any individual later brood in either experiment, with the sole exception of the last sizable brood, D, of Experiment I. Here, b = f0.929, a relatively large positive value [t = 3.54; df = 2; P = .07].
ARTHUR P. MANGE
I I I
LATER BROODS
0 5 10 15 0 5 10 15
FATHER'S AGE AT START OF MATING (DAYS)
FIGURE 3.-Main Experiments I and 11: a comparison of the A Broods and the pooled results of the Later Broods. Mothers' ages at the start of mating were constant at five days. For each data point the average number of offspring per vial is indicated. Significant linear regressions and their slopes are indicated.
TABLE 2
Changes in sex ratio in Experiments I and II in going from A Broods to Later Broods
MPERlMENT I MPERIMEXiT I1
Age of
m
Brood Broods Chanm Brood Broods ChaneeA Iater A Later
1 54.4 48.7
-
5.7 52.9 49.7 -3.22 53.2 47.6
-
5.6 50.8 50.2-
a.69 48.5 48.4
-
0.2 49.1 51.2 +2.113 44.7 49.9 '5.2 47.9 48.4 +0.5
9- and 13-day old fathers, generally female offspring appear preferentially in the first broods.
they are slanted down somewhat more, reflecting the proportion of the total flies that occur in the
A
broods. The corresponding regression lines are slightly nega- tive but not at all significantly different from zero slope.DISCUSSION
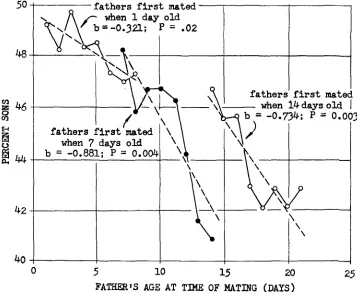
I have summarized the results of YANDERS (1965) in Figure 4 since there is an interesting point of agreement with the current experiments. YANDERS aged his Oregon-R males by transferring them daily to new, constant-aged, virgin, Oregon-R females, all flies being raised at 23°C. This he did for 8 mating periods beginning with either I-, 7-, or 14-day old virgin males. Each female was isolated and allowed to lay eggs in a single vial for 9 days. Microscopic examination of the storage organs of random females indicated that after this period of time no sperm were left. Daily counts of offspring were made until the vials were exhausted, the progeny exceeding a total of IO5. Figure 4 represents only part of
YANDERS' experiments, namely the untreated controls for irradiated flies.
50 i f a t h e r s f i r s t mated I I
when
1 day old 0.323.; p =.02
48
f a t h e r s f i r s t mated when
14
days old1
b =-0.734;
P
=
0.C46
m
6
when7
days oldb
=-0.881;
P = 0.004I \
3
a44
'3
5
10 15 20 250
FATHER'S AGE
AT TlMEOF
MATING(DAYS)
FIGURE 4.-The Experiments of YANDERS (1965): Sex ratio of offspring from fathers of increasing age. Mothers' ages at the start of mating were between 4 and 6 days. The dashed lines indicate the linear regressions within each of the three groups of fathers. The slopes and the
102 ARTHUR P. M A N G E
Considering the differences in experimental conditions and procedures, it is curious that YANDERS’ results also exhibit the steady decrease in sex ratio until the fathers are about two weeks old, followed by a sharp increase and subsequent decrease. The sharp increase is obscured due to its coming just between the second and third groups of males. (A sudden rise in the sex ratio among the progeny from increasingly older males has also been noted by OFTEDAL [ 19631 .) Considering as a single group all fathers between 1 day old and 14 days old, the slope of the regression is b = -0.516 [t = 6.12; df = 14; P
<
.001], a value which is inter- mediate between the main experiments reported here. The major discrepancy is that this significant sex ratio decrease in YANDERS’ experiments applies to all offspring of sperm-depleted females, while, in my experiments, it applies only to the first brood of offspring. It is possible that, in YANDERS’ studies, the first-laid eggs enjoyed a higher probability of attaining adulthood than the eggs laid as much as 9 days later in the same vial. This might have occurred if the vials became at all crowded after the first few days, and to the extent that it did, YANDERS’ results are weighted toward initial broods, and thus toward agreement with my results.The mechanism by which younger fathers produce, on the average, sons before daughters, while older fathers do the opposite (Table 2) is obscure. This mechanism cannot rely only on differential production or only on differential death of the two sperm types since, for any age of father, the overall proportion of the two sexes does not change. A balanced combination of meiotic drive and sperm selection could predict the present results, but this model seems unneces- sarily complex. Maternal conditions, per se, would not seem a likely explanation since the mothers were similar to each other for all ages of fathers. Nor does it seem possible that cultural conditions affecting the larvae or pupae differentially could easily explain the present results since these were also held constant. This last point could be checked by using attached-X mothers to reverse the relation between a sperm’s sex-chromosomes and the sex of the resulting zygote.
The sex ratio difference between A Broods and Later Broods may be related
It should be emphasized again that, in my experiments, there is no ouerall shift in the progeny sex ratios based on paternal age. Looking at Table 2 we need to explain why the order of appearance of males and females among the progeny depends on paternal age. Formally this effect can be described on the basis of the relative “priority” of X- and Y-bearing sperm. “Priority,” following Merriam- Webster dictionaries is to be understood as a “preferential rating.
.
.
prescribing the order in which assignments,” that is, fertilizations, “are to be attended to.” From younger males (1 and5
days old) Y-bearing sperm would have higher priority,” while from older males (9 and 13 days old) X-bearing sperm would have higher “priority.” While the idea of differing “priorities” in sperm utiliza- tion is reasonable and convenient, other interpretations involving the sequence of events from the unseen primary spermatocytes in a father to the observed sex ratio among his adult offspring are possible. This sequence involves sperm maturation and storage in the male, sperm transfer to and storage in the female, the fertilization, deposition, and hatching of eggs, and, finally, the development of the larvae, pupae, and adults up to the time of observation.Experiments similar to those described here were done many years ago by MULLER and SETTLES (1927). These investigators compared the sex ratio from eggs laid immediately after insemination (an “A Brood” in my terminology) with that from eggs laid 7 to 14 days after the female had been separated from the male (a “Later Brood”). No significant difference in sex ratios was found, from which the authors conclude that
X -
and Y-linked genes do not function in the sperm. However, MULLER and SETTLES used, apparently, only one age of father and they do not state what this age was; if I had used only about 8-day old fathers no change would have been likely either.A possible mechanism for the differential recovery of X- and Y-bearing sperm not involving the functioning of genes within sperm is based on the meiotic segregation of cytoplasmic granules to only one of the two first division cells
(PEACOCK and ERICKSON 1964). This visible polarity has been shown to be associated with the segregation of the sex chromosomes in males whose X-chromo- some carried the sc4-sc8 inversions, and whose Y-chromosome carried a small X-chromosome translocation (YANDERS, BREWEN, PEACOCK and GOODCHILD 1968). The association was particularly pronounced when nondisjunction oc- curred in these males. In a control stock carrying a morphologically normal X- chromosome, however, no association existed between the granular polarity and the sex chromosome segregation. I t would thus seem that this phenomenon is not general.
A type of reaction between the male ejaculate and the female tract in
D.
melanogaster has been demonstrated by DEVRIES (1 964). Her studies involved all pairwise crosses among four wild-type strains. Particular male-female combi- nations differed in the numbers of sperm stored in the ventral receptacle, and the rate of sperm loss from this storage organ. These strain-dependent interactions indicate that sperm storage and usage can be affected by internal conditions. I n this connection it would be interesting to repeat my experiments within and between a number of different wild-type strains.
104 A R T H U R P. M A N G E
That comparable sperm may behave differently in females of differing geno- type is further shown by the work of ZIMMERING and FOWLER (1968). Here Oregon-R males inseminated both Oregon-R females and a mutant strain of yellow females. Counts of the numbers of sperm stored in the females were made 3 h r after a single copulation. For both strains of females these counts were similar, averaging close to 500. However, the number of offspring produced by the two strains was quite different, averaging 69% of the number of stored sperm for Oregon-R females, and 43% for yellow females. The authors postulate that the ability of a sperm to function in fertilization depends upon the genotype of the female entirely, or on an interaction between the genotype of the female and that of the sperm. Of course, the question of comparable sperm behaving differ- ently in different females is not the same as whether different sperm ( X and Y ) can behave differently within a single female.
Females store sperm in both their paired spermathecae and in the much larger organ, the ventral receptacle. In other work of ZIMMERING and FOWLER (1966) Oregon-R females stored, on the average, 36 sperm in the spermathecae and 300 sperm in the ventral receptacle. Many more than this-up to several thousand- are normally deposited by a “middle aged” male into the female genital chamber
(KAUFMANN and DEMEREC 1942), but most of these are probably swept out with the laying of the first egg. The authors noted that when smaller numbers of sperm (from irradiated males) were stored the percentage which ended up in the spermathecae was higher, indicating, perhaps, that the smaller storage organs were filled preferentially. This becomes important since young males-less than a day or two old-transfer fewer sperm than the several hundred that the female can store. I t would be nice to know the order in which sperm are utilized from the storage organs, and whether a difference in storing selectivity between the spermathecae and the receptacle exists.
Maternal aging effects upon the sex ratio of offspring have been investigated thoroughly by HANNAH (1955) using several temperatures and a variety of mutant stocks-in particular. a stock carrying a ring-X-chromosome. Matings of females to ring-X males produced significant increases in the proportion of sons
as the mothers were aged as virgins for 7, 14, or 21 days. Similar, although not significant changes were noted using wild-type Samarkand fathers.
In other observations using ring-X males, HANNAH compared the sex ratio of offspring developing from eggs laid during the first 24 h r after mating (an A Brood) us. the second 24 h r (a B Brood). In these experiments the fathers were
a few hours old at the time of first mating and all parents were transferred from the A to the B broods. She found that the first period usually produced a higher percentage of sons than the second. This was interpreted as a difference between stored, that is, aged, eggs in the A Broods and freshly matured eggs in the B Broods. The heterogeneity between replicated cultures could be ascribed in part
to the amount of egg-laying by individual females while being stored as virgins. Some laid many eggs during the virgin period, and, among these, the sex ratio shift was not very great. I n contrast, those females that retained their eggs prior
characteristic of the first 24 h r after mating and a lowering of this proportion thereafter.
The two effects observed by HANNAH appear to be somewhat similar: the probability of a son increases either as his mother ages (from the time of her eclosion) or as the egg from which he develops ages (from the time of its matura- tion). HANNAH interpreted these changes as age dependent modifications of the egg which caused a greater likelihood of male survival compared to females, or, to a lesser degree, a greater likelihood of the ring-X being eliminated, thus con- verting a presumptive XX (ring/rod) female into an XO male.
In my experiments, the age of the mothers was not varied for the different ages of fathers, so that the first aspect of HANNAH’S observations is not pertinent. Her observation of a high percentage of sons in first broods compared with second broods is consistent with my results for fathers 1 day old at the time of mating
(recalling that her fathers were also very young). Her explanation based on egg modification is adequate for these data. But to account for my results from 9-
and 13-day old fathers this explanation would need to be exactly reversed since the sex ratio change from first to second broods is reversed. On the other hand, the formalism of varying sperm “priorities” accounts for both the sex ratio shift within A broods as a function of paternal age as well as the necessarily related sex ratio shift from the A to Later Broods for any given paternal age.
SUMMARY
Oregon-R males varying in age from 1 day to 13 days were mated to 5-day old females for 24 hours. The females were transferred successively to fresh food to utilize all of their stored sperm. The experimental conditions were carefully con- trolled to achieve roughly constant numbers of developing larvae in any vial. Among the offspring, the overall sex ratio from any given age of male did not vary much from SO%, in contrast to previously published results. Among roughly the first 20% of the eggs laid by the females, however, there was a marked decrease i n the proportion of sons, from 54% down to 46%, as their fathers aged from 1 day to 13 days. This decrease was absent, or possibly reversed, among later eggs. A simple explanation of these results is that Y-bearing sperm from younger males, but X-bearing sperm from older males, is preferentially utilized.
LITERATURE CITED
DE VRIES, J. K., 1964 18: 271-282.
Insemination and sperm storage in Drosophila melanogaster. Evolution
Are deviant sex ratios in normal strains of Drosophila caused by aberrant segregation? Genetics 52: 259-266. -, 1969 A deviant sex ratio in Drosophila melanogaster. Genetics 61 : 595-606.
The effect of aging the maternal parent upon the sex ratio in Drosophila melanogaster. 2. Ind. Abst. Vererbl. 86: 574599.
Utilization of sperm by the female Drosophila HANKS, G. D., 1965
HANNAH, A., 1955
106 ARTHUR P. MANGE
LEVY, A., 1965
Naturalist 99 : 426-429. MANGE, A. P., 1969
Serv. 44: 79-80.
MANGE, A. P. and A. R. ALEXANDER, 1968
gaster males given varying numbers of females. Drosophila Inform. Serv. 43 : 132-133. MULLER, H. J. and F. SETTLES, 1927
Abst. Vererbl. 43: 285-312. OFTEDAL, P., 1963
PEACOCK, W. J. and J. ERICKSON, 1964 An indication of polarity in the spermatocyte? Drosophila Inform. Serv. 39: 107-108.
YANDERS, A. F., 1965 A relationship between sex ratio and paternal age in Drosophila. Genetics
YANDERS, A. F., J. G. BREWEN, W. J. PEACOCK and D. J. GOODCHILD, 1968 Meiotic drive and visible polarity in Drosophila spermatocytes. Genetics 59 : 245-253.
ZIMMERING, S. and G. L. FOWLER, 1966 X-irradiation of the Drosophila male and its effect on the number of sperm transferred to the female. Z. Vererbl. 98: 15G151. 1968 Progeny: sperm ratios and nonfunctional sperm in Drosophila melanogaster. Genet. Res. 12: 359-363.
Sex ratio in isogenic laboratory populations of Drosophila melanogaster. Am.
Larval density versus sex ratio in D . melanogaster. Drosophila Inform.
Fecundity of virgin versus non-virgin D . melano-
The non-functioning of the genes in spermatozoa. Z. Ind.
Sex ratio in broods. Drosophila Inform. Serv. 37: 114-115.
51 : 481-486.