Case Report
Micronodular thymoma with lymphoid stroma:
report of two cases and review of literature
Yuanyuan Liu*, Youjie Gong*, Qiong Wu, Menghui Wang, Jinlian Zhang, Zelong Cheng, Yurong Ou
Department of Pathology, The First Affiliated Hospital of Bengbu Medical College, Bengbu Medical College, Bengbu 233004, Anhui, China. *Equal contributors.
Received June 1, 2019; Accepted July 23, 2019; Epub September 1, 2019; Published September 15, 2019
Abstract: Micronodular thymoma with lymphoid stroma (MNT) is a rare subtype of organoid thymic epithelial tumor that occurs in middle-aged and elderly people. Histologically, MNT is characterized by multiple epithelial nodules that are scattered or fused and abundant lymphocyte stroma with prominent germinal centers. It has a benign course and a good prognosis, and its diagnosis mainly depends on histopathologic and immunohistochemical mark-ers. This article reports two cases of MNT to explore its clinicopathologic features, diagnosis and differential diagno-sis to improve understanding of this subtype. In addition, we review previously reported cases of MNT.
Keywords: Thymic neoplasms, micronodular thymoma with lymphoid stroma, clinical pathology, immunohisto-chemistry, prognosis
Introduction
Micronodular thymoma with lymphoid stroma (MNT)is an exceedingly rare subtype of thymic neoplasm. It accounts for about 1%-5% of all thymomas, and is rarely reported [4, 7] (Table 2). The typical histopathologic features of MNT include multiple diffuse or fused epithelial nod-ules and abundant lymphocyte stroma with prominent germinal centers [2, 19]. Microno- dular thymoma with B lymphocyte hyperplasia was first reported by Suster and Moran [1], and was later renamed as micronodular thymoma with lymphoid stroma (MNT) and included in the WHO book (2004). The pathology and gene- tics of lung, pleura, thymus, and cardiac tumors have been listed separately. In this article, two cases with MNT are reported to explore its clini-copathologic features, diagnosis, and differen-tial diagnosis to improve understanding of this subtype.
Case presentation
Clinical history
A 72-year-old woman with MNT was treated at First Affiliated Hospital of Bengbu Medical College in 2018, and a 72-year-old man was from an outside consult. There was no history
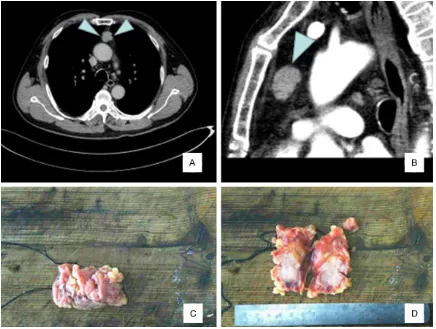
of myasthenia gravis or autoimmune disease in the 2 cases. Case 1 was a 72-year-old female who was admitted to the hospital due to chest tightness and asthma for 20 days. Laboratory examination revealed no abnormalities. Chest CT showed a mass of soft tissue density in the anterior mediastinum, wherein the boundary was clear, and enhanced scan showed uniform enhancement, suggestive of thymoma. The pa- tient then underwent thoracoscopic descend-ing mediastinal mass resection. The largest diameter of the tumor was 5 cm, and the cap-sule was intact.Case 2 was a 72-year-old male, with no abnormalities by laboratory examina-tion. Chest CT showed an elliptical mass in the anterior mediastinum with a maximum diame-ter of 2.8 cm, that was solid with a clear bound-ary (Figure 1A, 1B). The 2 cases of micronodu-lar thymoma with lymphoid stroma were treated by lumpectomy, and no other treatment was performed after operation. Case 1 was followed up for 5 months, and showed no evidence of recurrence or distant metastasis. Case 2 was a consultation from a foreign hospital, and there was no follow-up.
Materials and methods
The sections were cut at 4 μm, and were H&E, and immunohistochemically stained using En- Vision method. All antibodies including CK, CK19, P63, CD5, CD20, CD1a, CD99, CD117, TdT and Ki-67 used are presented in Table 1. Gross features
Grossly, case 1 showed a gray red nodule of 5.0 cm × 4.5 cm × 4.5 cm. The cut surface was gray brown, medium in texture, and covered by a capsule. In case 2, the nodule appeared as an elliptical gray-yellow gray-red tissue of 2.8 cm × 2.0 cm × 1.9 cm (Figure 1C). The cut sur-face was gray red, and covered by an intact capsule locally (Figure 1D).
Histologic features
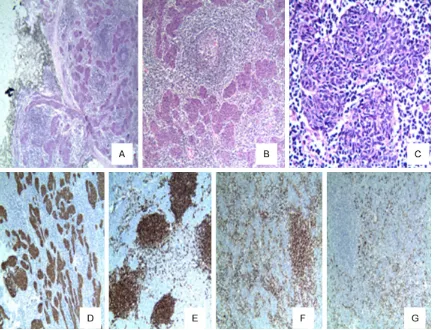
Microscopic examination of the tumors of the 2 cases showed that the boundary was clear and the local surface had a thin fibrous envelope (Figure 2A). In the solid area of the tumor, the epithelioid tumor cells showed a micronodular distribution, and some of the regions were merged into a small piece or a cord-like struc-ture (Figure 2B).At high magnification, the epi-thelioid tumor cells appeared as micronodular or fused nodules with flaky growth, bland mor-phology, rich interstitial lymphocytes, and lym-phoid follicle formation with germinal centers in some areas. The epithelioid tumor cells were composed of bland short fusiform or oval cells, the nucleus was oval in shape, no prominent nucleolus (Figure 2C), no obvious atypia or mitotic figures, and no obvious bleeding and necrosis.A small amount of lymphocytes were scattered in the epithelial-like tumor cell nests, while a large number of lymphocytes divided
the tumor cell nests in the interstitium.In some areas, follicle formation with a distinct germinal center was seen, which was surrounded by a cuff and a widened marginal zone, and few thin-walled vessels were visible in the surround-ing interstitium without thymic bodies or peri-vascular spaces.
Immunohistochemical staining
Immunohistochemical staining mainly detected the epithelial tumor cells and interstitial lym-phocytes. The 2 cases showed tumor epithelial cells that were positive for CK, CK19 (Figure 2D), and P63, and negative for CD5, CD117 and CD20. The T cells and B cells were among interstitial cells, and CD20-positive B cells were mainly located in the follicular area (Figure 2E), and were surrounded by CD5-positive T lym-phocytes (Figure 2F), CD1a and CD99-positive immature T cells. These were mainly distribut-ed in the T lymphocyte stroma around the epi-thelial-like cell nests, where CD1a-positivity showed that Langerhans cells were scattered in the epithelial cells (Figure 2G). The Ki-67 pro-liferation index showed low expression in the epithelial nodules and high expression in the immature lymphocytes.
Discussion
Thymoma that originates in the thymic epitheli-al cells or differentiates into thymic epitheliepitheli-al cells, regardless of the presence of non-neo-plastic lymphocytes. According to differing mor-phology of tumor epithelial cells and distribu-tion of T lymphocytes, thymoma is divided into various types. MNT is a very rare thymic tumor with a unique morphology. It initially originated from thymic medullary epithelial cells, and mostly occurs in middle-aged and elderly peo-ple, with an age of onset between 41 and 80 years. There is no obvious gender difference. The tumor mostly occurs in the anterior medi-astinum, and there are few reports of cervical ectopic MNT [5, 6, 12, 17]. Patients included in this report were elderly, with 1 male and 1 female, and had thymomas in the anterior mediastinum. This was consistent with the lit-erature. The vast majority of patients have a history of being asymptomatic or myasthenia gravis, usually unintentional. At times, on physi-cal examination, the patients had symptoms, such as thymic cyst [11], cardiac myxoma, and
Table 1. Antibodies and dilutions used in the evaluation of MNT
Antibody Clone Dilution Source
CK AE1/AE3 1:20 Abcam
CK19 EP1580Y 1:400 Abcam
P63 EPR5701 1:2500 Abcam
CD5 4C7 1:50 Novocastra
CD20 EP459Y 1:50 Abcam
CD1a EP3622 1:250 Abcam
CD99 MIC2 1:50 Dako
CD117 YR145 1:400 Abcam
TdT 41A 1:100 Abcam
Table 2. Summary of MNT clinical data reported in literature
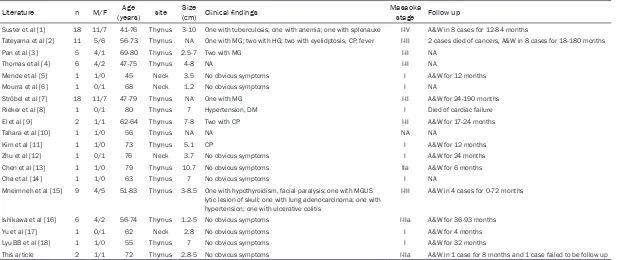
Literature n M/F (years)Age site (cm)Size Clinical findings Masaoka stage Follow up
Suster et al [1] 18 11/7 41-76 Thymus 3-10 One with tuberculosis; one with anemia; one with splenauxe I-IV A&W in 8 cases for 12-84 months
Tateyama et al [2] 11 5/6 56-73 Thymus NA One with MG; two with HG; two with eyelidptosis, CP, fever I-III 2 cases died of cancers, A&W in 8 cases for 18-180 months
Pan et al [3] 5 4/1 69-80 Thymus 2.5-7 Two with MG I-II NA
Thomas et al [4] 6 4/2 47-75 Thymus 4-8 NA I-II NA
Mende et al [5] 1 1/0 45 Neck 3.5 No obvious symptoms I A&W for 12 months
Mourra et al [6] 1 0/1 68 Neck 1.2 No obvious symptoms I NA
Ströbel et al [7] 18 11/7 47-79 Thymus NA One with MG I-II A&W for 24-190 months
Rieker et al [8] 1 0/1 80 Thymus 7 Hypertension, DM I Died of cardiac failure El et al [9] 2 1/1 62-64 Thymus 7-8 Two with CP I-II A&W for 17-24 months
Tahara et al [10] 1 1/0 56 Thymus NA NA NA NA
Kim et al [11] 1 1/0 73 Thymus 5.1 CP I A&W for 12 months
Zhu et al [12] 1 0/1 76 Neck 3.7 No obvious symptoms I A&W for 24 months
Chen et al [13] 1 1/0 79 Thymus 10.7 No obvious symptoms IIa A&W for 6 months
Cha et al [14] 1 1/0 63 Thymus 7 No obvious symptoms I NA
Mneimneh et al [15] 9 4/5 51-83 Thymus 3-8.5 One with hypothyroidism, facial paralysis; one with MGUS lytic lesion of skull; one with lung adenocarcinoma; one with hypertension; one with ulcerative colitis
I-III A&W in 4 cases for 0-72 months
Ishikawa et al [16] 6 4/2 56-74 Thymus 1.2-5 No obvious symptoms I-IIa A&W for 36-93 months
Yu et al [17] 1 0/1 62 Neck 2.8 No obvious symptoms I A&W for 4 months
Lyu BB et al [18] 1 1/0 55 Thymus 7 No obvious symptoms I A&W for 32 months
This article 2 1/1 72 Thymus 2.8-5 No obvious symptoms I-IIa A&W in 1 case for 8 months and 1 case failed to be follow up
salivary gland damage [5]. According to ITMIG statistics, 96% of MNT clinical stages are most-ly at stages I and II [20]. About 62% of MNT cases have a complete capsule, i.e., the clinical stage is stage I, but 36% of cases are accompa-nied by microinfiltration, i.e., the clinical stage is stage II [1, 4, 7]. So far, only one case of MNT has been reported to be extensively invasive with multiple pleural implants [7]. One of the two cases in this report had capsular invasion, and the Masaoka stage was stage IIa.
MNT is generally between 3 and 15 cm in diam-eter, and the cystic areas are generally of differ-ent sizes [8]. The typical histopathologic fea-ture of MNT includes multiple diffuse or fused epithelial nodules and abundant lymphocyte stroma. The tumor cells are short fusiform or oval in shape, the nucleus is oval, the nucleoli are not obvious, atypical mitotic figures are absent, there is no obvious hemorrhage and necrosis, no clear thymus bodies are visible,
and there are fewer lymphocytes in the epithe-lial cells. Also the interstitium is rich in lymphoid tissue, the lymphoid follicles have distinct minal centers in the lymphatic stroma, the ger-minal center is surrounded by a wrap around area and a widened marginal zone, and a few plasma cells occur.
Tumor cells express CK pan, CK5/6, and CK19; CD117 and CD20 are usually negative [3]. There are scattered T lymphocytes that can be seen in the epithelial cell nests, and T cells and B cells occur in the interstitium [4, 7]. In this report, CD20-positive lymphoid follicles were observed, and CD5-positive T lymphocytes were observed around them. The distribution of CD1a-positive Langerhans cells in the epitheli-al nests is consistent with the previous litera-ture [16].
[image:4.612.89.525.71.400.2]pathology and immunohistochemistry, but it needs to be differentiated from the following tumors: ① Type A thymoma is mainly compos- ed of mild fusiform or oval-shaped epithelial cells, and are diffuse in large patches, with no micronodules formed, and there are no or few lymphocytes in the interstitium. ② Type B thy-moma is mainly composed of immature T lym-phocytes, which are lobulated, and a perivascu-lar space is common, while MNT is mainly composed of B lymphocytes, and a perivascu-lar space is rarely observed. ③ Type AB thymo-ma is most often confused with MNT. This type is lobulated, and a fibrous interstitium sepa-rates the tumor into a jigsaw-like structure. It is mainly composed of a combination of type A thymoma with fewer lymphocytes and type B thymoma rich in lymphocytes. Among these, the B-type component is rich in lymphocytes
with mixed distribution of epithelial cells, while in MNT, the lymphatic stroma divides the epi-thelioid tumor cell nests to form a cord-like structure, the boundary between the epitheli-oid tumor cells and the lymphocytes is clear, and there are no mixed epithelial cells in the lymphoid stroma.④ Micronodular thymic carci-noma with lymphoid stroma: Weissferdt et al. [21] have reported that this is a rare variant of thymic carcinoma, that is similar in morpholo- gy to MNT and can be characterized by micro- nodular distribution. However, it often presents with invasive growth pattern, abnormally shap- ed epithelioid cells, and often capsule invasion and necrosis are observed. Epithelial cells are positive for CD117 and CD5, and interstitial lymphocytes are negative for TdT. ⑤ Primary thymic MALT lymphoma, or B lymphocyte lym-phoma: The tumor cells are mainly B lympho-Figure 2.A. The boundary of the tumor was clear and the local surface had a thin fibrous envelope. B. The epithelioid
[image:5.612.91.525.73.403.2]cytes, and the remaining thymic tissue and lym-phatic epithelial lesions can be seen, and if necessary, they can be identified by gene rear-rangement. ⑥ Thymic lymphoepithelial
carci-noma: The pleomorphism of the cancer cells in this type is obvious, with vacuolated nuclei, and the mitotic figures are easily found. The interstitial lymphocytes are peripheral mature lymphocytes, rather than naïve thymic T lym-phocytes that are different from MNT. Immu- nohistochemical markers of TdT and EBV are helpful for identification.
At present, the treatment of this tumor mainly involves surgical resection, but MNT is a rare primary tumor of the thymus, that is different from other types of thymoma. It is currently considered to be a borderline tumor with good prognosis, rare recurrence and metastasis. Postoperative death due to severe left ventricu-lar function was reported only in 1 case [8]. Relevant literature reports showed that patients with cystic solid tumors have a better progno-sis, while those with solid and capsule invasion have a higher risk index [21]. One patient in this report was followed up for 8 months. Currently, the general condition of the patient is good. The second was a consultation case, and so could not be followed up.
Acknowledgements
This work was supported by the Nature Scien- ce Key Program of College and University of Anhui Province (no. KJ2016A468) and the Pro- vincial Innovation Project of College Students (no. 201810367083).
Disclosure of conflict of interest
None.
Address correspondence to: Yurong Ou, Department
of Pathology, The First Affiliated Hospital of Bengbu
Medical College, Bengbu Medical College, Bengbu 233004, Anhui, China. E-mail: oy1988527@163. com
References
[1] Suster S, Moran CA. Micronodular thymoma with lymphoid B-cell hyperplasia: clinicopatho-logic and immunohistochemical study of eigh-teen cases of a distinctive morphologic variant of thymic epithelial neoplasm. Am J Surg Pathol 1999; 23: 955-962.
[2] Tateyama H, saito Y, Fujii Y, Okumura M, Naka-mura K, Tada H, Yasumitsu T, Eimoto T. The spectrum of micronodular thymic epithelial tu-mours with lymphoid B-cell hyperplasia. Histo-pathology 2001; 38: 519-527.
[3] Pan CC, Chen WY, Chiang H. Spindle cell and mixed spindle/lymphocytic thymomas: an inte-grated clinicopathologic and immunohisto-chemical study of 81 cases. Am J Surg Pathol 2001; 25: 111-120.
[4] Thomas De Montpréville V, Zemoura L, Dulmet E. Thymoma with epithelial micronodules and lymphoid hyperplasia: six cases of a rare and equivocal subtype. Ann Pathol 2002; 22: 177-182.
[5] Mende S, Moschopulos M, Marx A, Laeng RH. Ectopic micronodular thymoma with lymphoid stroma. Virchows Arch 2004; 444: 397-399. [6] Mourra N, Duron F, Parc R, Flejou JF. Cervical
ectopic thymoma: a diagnostic pitfall on frozen section. Histopathology 2005; 46: 583-585. [7] Ströbel P, Marino M, Feuchtenberger M,
Rouz-ière AS, Tony HP, Wulbrand U, Förster R, Zettl A, Lee Harris N, Kreipe H, Laeng RH, Müller-Her-melink HK, Marx A. Micronodular thymoma: an epithelial tumour with abnormal chemokine expression setting the stage for lymphoma de-velopment. J Pathol 2005; 207: 72-82. [8] Rieker RJ, Aulmann S, Schnabel PA, Sack FU,
Otto HF, Mechtersheimer G, Schirmacher P, Bläker H. Cystic thymoma. Pathol Oncol Res 2005; 11: 57-60.
[9] El MF, Braham E, Ayadi A, Ismail O, Kilani T. Micronodular thymoma with lymphoid stroma: report of two cases and particular association with thymic lymphoid hyperplasia in one case. Pathology 2006; 38: 586-588.
[10] Tahara S, Takami K, Omiya H, Kuriyama K, Ko-dama Y, Tsujinaka T. Micronodular thymoma with lymphoid stroma (MNT). Kyobu Geka 2012; 65: 1045-1048.
[11] Kim NR, Lee JI, Ha SY. Micronodular thymoma with lymphoid stroma in a multilocular thymic cyst: a case study. Korean J Pathol 2013; 47: 392-394.
[12] Zhu P, Yan F, Ao Q. Langerhans cells prolifera-tion in ectopic micronodular thymoma with lymphoid stroma: a case report. Int J Clin Exp Pathol 2014; 7: 7262-7267.
[13] Chen CW, Chuang SS, Pan ST. Micronodular thymoma with lymphoid stroma diagnosed with core needle biopsy: a case report. Anal Quant Cytopathol Histpathol 2015; 37: 206-210.
[14] Cha YJ, Han J, Kim J, Lee KS, Shim YM. A rare case of mixed type a thymoma and micronodu-lar thymoma with lymphoid stroma. J Pathol Transl Med 2015; 49: 75-77.
neoplasms: case series and literature review with emphasis on the spectrum of differentia-tion. Mod Pathol 2015; 28: 1415-1427. [16] Ishikawa Y, Tateyama H, Yoshida M, Takami K,
Matsuguma H, Taniguchi T, Usami N, Kawagu-chi K, Fukui T, Ishiguro F, Nakamura S, Yokoi K. Micronodular thymoma with lymphoid stroma: an immunohistochemical study of the distribu-tion of Langerhans cells and mature dendritic cells in six patients. Histopathology 2015; 66: 300-307.
[17] Yu M, Meng Y, Xu B, Zhao L, Zhang Q. Ectopic micronodular thymoma with lymphoid stroma in the cervical region: a rare case associated with Langerhans cells proliferation. Onco Tar-gets Ther 2016; 9: 4317-4322.
[18] Lyu BB, Yao ZG, Wang Z. Micronodular thymo-ma with lymphoid strothymo-ma: report of a case. Zhonghua Bing Li Xue Za Zhi 2017; 46: 197-198.
[19] Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Pow-ell CA, Tsao MS, Wistuba I; WHO Panel. The
2015 World Health Organization classification
of lung tumors: impact of genetic, clinical and
radiologic advances since the 2004 classifica -tion. J Thorac Oncol 2015; 10: 1243-1260. [20] Weis CA, Yao X, Deng Y, Detterbeck FC, Marino
M, Nicholson AG, Huang J, Ströbel P, Antoni-celli A, Marx A; Contributors to the ITMIG Retro-spective Database. The impact of thymoma histotype on prognosis in a worldwide data-base. J Thorac Oncol 2015; 10: 367-372. [21] Weissferdt A, Moran CA. Micronodular thymic
carcinoma with lymphoid hyperplasia: a clini-copathological and immunohistochemical stu-
dy of five cases. Mod Pathol 2012; 25: