MERODIPLOIDY
I N ESCHERICHIA COLI-SALMONELLA
TYPHIMURIUM
CROSSES: T H E ROLE OF UNEQUAL
RECOMBINATION BETWEEN RIBOSOMAL RNA GENES
ANDREAS F. LEHNER' AND C. W. HILL
Department of Biological Chemistry and The Cancer Research Center, The Milton S. Hershey Medical Center, The Pennsylvania State University, Hershey, Pennsylvania 17033
Manuscript received September 24, 1984 Revised copy accepted March 1, 1985
ABSTRACT
Previous workers have shown that intergeneric crosses between Salmonella typhimurium and Escherichia coli produce a high proportion of merodiploid recombinants among the viable progeny. We have examined the unequal cross- over event that was responsible for a number of intergeneric merodiploids. The merodiploids that we studied were all heterozygous for the metB-argH
interval and were the products of intergeneric conjugal crosses. We found that when the S. typhimurium donor had its transfer origin closely linked to metB
and argH, all recombinants examined were merodiploid, and they generally arose as F-prime factors. Many of these F-prime factors had been created by recombination between flanking rrn genes in the donor. When the S. typhi- murium Hfr transfer origin was more distant from the selected markers, quite different results were obtained. (1) Depending on the donor, 19-47% of the recombinants that acquired the donor argH+ or metB+ genes were merodiploid for these loci, but none of the recombinants were F-prime. (2) A majority of the merodiploids had a novel (nonparental) r m gene, indicating that unequal recombination between nonidentical rrn genes was a prevalent mechanism for establishing the merodiploidy. (3) Both tandem and nontandem duplications were found. (4) Some of the merodiploids duplicated E. coli genes in addition to acquiring S. iyphimurium genes. (5) Some merodiploids contained the oriC
region from each parent. Of a total of 11 8 intergeneric merodiploids charac- terized from all donors, 48 different genotypes were observed, and 38 of the 48 had one or more nonparental rrn operons.
ERODIPLOIDS are common among the progeny of Salmonella typhimu-
M
rium-Escherichia coli intergeneric crosses (BARON et a l . 1968; SANDERSON1976). T h e merodiploidy is maintained through F-prime factors in some of these recombinants, but in others the extra DNA is inserted into the recipient chromosome (JOHNSON et al. 1973, 1975). Despite the potential importance of intergeneric merodiploids in bacterial evolution, little attention has been paid to the illegitimate recombinational events responsible for the insertional type. Considerable information is available, however, on insertion merodiploids aris- ing within E. coli or within S. typhimurium. In E. coli, for example, homologous
' Current address: Departments of Medicine and Endocrinology/BD-13 1, Medical College of Georgia, Au- gusta, Georgia 309 12.
366 A. F. LEHNER AND C. W . HILL
recombination between different ribosomal RNA genes (rrn) can generate both tandem (HILL et al. 197’7) and transposed duplications (HILL and HARNISH
1982). In a survey of duplications occurring in S. typhimurium, it was found that loci lying between directly repeated rrn genes were more likely to become duplicated than loci elsewhere on the chromosome (ANDERSON and ROTH
1978). T h e r m operons seemed ideal targets for the type of unequal crossover required to establish intergeneric merodiploids, considering the degree of in- tergeneric conservation of both the nucleotide sequence (KOHNE 1968) and the map position of the seven r m operons (ANDERSON and ROTH 1978; LEH-
NER and HILL 1980; LEHNER, HARVEY and HILL 1984), and this paper ad- dresses their contribution.
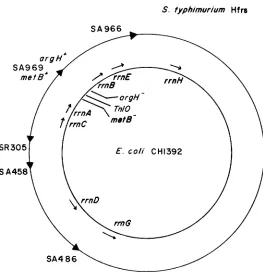
T h e map positions and orientations of the rrn operons is shown in Figure 1. Given that there are seven of these operons, two of which are oriented in opposition to the other five, there are a large number of potential interactions that could lead to merodiploidy. Seven distinct mechanistic types that will be of importance in our results are depicted in Figure 2. In each mechanism shown, the novel joint(s) is created by recombination between different rrn operons. T h e merodiploids produced can be tandem duplications (A, B and
C), transposed duplications (D and E) or more complex types (F and G). Of course, inserted merodiploids could be created by illegitimate recombination between sites other than rrn operons, and each mechanism in Figure
2
should have a counterpart that does not use rrn operons for unequal crossover. Acommon feature of all of the mechanisms of Figure 2 is that at least one of the partners in the creation of each novel joint is donor DNA. Mechanisms can also be imagined where both partners in forming the novel joint are recipient
DNA. Three such mechanisms are shown in Figure 3. We will describe a set of merodiploid recombinants whose properties fit all of the types proposed in Figures 2 and 3 except type H.
T o obtain intergeneric merodiploids, we conducted a series of crosses using an E . coli F- recipient and a variety of S. typhimurium Hfr donors. T h e donor markers selected were metB+ or argH+ which occur within the r r A - r m B in- terval. T h e progeny were classified as to whether they were haploid or diploid for these selected markers and analyzed as to the number and identity of the rrn operons present. This analysis took advantage of the fact that each of the seven E . coli and each of the seven S , typhimurium rrn operons occur within a distinct BamHIIPstI fragment (LEHNER, HARVEY and HILL 1984). Since the positions of the flanking restriction sites for all operons, except S. typhimurium
r m H , have been mapped (BOROS, KISS and VENETIANER 1979; LEHNER,
HARVEY and HILL 1984) the sizes of recombinant r m operons can be predicted and used in their identification.
mosomal structures were considered only if they contained at least one rep- resentation, either donor or recipient, of all parts of the chromosome as well as a metB-argH duplication; ( 4 ) nonparental rrn fragment identifications were confirmed by comparing observed sizes with those predicted from the pub- lished restriction map of E. coli and S. typhimurium genes (LEHNER, HARVEY and HILL 1984); (5) in addition, the merodiploids were tested for the presence of F-prime factors.
MATERIALS AND METHODS
Bacterial strains: E. coli mutants used were K-12 derivatives. CH1392 was F- sbcB15 endA hsdR4
h s d W metBl zij-116::TnIO argHl thi gal (LEHNER, HARVEY and HILL 1984). CHI396 was derived by cotransducing recA56 with srl-300::TnIO into a trpA23 Pur+ derivative of AB336 (TAYLOR and ADELBERC 1960). S. typhimurium Hfr’s were obtained from the Salmonella Genetic Stock Center, University of Calgary, Calgary, Alberta, Canada, and have been described by SANDERSON et al. (1972). Their origins of transfer are shown in Figure 1.
Microbiological techniques: Media and techniques for noninterrupted mating, purification of re- combinants and storage of strains have been described (LEHNER, HARVEY and HILL 1984). Sensi- tivity of a strain to R17 phage was determined by placing a loopful of RI7 on the surface of a soft agar layer containing the bacterial mutant. Segregation frequencies were determined by grow- ing a culture in synthetic medium containing tetracycline but no methionine or arginine, spreading on glucose synthetic plates containing methionine and arginine to yield 150-350 colonies and replica plating to detect Met-, Arg- or Tet’ segregants.
Restriction enzyme digestion and hydridization: Methods for isolation of genomic DNA, restriction endonuclease digestion, gel electrophoresis, transfer to diazotized paper, hybridization to E. coli
[SsP]ribosomal RNA and autoradiography have been described previously (LEHNER, HARVEY and HILL 1984). F+ plasmid was prepared from an F+ segregant of KL96 (gift of M. CAPAGE) by CsCI- ethidium bromide centrifugation following lysozyme-RNase A-Sarkosyl treatment of logarithmically grown cells (HILL et a l . 1977). F plasmid preparations were radiolabeled to 107-108 dpmlpg with [a-”P]dCTP by the procedure of RICBY et a l . (1977).
RESULTS
Intergeneric crosses: One objective of this work was to determine how rrn genes might recombine to establish merodiploidy in intergeneric recombinants. Therefore, the metB and argH loci were chosen for study because they lie within a region containing several rrn genes (Figure 1). Specifically, we crossed various S. typhimurium Hfr donors with a metBl z~-114::TnlO argHl restriction deficient E . coli recipient, CH1392, and selected for either Arg+ or Met,+ recombinants. T h e TnlO that made the recipient Tet‘ was inserted between metB and argH, and its retention in Met+, Arg+ recombinants was anticipated to indicate merodiploidy of the region. For all of the donors except SA969, the coinheritance of Arg+ and Met’ was >99% regardless of which marker was selected; for SA969, which had its origin of transfer directly in the metB argH region, the coinheritance was 85%. For each cross conducted, the fre- quencies of Tet‘ and Tet” among Met+, Arg+ recombinants were determined, and these results are shown in Table 1. Tet’ recombinants constituted 19% or more of the viable recombinants in all intergeneric crosses.
368 A. F . LEHNER AND C. W. HILL
S. typhimurium Hfrs
FIGURE 1.-The map of the rrn operons and other relevant loci in E. coli and S . typhimurium
(not to scale). T h e rrn operons map in closely analogous positions in both species (ANDERSON and ROTH 1978; BACHMANN 1983; LEHNER, HARVEY and HILL 1984), and their direction of transcrip- tion is indicated by the arrows. T h e T n l O insertion is zij-116::TnIO. T h e arrowheads on the outer circle indicate the transfer origins of the S. typhimurium Hfr's (SANDERSON et al. 1972).
merodiploid. All 1 18 Tet' recombinants had eight or more [32P]rRNA-hy- bridizable fragments, several examples of which are shown in Figure 4 (lanes
3, 4, 6,
7
and 8). Fifty-seven of the 58 Tets recombinants each had seven hy- bridizable fragments, the normal haploid number. T h e haploid, Tets, recom- binants have been described previously (LEHNER, HARVEY and HILL 1984).Merodiploidy d u e to F-prime formation: It might have been expected that a large portion of the merodiploids would be F-primes. In fact, F-primes were found only among the progeny of SA969, whose progeny were largely F- primes. T h e SA969 origin of transfer is probably between metB and argH, metB
being transferred early (see DISCUSSION). Seven SA969-derived intergeneric recombinants were tested for their ability to transfer Arg+ to a recA E . coli
recipient (CH 1396), a capacity anticipated of F-prime merodiploids (Low 1972). All seven behaved as F-primes in this test. Of 17 SA969 progeny characterized by restriction digestion and Southern analysis, 14 possessed a novel r m gene. Two examples are shown in Figure 4 (lanes 3 and 4). Of these
14, 1 1 were like M 2 0 8 (Figure 4 , lane 3) in that each had an 8.9-kb novel rrn
TABLE 1
Summary of recombinants
Recombinant designations
% merodiploid Hfr donor" Selection Met+ Tet' Arg+ Met+ Tets Arg+ Otherb (Tet')
SA966 Arg+ A001-A025 A 101-A120 19
Met+ M001-MO25 M101-M120
SA969 Arg' A201-A210 Met+ M20 1-M2 10
SA486 Arg+ A40 1 -A4 10 A501-A5 10 Met+ M401-M410 M501-M510
SR305 Arg+ A601-A609 A701-A710 Met+ M601-M610 M701-M710
SA458 Arg' A801-A810 A901-A910 Met+ M801-M810 M901-M910
A301, A303 >95 M302
47
25
22
' E . coli CH1392 was the recipient in all cases.
A301 and A303 were Met-, TetR, Arg'; M302 was Met:, TetR, Arg-.
episome (nomenclature of SCAIFE 1967) produced by excision between rrnA and rrnB within the parental SA969 chromosome. Another mutant, M204 (Figure 4, lane 4), acquired S. typhimurium fragment b in addition to a 12.1- kb novel fragment corresponding in size to (ela). This type I1 F-prime most probably was produced by excision between rrnA and rrnE in the donor. Others of the F-primes had no nonparental rrn fragments and appeared to be type IA (transferring metB+ and iZvA+ but not argH+) or type IB (transferring
argH+ but not me&).
Two conclusions were drawn from the analysis of SA969-derived mero- diplids. First, when the origin of transfer was closely linked to the selected donor markers, F-primes predominated among intergeneric merodiploids. Sec- ond, when directly repeated rrn genes flanked the integrated F, unequal re- combination between these genes could readily cause F-prime excision. This mechanism for generating type I1 F-primes has been demonstrated by DEONIER et al. (1974) and by BLAZEY and BURNS (1983).
3 70 A . F. L E H N E R A N D C . W. HILL
the presence of [32P]F-hybridizable bands in total DNA digests, corresponding in size to F+ BamHIIPstI fragments. A positive result in one test correlated with a positive result in any other. Approximately half of the 101 recombinants possessed F, but none behaved as F-prime. F+-sized plasmids would not hy- bridize with [’*P]rRNA (data not shown). Since donor rrn loci were gained in all merodiploids, these F-sized plasmids could not be responsible for maintain- ing the merodiploidy. Furthermore, of 20 merodiploid recombinants tested for the ability to transfer Arg+ to an argH recA E. coli recipient (CH1396), none could transfer. We concluded that the F factors in the original Hfr donors were somewhat unstable and could be transferred to and maintained in the recombinants as free F+. J O H N S O N et al. (1 973) also reported intergeneric mer- odiploids that coincidentally became F+, without the F factor being involved in maintenance of the merodiploidy. Recombinants that possessed F+ are des- ignated as such by an “ f ” suffix to the strain designation.
Merodifdoids with nonparental rrn restriction fragments: Of the intergeneric merodiploids without F-prime factors, 63 of 101 exhibited a novel (nonpar- ental) rrn fragment on BamHI/PstI double digestion. This indicated that un- equal recombination between rrn genes had occurred. Of the 63, 51 had profiles in which unequal rrn recombination alone could account for establish- ment of merodiploidy (Figure 2). These 51 are listed in Table 2 where they are grouped according to the mechanism that best explains their genotype. Note that many subtypes are possible for each of the seven mechanistic types depicted in Figure
2;
e.g., Type A could be produced by crossover between donor rrnE and recipient rrnC just as well as by the rrnB with rrnA crossover depicted. All of the recombinant rrn genes assigned in Table2
matched the sizes predicted by the restriction maps of the parental operons ( L E H N E R , HARVEY and H I L L 1984) within 3%. In most cases, the identities were verified by using either PstI or BamHI digestion alone.T h e application of the rationale described in the introduction to assigning genotypes to these 51 recombinants can be illustrated by considering A009 (Figure 4, lane 6). A009 displayed rrn fragments identical with E. coli G, B , E and H and to S . typhimurium d , c and a. In addition it had a nonparental 7.1- kb rrn fragment. Since duplication of the metB-argH region in the rrnA-rrnB interval was required, the most likely donor contribution to the nonparental rrn operon was the promoter proximal portion of b , since parental a but not
6 was present. Similarly, the most likely recipient contribution was the pro- moter distal portion of A . In other words, the nonparental rrn operon in A009 was expected to be ( b / A ) arising via type A merodiploidy (Figure 2) with the Gdca( b/A)BEH genotype. This deduction was strongly supported by the fact that the 7.1-kb measurement of the A009 novel fragment agreed well with the 7.0-kb size predicted for ( b / A ) from our previous work ( L E H N E R , HARVEY and H I L L 1984).
A d c o + b e
C
D
E
0
G D C A B € H
-4
Q
. .G D C A E f H
-4
t +
b o c d
F
,
G b C A B € H
-c - +
t
G G D C A B € H
-I,
-
G k / D b G/O D C A B P H - - - - - A-U
? _ _ - - -G D C A E € H
t
FIGURE 2.-Schemes for creating merodiploid recombinants by rrn crossovers. Throughout this work we will use the following conventions for designating the rrn operons and the restriction fragments that contain them: E. coli operons by upper case letters; analogous S. typhimurium
operons by lower case letters; recombinant operons by a symbol such as ( E / a ) which designates an operon whose promoter proximal sequences came from E. colt rrnE and whose promoter distal sequences came from S. typhimurium r r A . In all seven schemes the novel joint(s) that is essential for merodiploidy is produced by an unequal recombination between a donor rrn operon and a second rrn operon which may be either donor or recipient. The type B and G mechanisms require the participation of two recipient chromosomes or chromatids. The type C and D mechanisms require circularization of the donor fragment before integration into the recipient chromosome. Note that many subtypes are possible for each of the seven mechanistic types depicted; e.g., type A could be produced by crossover between donor rrnE and recipient rrnC just as well as by the
372 A . F. LEHNER AND C. W . HILL 1 A B L E 2
Intergenertc merodaplotds wzth a novelloant dertved at least tn part from donor D N A
Probable Recombinant
iiiecliriiiisinY designationb Constitution'
A AOOY, A023, A408f, A409f, Gdca(b/A)BEH
M430f
A008f, M007, MO08 GDCAB(E/a)beH
MO01 GDCA(B/a)bEH
M601, A 6 0 2 , A605, A608 GDCA(B fc)abEH
A609 GDCa(b/A)BEH
M 8 0 5 G Dca b(e/C)ABEH
A002, A012, A 0 1 4 , MO11 GDCAB(E fc)abeH
A001, A010, A017f, M002f Gdcab(e/A)BEH
A003f GDca b(e/A)BEH
A007 Gdca(b/C)ABEH
A O l l f GDCAB(E fc)ab(e fE)H
A809 GDC(A/aXb fA)BEH
A 0 1 3 , M603, A807 GD(C/c)a(b/C)ABEH
B
c
L) o r E
M010, M604f, M606, M609, GDCAB(E/c)abEH
A 0 1 9 GDCA B(E/a)bEH
A802, A 8 0 3 , M801
A607 A601f, M602 A015f
A 8 0 5 , M803, M605 M610
A 6 0 3 M802 A801
GDCAb(e/c)aBEH GDCA(b/c)aBEH GDCAb(e/a)BEH GDCAB(E/c)a(b/E)H G(e/D)ba(D/c)CABEH (e/G)ba(G/c)DCABEH GDCAB(E/aXb fE)H (b/G)a(G/c)DCABEH
F MO24 G(b fD)ac(d fA)BEH
G MO 12f G(e/D)b(G fa)DCABEH
' See Figure 2 for designation of t h e mechanistic type.
Parents a n d phenotypes of recombinants a r e specified in T a b l e 1. T h e suffix "f' denotes that t h e recombinant was F+ (see text).
' T h e rrn restriction fragments were identified by size, a n d t h e identities of t h e nonparental ones were deduced as described in t h e text. I n all cases t h e observed sizes agreed within 3% of t h e predicted sizes. T h e actual o r d e r of t h e rrn operons could not he deduced from t h e data.
Another common type was type B. T h e salient feature of type B recombi- nants is that a portion of the recipient chromosome appears in both copies of the duplication. In the case of MO10 (Table 2) this was the promoter proximal restriction site of E . coli rrnE which appeared in both ( E / c ) and in E. This phenomenon can best be explained by invoking the participation of two recipient
4
c a b e
-
_ _ - , d - e - G D C A B E . / A B E E : ' H - ~ - G 0 C A B E K a b e H -
H G D C A B € H
I -
G D C A B E H
+
d c a b e
7
I G D C A B E H
< _ - -
_ _
_ - - - - /-e
G D C A B E H i D C A 8 E/ H --
G D C A E E H d c 0 b e H - G D C A B E Hd c a b ?
G D C A B E H
_.-,
- e _
G D C A E E H / D C A E E H - e - G D C A B E H d c a b e/H-
J
-. .- - - -
4 r -
G O C A B E H
FIGURE S.-Schemes for creating merodiploid recombinants. Conventions are as specified in Figure 2. These schemes differ from those in Figure 2 in that the novel joint essential for creating merodiploidy is produced by a crossover between two recipient DNA segments. Note that the J scheme calls for the production of a second novel joint through a crossover between a donor and a recipient rrn operon.
Four recombinants had novel rrn operons produced by recombination be- tween two donor r m operons. These could be produced by a type C sequence (Table
2
and Figure 2) wherein the donor DNA circularizes and then inte- grates by an equal crossover to produce a tandem duplication.There were seven mutants that were transpositions. These could have been produced by either the two-step type D mechanism or the concerted type E one. T h e D mechanism, like C, calls for the circularization of the donor frag- ment. However, the circle intergrates elsewhere in the chromosome, using the homology provided by the rrn operons. T h e E mechanism accomplishes much the same end as D, except that no circular intermediate is formed. T h e D and E mechanisms cannot be distinguished by examination of the rrn profiles of the recombinants. Transpositions into the recipient rrnD, rrnE and rrnG op- erons were observed. T h e largest transpositions were of the entire donor rrnC
to rrnE interval into either rrnD (mutant M610) or into rrnG (mutant A603).
Two interesting mutants each had two novel joints involving four rrn op- erons. These were MO24 and MOlZf, which could be explained by type F and type G mechanisms, respectively. Note that MO24 acquires an inversion of the rrnD to rrnA interval as well as the duplication.
As mentioned above a total of 63 recombinants had novel rrn operons, and
5 1 of these are listed in Table
2.
Six of the remaining 12 will be described in the next section, whereas the other six appeared to result from more complex mechanisms.3 74 A . F. LEHNER AND C. W . HILL TABLE 3
Intergeneric merodiploids with a novel joint derived completely from recipient
DNA
Probable Recombinant
niechanism" designationb Constitutionc 1 M 4 0 7 , M 4 0 9 , M410
M 4 0 1 A810, M807f A 8 0 8 , M 8 0 6 , M 8 0 8 f
M023f
J
M 4 0 4 f , M 4 0 5 A407, M 4 0 6 f A 8 0 6 , M 8 1 0 fGDCABEHdcabeH GDCABEHdcabEH GDCABEHcabeH GDCABEHcabEH
GDcabeDCABEH
GDCABEHdca(b1H) GDCABEHdcab(e1H) G DCABEHcab(e1H)
a See Figure 3 for designation of the mechanistic type.
Parents and phenotypes of recombinants are specified in Table 1 . The suffix "f' denotes that the recombinant was F+ (see text).
The rrn restriction fragments were identified by size, and the identities of the nonparental ones were deduced as described in the text. In all cases the observed sizes agreed within 3% of the predicted sizes. The actual order of operons could not be deduced from the data.
was not the novel joint essential for merodiploidy but was rather the site of the completion of the integration. T h e essential novel joint in these six may have joined recipient DNA to recipient DNA (Figure 3, type J). Therefore, these six are more logically grouped with the type I and listed in Table 3 rather than grouped with those listed in Table 2.
T h e last major category had no nonparental r m operons and simply had one or more donor r m operons added to a full complement of recipient operons. There were 27 of these. One added only donor 6 , whereas the other 26 added donor d , c , a , b and e . We have named this last type, type K. M006, shown in Figure 4 (lane 8), was an example of these.
T h e final one of the 101 had no apparent donor r m operons but did appear to duplicate the recipient r m B .
Genetic instability: A classical property of genetic duplications is genetic in- stability. T o test for instability, several intergeneric merodiploids were grown
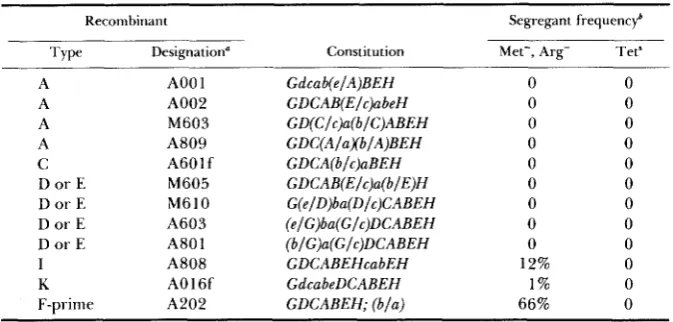
to stationary phase in glucose synthetic medium (selects Metf, Arg+), diluted and spread to yield 150-350 colonies on a nonselective plate. These were then screened for either Met-, Arg- or Tets segregants by replica plating. Most intergeneric merodiploids produced no detectable segregants (Table 4). Excep- tions were the b/a F-prime merodiploid, A202, which proved highly unstable, the type I cabEH duplication, A808, and the type K dcabe duplication, A016f. Fourteen segregants each from A808 and from A016f were examined for remaining r m genes, and in all cases the segregants had lost all S. typhimurium
I
2
e l l @
t
C I )
c m
G8 A
3
4
5
6
7
me
RI1
*-
e mFIGURE 4.--BamHI/Pstl digestion of genomic DNA from various intergeneric merodiploids. DNA isolation, digestion, gel electrophoresis, transfer and hybridbation to E. coli ['*P]rRNA and autoradiography were as specified in MATERIALS A N D METHODS. The E. coli and S. lyphimurium parental r m fragments served as internal size standards (LEHNER, HARVEY and HILL 1984) for measurement of the novel rrn fragments. Lanes 1-4 came from one gel; lanes 5-8 from a second. Lane I , E. coli K-12 strain CH439; lane 2, S. lyphimurium LT2; lane 3. F-prime recombinant M208; lane 4, F-prime recombinant M204; lane 5, a mixture of E. coli K-12 and S. lyphimurium LT2 DNA; lane 6, a type A merodiploid, A009; lane 7, a type I merodiploid. A810; lane 8, a type K merodiploid M006.
DISCUSSION
376 A . F . LEHNER AND C. W. HILL TABLE 4
Stability of intergeneric merodiploids
Recombirran t Segregant frequencyb
Type Designation" Constitution Met-, Arg- Tet*
A A A A
c
D or E D or E D or E D or E I K F-prime A00 1 A002 M603 A809 A60 1 f M605 M610 A603 A801 A808
A 0 16f A 2 0 2
Gdcab(e fA)BEH GDCAB(E/cja beH GW(C fc)a(b/C)ABEH GDC(A faxb/A)BEH GDCA(b fc)aBEH GDCAB(E/c)a(b/E)H G(e/D)ba(D fc)CABEH (e/G)ba(G/c)DCABEH (b/G)a(Glc)DCABEH GDCABEHcabEH GdcabeDCABEH GDCABEH; (b fa )
0 0
0 0
0 0
0 0
0 0
0 0
0 0
0 0
0 0
12% 0
1 % 0
66% 0
a Parents and phenotypes of recombinants are specified in Table 1 .
*
0 indicates none detected out of 150-350 colonies.mechanisms could be derived. With regard to the 51 recombinants making up the 25 genotypes listed in Table
2,
all can be explained by mechanisms that require only two homologous crossovers, at least one being an unequal cross- over between r r n operons to create the novel joint(s).T h e nature of the merodiploids varied with the particular Hfr donor used. Of the five Hfr strains, only SA969 was observed to produce F-primes. In this case the majority of the recombinants were type I1 F-prime (according to the nomenclature of SCAIFE 1967). In somewhat analogous experiments, JOHNSON
et al. ( 1 973) found Mal', Xyl' merodiploid F-prime recombinants when they mated a S. typhimurium Hfr (transfer origin between mal and xyl) with an E.
coli F-. Most of the F-prime factors in our cross were created by excision between directly repeated rrn operons in the donor. DEONIER et al. (1974) and
BLAZEY and BURNS (1983) have previously described the creation of a type I1 F-prime by excision between directly repeated r m genes. One conclusion in- dicated by analysis of SA969 progeny was that the origin of transfer of SA969
must lie between metB and argH. SANDERSON et al. (1972) have suggested that the point of origin lies further clockwise than this because SA969 transferred
thi efficiently. (The thi locus is in the rmB-rrnE interval.) Our results suggest that thi is distal in SA969 and that transfer of thi could occur by frequent generation of type I1 F-prime factors.
itance of very distal genes in this conjugal cross contrasts strongly to the observation that inheritance of distal markers decreases exponentially in simple intraspecies crosses (JACOB and WOLLMAN 196 1). Whether this represents a restrictive requirement for coinheritance of proximal and distal donor genes, or whether this simply reflects the formation of merodiploids through some opportunistic mechanism, must be decided by further experimentation. Yet another example of dependency of progeny type on the donor was the group listed in Table 3. Fifteen of 16 recombinants listed in Table 3 were progeny of either SA458 or SA486. T h e common features of these 15 were that the merodiploid region extended beyond rrnH and may have contained a recipient- by-recipient novel joint.
One type of merodiploid was notably absent from the ones we studied. These were the type H diagrammed in Figure 3. From previous work in E. coli (HILL and COMBRIATO 1973) and S. tjfihimurium (ANDERSON and ROTH 1978), it is known that approximately one cell in lo3 spontaneously carries a duplication of one of the intervals between directly repeated r m operons. These are likely produced by the crossover specified in the first step of the
type
H mechanism (Figure 3). Such cells, when mated with an Hfr cell, would yield merodiploids with high efficiency. Nevertheless, none of the 1 18 merodiploids examined appeared to betype
H. Analogously, it might have been anticipated that mer- odiploids would result from unequal recombination in the donor before con- jugation. This was clearly the case for the type I1 F-prime progeny of SA969. T h e recombinants classified as type C in Table2
required unequal crossover between two donor ~ r n operons. This unequal crossover might have occurred in the recipient after conjugation, as depicted in Figure2.
Alternatively, itmight have occurred in the donor to produce a tandem duplication before conjugation. All of the other merodiploid types listed in Table
2
have donor and recipient DNA joined to form the novel joint, and this event must have occurred after conjugal transfer.378 A. F. LEHNER AND C. W . HILL
has not been identified, and, consequently, we do not know whether the extra DNA was inserted into the chromosome. Since the origin of replication, oriC,
maps in the rrnD-rrnC interval, rather close to rrnC (BACHMANN 1983), those that have the rrnD-rrnC region from both E. coli and S. typhimurium (type K)
have two replication origins. We have in fact confirmed that both origins are present by hybridization experiments using the cloned S. typhimurium oriC
region (ZYSKIND and SMITH 1980) as probe (A. F. LEHNER and C. W. HILL, unpublished results). For lack of evidence to the contrary, the possibility that the duplicated DNA was a separate replicon using the S. typhimurium origin of replication must be considered. If this were true, replication of the extra DNA must have been efficient since spontaneous segregants were produced relatively infrequently as illustrated by type K recombinant A016f in Table 4.
Most types of intergeneric merodiploids were quite stable, losing the extra genes at frequencies of less than 1%. This contrasts with E. coli tandem dupli- cations which are substantially less stable (HILL and COMBRIATO 1973). Two factors likely contribute to the apparent stability of tandem duplications such as A001 (Table
4).
First, the frequency of excision by looping out would be reduced because the S. typhimurium copy of the duplication would have limited homology with the E. coli copy. Second, some haploid segregants might not be viable because they would be left with unfavorable combinations of S. typhimurium and E. coli genes. The F-prime merodiploid type (see A202 in Table 4), by contrast, was extremely unstable. In their studies, JOHNSON et al.(1973, 1975) also found that F-prime merodiploids were substantially less stable than merodiploids whose exogenote was not associated with F.
This paper has discussed the chromosomal structure of intergeneric mero- diploids and in the process has extended the findings of other workers (BARON
et al. 1968; SANDERSON 1976) concerning the high frequency of merodiploids among the progeny of intergeneric crosses. An important question is whether merodiploids are more likely to occur on a per mated pair basis in intergeneric crosses than they are in intraspecific crosses or whether they are simply re- covered in preference to haploid recombinants. We found that approximately 20% of recovered progeny were merodiploid in these intergeneric crosses (Table l ) , compared to 1 - 2 s in similar intraspecific crosses. However, the recovery of total progeny from the intergeneric crosses was reduced at least two orders of magnitude relative to intraspecific crosses (see LEHNER, HARVEY
metB and rrnC and 56 times either within or beyond rrnC. In contrast, the requisite clockwise crossover occurred 34 times in the 0.9-min interval between
argH and rrnE and 23 times beyond rrnE. This strong bias against crossovers in the region just counterclockwise from metB relative to the region just clock- wise from argH could reflect lack of homology for the crossover or incompat- ibility of certain gene combinations. Whatever the cause, it clearly shows a restriction on recoverable recombinants.
We thank MIKE CAPAGE and KENNETH SANDERSON for the gift of bacterial strains. This work was supported by United States Public Health Service research grant GM16329 from the National Institutes of Health and by United States Public Health Service grant 1 P30 CA18450 awarded by the National Cancer Institute.
LITERATURE CITED
ANDERSON, R. P. and J. R. ROTH, 1978 Gene duplication in bacteria: alteration of gene dosage
BACHMANN, B. J., 1983 Linkage map of Escherichia coli K-12, edition 7. Microbiol. Rev. 47: 180-
BARON, L. S., P. GEMSKI, JR., E. M. JOHNSON and J. A. WOHLHIETER, 1968 Intergeneric bacterial matings. Bacteriol. Rev. 32: 362-369.
BLAZEY, D. L. and R. 0. BURNS, 1983 recA-dependent recombination between rRNA operons generates type 11 F’ plasmids. J. Bacteriol. 156: 1344-1348.
BOROS, I., A. KISS and P. VENETIANER, 1979 Physical map of the seven ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 6: 1817-1830; 2961 (erratum).
DEONIER, R. C., E. OHTSUBO, H. J. LEE and N. DAVIDSON, 1974 Electron microscope hetero- duplex studies of sequence relations among plasmids of Escherichia coli. VII. Mapping the ribosomal RNA genes of plasmid F14. J. Mol. Biol. 8 9 619-629.
Genetic duplications induced at a very high frequency by ultraviolet irradiation in Escherichia coli. Mol. Gen. Genet. 127: 197-214.
Tandem duplications resulting from recombination between ribosomal RNA genes in Escherichia coli. J. Mol. Biol.
HILL, C. W. and B. W. HARNISH, 1982 Transposition of a chromosomal segment bounded by redundant rRNA genes into other rRNA genes in Escherichia coli. J. Bacteriol. 1 4 9 449-457.
JACOB, F. and E. L. WOLLMAN, 1961 Sexuality and the Genetics of Bacteria, p. 152. Academic Press, Inc., New York.
JOHNSON, E. M., W. G. CRAIG, JR., J. A. WOHLHIETER, J. R. LAZERE, R. M. SYNENKI and L. S. BARON, 1973 Conservation of Salmonella typhimurium deoxyribonucleic acid in partially dip- loid hybrids of Escherichia coli. J. Bacteriol. 115: 629-634.
Conservation of Salmo- nella typhimurium deoxyribonucleic acid by chromosomal insertion in a partially diploid Esch- erichia coli hybrid. J. Bacteriol. 123: 1-6.
Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145: 1365-1373.
Isolation and characterization of bacterial ribosomal RNA cistrons. Biophys.
Mapping and spacer identification of rRNA by sister chromosome exchanges. Cold Spring Harbor Symp. Quant. Biol. 43: 1083-1087.
230.
HILL, C. W. and G. COMBRIATO, 1973
HILL, C. W., R. H. GRAFSTROM, B. W. HARNISH and B. S. HILLMAN, 1977
1 1 6 407-428.
JOHNSON, E. M., B. P. PLACEK, N. J. SNELLINCS and L. S. BARON, 1975
KADO, C. I. and S.-T. LIU, 1981
KOHNE, D. E., 1968 J. 8: 1104-1 118.
380 A. F. LEHNER AND C. W. HILL
LEHNER, A. F. and C. W. HILL, 1980 Involvement of ribosomal ribonucleic acid operons in
Salmonella typhimurium chromosomal rearrangements. J. Bacteriol. 143: 492-498.
607.
Low, K. B., 1972 Escherichia coli K-12 F-prime factors, old and new. Bacteriol. Rev. 3 6 587-
RIGBY, P. W . J., M. DIECKMANN, C. RHODES and P. BERG, 1977 Labelling deoxyribonucleic acid
to high specific activity in vitro by nick translation with DNA polymerase 1. J. Mol. Biol. 113:
237-251.
Genetic relatedness in the family Enterobacteriaceae. Annu. Rev. Micro-
F', Hfr, and F' strains of SANDERSON, K. E., 1976
biol. 30: 327-349.
SANDERSON, K. E., H. ROSS, L. ZIECLER and P. H. MAKELA, 1972
Salmonella typhimurium and Salmonella abony. Bacteriol. Rev. 36: 608-637. Episomes. Annu. Rev. Microbiol. 21: 601-638.
SCAIFE, J., 1967
TAYLOR, A. L. and E. A. ADELBERG, 1960 Linkage analysis with very high frequency males of
Nucleotide sequence of the Salmonella tyfhimurium origin
Communicating editor: B. W. GLICKMAN
Escherirhia colt. Genetics 45: 1233-1 243.
ZYSKIND, J. W. and D. W. SMITH, 1980