MAGNETIC RESONANCE IMAGING FOR THE DETECTION OF AGE- AND DISEASE-RELATED CHANGES IN THE
HUMAN HEART
Shona Matthew
A Thesis Submitted for the Degree of PhD at the
University of St Andrews
2012
Full metadata for this item is available in Research@StAndrews:FullText
at:
http://research-repository.st-andrews.ac.uk/
Please use this identifier to cite or link to this item: http://hdl.handle.net/10023/3558
This item is protected by original copyright
Resonance Imaging for the Detection of Age- and
Disease-Related Changes in the Human Heart
Shona Matthew
This thesis is submitted in partial fulfilment for the
degree of PhD at the University of St Andrews
This thesis is dedicated to my foster parents
Frank & Mary Dow
and
Christine & Jimmy Craig
Abstract and scope i
Abstract i
The Scope of the Thesis ii
Acknowledgements vi
Significant events in the history of NMR and MRI 2
1. The Basics of MRI 3
1.1 Diagnostic Imaging 4
1.2 Magnetic Resonance Imaging 4
1.3 Nuclear Magnetic Resonance 5
1.4 Spin Angular Momentum 5
1.4.1 Gyromagnetic Ratio 6
1.5 Hydrogen Nuclei 7
1.6 The Zeeman Effect 7
1.7 The Boltzmann Distribution 8
1.8 Larmor Equation 9
1.9 Net Magnetisation Vector 9
1.10 The Main Magnetic Field 11
1.10.1 Superconducting Magnets 11
1.11 The Radio Frequency Field 12
1.12 Resonance 13
1.12.1 Rotating Frame of Reference 13
1.12.2 Flip Angles 14
1.12.3 Partial Flip Angles 15
1.12.4 Free Induction Decay 15
1.12.5 T1 Relaxation 16
1.12.6 T2 Decay 17
1.12.7 T2* Relaxation 18
1.13 Image Formation 19
1.13.1 Gradient Magnetic Fields 20
1.13.2 Slice Select Gradient 20
1.13.3 Frequency Encoding 21
1.13.4 Phase Encoding 22
1.13.5 Repetition Time 24
1.13.6 Echo Time 25
1.13.7 Biological Parameters 25
1.14 Pulse sequences 25
1.14.1 Spin Echo 26
1.14.2 Spin Echo – T1 Weighted Images 27
1.14.3 Spin Echo – T2 Weighted Image 27
1.14.7 Pathology Scans 31
1.14.8 Proton Density Scans 31
1.15 Coils 32
1.15.1 Body Coil 32
1.15.2 Shim Coil Sets 32
1.15.3 Surface Coils 33
1.15.4 Phased Array Coils 33
1.16 K-Space 34
1.17 SAR limitation 38
1.18 Cardiac Magnetic Resonance Imaging 39
1.19 Other Techniques and their Limitations 40
1.19.1 SPECT and PET 40
1.19.2 Computed Tomography 41
1.19.3 Echocardiography 42
1.20 The Advantages of Cardiac Magnetic Resonance Imaging 43 1.21 The Limitations of Cardiac Magnetic Resonance Imaging 44
References 46
2. The Human Heart 49
2.1 The Chambers of the Heart 50
2.2 The Valves of the Heart 51
2.3 The Papillary Muscles 52
2.4 The Myocardium 53
2.5 Path of Blood through the Heart 54
2.6 The Coronary Arteries 56
2.7Blood Pressure 56
2.8 The Cardiac Cycle 57
2.9 The Electrocardiogram 57
2.10 Diseases of the Heart 58
2.10.1 Coronary Artery Disease 58
2.10.2 Valve Disease 60
2.10.3 Left Ventricular Hypertrophy 61
2.10.4 Cardiac Arrhythmias 62
2.10.5 Heart Failure 62
2.11 Acknowledgments 63
References 64
3. The Development of Cardiac Magnetic Resonance Imaging 66
3.1 Hardware Developments 67
3.1.1 Higher Fields 67
3.1.2 Gradient Capabilities 68
3.1.3 Phased Array Coils 69
3.1.4 Parallel Imaging 71
3.2.3 Echo Planar Imaging 75
3.3 Cardiac Applications 76
3.3.1 Breath-Holding Techniques 76
3.3.2 Gating and Triggering Techniques 76
3.3.2.1 Prospective Triggering 77
3.3.2.2 Retrospective Gating 77
3.4 Contrast Agents 78
3.5 Conclusion 79
References 80
4. The Advantages, Challenges and Limitations of Imaging at 3.0T in Comparison
to 1.5T 83
4.1 Introduction 83
4.2Technical Details of the Magnetic Fields 84
4.3 The Images 84
4.4 The Signal 85
4.5 The Noise 86
4.5.1 Signal-to-Noise Ratio 87
4.6 The Advantages of CMRI at 3.0T 88
4.7 The Challenges and Limitations of CMRI at 3.0T 89
4.7.1 Chemical Shift 89
4.7.2 Chemical Shift of the Second Kind 92
4.7.3 Dielectric Effect 94
4.7.4 B1 Inhomogeneities 94
4.7.5 Changes in Power Deposition and the Impact on SAR limitations 95 4.7.6 FieldInhomogeneities and Susceptibility 97
4.7.7 Susceptibility Effects 98
4.7.8 Metal Artefact 99
4.7.9 Shimming 100
4.7.10 Parallel imaging 101
4.7.11 Coils 102
4.7.12 The Magnetohydrodynamic Effect 102
4.7.13 Vector Cardiogram 103
4.7.14 Contrast to Noise Ratio 104
4.8 The Changes in Tissue Relaxation Times 104
4.9 Contrast Enhancement 105
5. Cardiac Magnetic Resonance Imaging Protocols 106
5.1 Introduction 106
5.2 Fast Gradient Echo 106
5.3 TrueFISP 107
5.3.1 Equilibrium 107
5.3.5 Optimal Flip Angle 111
5.3.6 Optimal Signal 111
5.3.7 Artefacts 112
5.4 TurboFLASH 113
5.5 The Images 113
5.5.1 Reference Images 113
5.5.2 The Localisers 113
5.6 Functional Imaging 116
5.6.1 Clinical Information 117
5.7 First Pass Perfusion Imaging 118
5.7.1 Clinical Information 119
5.8 Post Processing of Cardiac Images 119
5.8.1 Cardiac Volumes 119
5.8.2 Cardiac Mass 119
5.8.3 Cardiac Output and Ejection Fraction 120
References 121
6. Assessment of clinical differences at 1.5T between quantitative right and left
ventricular volumes and ejection fractions using MRI 122
6.1 Introduction 122
6.2 Aims 122
6.3 Cohorts 123
6.4 Acquisition Protocol 124
6.5 Image Analysis 124
6.6 Statistical Analysis 125
6.7 Results 126
6.8 Discussion 129
6.8.1 Reproducibility 129
6.8.2 Significant Changes in Cardiac Parameters 130
6.9 Conclusion 130
References 131
7. Normal Ranges of Left Ventricular Functional Parameters at 3 Tesla 132
7.1 Part 1 132
7.2 The Normal Healthy Volunteers 134
7.2.1 Exclusion Criteria 134
7.2.2 The Cohorts 134
7.3 Imaging Parameters 135
7.3.1 Image Optimisation 135
7.3.2 Localised Shimming 136
7.3.3 Frequency Scout 136
7.4 Image Analysis 137
7.5 Calculated Values of Cardiac Function for the Age- and Gender-Defined
7.8 Conclusion 139
7.9 Part 2 141
7.10 The Cohort 141
7.11 Image Analysis and Post-processing 141
7.12 Like for Like Comparison 142
7.13 Conclusion 143
7.14 Future work 144
References 145
8. Left Atrial Dimensions as Determinants of Cardiac Dysfunction 146
8.1 Methods and Materials 150
8.2 Image Analysis 152
8.3 Results 154
8.4 Discussion 159
8.5 Conclusion 164
References 165
9. The Impact of Contrast Agents on Quantitative Parameters in Cardiac MRI 169
9.1 Introduction 169
9.2 Methods and Materials 171
9.3 Image Analysis 172
9.4 Statistical Testing 174
9.5 Results 174
9.5.1 Reproducibility 177
9.6 Discussion 181
9.7 Conclusion 183
References 184
10. Conclusion 185
10.1 FutureDevelopments 187
10.2 Present and Future Work 189
10.2.1 Novel Work 190
10.2.2 Present Challenges 191
10.3 Presentations, Posters and Publications 192
10.3.1 Oral Presentations 192
10.3.2 Posters 193
10.3.3 Publications 194
10.3.4 Submissions – Under peer review: 194
A1 The Bloch equations viii
A1.1 FLASH and TrueFISP xii
A2 Quality Assurance Checks on Contrast Measurements in MRI xv
A2.1 Signal Intensity Measurements xv
A2.2 The Images xv
A2.3 The Environment xviii
A2.4 The Aim xviii
A2.5 The Code xviii
A2.6 The Files xix
A2.7 Renaming the Files xix
A3 Measuring the Signal xxiii
A3.1 Plotting the data xxv
A4 Calculating T1 Relaxation Rates xxvi
A4.1 TE Data xxix
A4.2 Flip Angle Data xxxi
References xxxv
Appendix B. Matlab® code xxxvi
B1 Header xxxvi
B2 Class data members xxxviii
B3 Class methods (functions) xxxix
B3.1 Static method block xxxix
B3.2 Public method block xlii
B3.3 Protected method block liii
Page | i
Abstract
Cardiovascular disease (CVD) is a term used to describe a variety of diseases
and events that impact the heart and circulatory system. CVD is the United
Kingdom’s (UKs) biggest killer, causing more than 50,000 premature deaths
each year. Early recognition of the potential for magnetic resonance imaging
(MRI) to provide a versatile, non-ionising, non-invasive, technique for the
assessment of CVD resulted in the modality becoming an area of intense
interest in the research, radiology and cardiology communities. The first half of
this thesis reviews some of the key developments in magnetic resonance
hardware and software that have led to cardiac magnetic resonance imaging
(CMRI) emerging as a reliable and reproducible tool, with a range of
applications ideally suited for the evaluation of cardiac morphology, function,
viability, valvular disease, perfusion, and congenital cardiomyopathies. In
addition to this, the advantages and challenges of imaging at 3.0T in
comparison to 1.5T are discussed. The second half of this thesis presents a
number of investigations that were specifically designed to explore the
capability of CMRI to accurately detect subtle age and disease related changes
in the human heart. Our investigations begin with a study at 1.5T that explores
the clinical and scientific significance of the less frequently used measure of
Page | ii
then shifts to imaging at 3.0T and the challenges of optimising cardiac imaging
at this field strength are discussed. Normal quantitative parameters of cardiac
function are established at this field strength for the left ventricle and the left
atrium of local volunteers. These values are used to investigate disease related
changes in left ventricle and left atrium of distinct patient cohorts. This work
concludes by investigating the impact of gadolinium-based contrast agents on
the quantitative parameters of cardiac function.
The Scope of the Thesis
This thesis is concerned with studies regarding both the basic principles and
clinical practice of CMRI when performed at 1.5T and 3.0T. Chapters 2–5
provide the platform of knowledge necessary to appreciate the experimental
work presented in 6–9 of this thesis.
Chapter 1 begins with a brief introduction to the physics of magnetic resonance
imaging before comparing the various cardiac imaging modalities routinely
available in British hospitals today.
Chapter 2 provides an overview of normal cardiac anatomy and function before
Page | iii
software developments that have led to a paradigm shift in CMRI over the past
three decades. The overall impact of these developments on this research is laid
into context.
Chapter 4 starts with a brief comparison of the radio frequency and gradient
fields of the 1.5T and 3.0T Siemens imaging systems utilised for the purpose of
this research. This is followed by a discussion on the advantages and challenges
of imaging at 3.0 Tesla in comparison to 1.5 Tesla.
Chapter 5 defines a cardiac magnetic resonance imaging protocol and discussed
two fast gradient echo sequences, both of which are fundamental to cardiac
imaging. In addition to this, chapter 5 contains examples of the images obtained
using these sequences together with a brief discussion of the clinical
information that can be gained from some of these images. The chapter
concludes with an introduction to image analysis and the important clinical
parameters that are obtained from post-processing short-axis cine images.
Chapters 6–8 investigate the clinical and scientific significance of novel or
infrequently used measures of cardiac function with the hypothesis that the
inclusion of this data may provide a more informative assessment of overall
Page | iv
reproducibility between a novice and experienced observer. In conjunction to
this, the quantitative right and left ventricular analysis of three distinct clinical
cohorts were examined with the hypothesis that the inclusion of RV data may
generate a more informative characterisation of cardiac function.
Chapter 7 Begins with a discussion on the challenges faced in optimising
ven-tricular imaging at 3.0T. This is followed by a study designed to define subtle
age-related functional changes in both male and female healthy volunteers in a
bid to provide normal ranges of cardiac function at 3.0T for the local
popula-tion. Measures of ejection fraction, end-diastolic volume, end-systolic volume,
and left ventricular mass for 100 volunteers are obtained and compared. This
chapter concludes by using these normal ranges to determine if disease-related
cardiac changes exist in a cohort of patients with Systemic Lupus
Erythemato-sus.
Chapter 8 An optimised LA protocol is implemented to identify possible
volu-metric variations between healthy volunteers and patient cohorts with carefully
defined clinical cardiac conditions.
Gadolinium-based contrast agents are used in CMRI protocols to enhance
imaging by shortening the inherent T1 values of tissue, effectively creating a
Page | v
ed cardiac variables, particularly on the value of LV mass. Significant
differ-ences are highlighted that may have important implications for the correct
in-terpretation of patient data in clinical studies.
Chapter 10 concludes this thesis and consolidates the achievements of chapters
6–9 before discussing the future of CMRI. This thesis is drawn to a close with an
overview of present studies and future work.
Appendix A: This appendix begins with a modification to the Bloch Equations
to provide explicate forms for Mx, My and Mz. This is followed by a valid
solution to an incoherent steady-state imaging technique where the transverse
magnetisation is essentially zero before the application of the excitation pulse
(FLASH). The equitation describing the magnetisation from a coherent
steady-state technique (TrueFISP) is given without proof.
Part two of this appendix describes an essential quality assurance test that is
routinely performed on all of our MRI scanners to ensure that they are
performing to the manufacturer’s specifications. Novel matlab code is
presented that efficiently post-processes the images acquired during testing.
Appendix B contains the MATLAB® code discussed in Appendix A of this
Page | vi
I would like to express my gratitude to Professor Malcolm H. Dunn of the
University of St Andrews for his loyalty, dedication, enthusiasm, support and
kindness. It has been a privilege and an honour to work under his supervision.
Thanks also to the significant others who helped make this such an enjoyable
journey:
IanLauraJessJosh Bobo
Professor Richard Lerski: Professor Graeme Houston: David Stothard: Solmaz Eradat Oskoui: David Walsh:
Stephen Gandy: Shelley Waugh: Ian Cavin: Elanne Knowles: Henry Knowles: Fiona Knowles:
Shona Ogilvie: Louise Lamont: Carole Wood: Audrey Kerr: Angela Little: Elaine Duncan: Audrey Barr: Jane Ross: Lesley Aitken: John Hughes: Sheila Weir: Elena Crowe: Patricia Martin: Darran Milne Lea Christina Heering:
Ken Welsh: Neil McGill: Graham Smith: Graham Turnbull: Mhairi Dennis: Ian Dennis: Norma Gourlay:
Page | vii
clinical patients, healthy volunteers and *TASCFORCE participants who kindly
agreed to their cardiac magnetic resonance images being used for teaching and
research purposes.
* Tayside Screening For risk of Cardiac Events and the effect of statin on risk reduction.
Finally, thanks to Dr Tom Gallacher of the University of St Andrews for his
substantial help in developing the novel MATLAB® code discussed in
Page | 1
13 June 1831 – 5 November 1879
“… the work of James Clerk Maxwell changed the world forever …”
Albert Einstein. New Scientist 1991; 130: 49.
t
B
E
Page | 2
Significant events in the history of NMR and MRI
Nuclear Magnetic Resonance: Isidor IsaacRabi (1898–1988); Nobel prize for Physics 1944.
Nuclear Magnetic Precision Measurements: Felix Bloch (1905–1983) & Edward Mills Purcell (1912–1997); 1952 Nobel Prize for Physics.
Magnetic Resonance Imaging: Paul Christian Lauterbur (1929–2007) & Sir Peter Mansfield (Born 1933); 2003 Nobel Prize for Physiology or Medicine.
Spin: Otto Stern (1888–1969); 1943 Nobel Prize for Physics. Spin: Walther Gerlach (1889–1979).
Nuclear Magnetic Resonance: Edward Mills Purcell (1912–1997); 1952 Nobel Prize for Physics.
Spin Angular Momentum: Wolfgang Ernst Pauli (1900–1958); 1945 Nobel Prize for Physics.
The Zeeman Effect: Pieter Zeeman (1865–1943); Nobel Prize for Physics 1902. The Boltzmann Distribution: Ludwig Eduard Boltzmann (1844–1906).
The Tesla (S.I. Unit of Magnetic Field): Nikola Tesla (1856–1943).
Superconductivity: Heike Kamerlingh Onnes (1853–1926); 1913 Nobel Prize for Physics.
The Larmor Equation: Joseph Larmor (1857–1942).
Pulsed Fourier Transform NMR. Richard R Ernst (Born 1933); Nobel Prize for Chemistry 1991.
Page | 3
1. The Basics of MRI
A healthy adult human heart expands and contracts an average of 100,000
times a day, continuously pumping approximately 7,000 litres of blood
through a system of veins, arteries and capillaries that is in excess of 96,000
kilometres long [1]. This continuous flow of blood through the cardiovascular
system is of paramount importance as it delivers nutrients, removes waste and
is central to the production of energy and other materials necessary for life
(metabolism).
Cardiovascular diseases (CVDs) are a group of disorders that negatively
impact the heart and blood vessels. It is estimated that CVDs will be responsible
for almost 23.6 million deaths globally per annum by 2030, with men
and women being almost equally effected [2]. Consequently, there is a
move towards the early identification of cardiovascular disease risk,
as proactive disease management may be effective in slowing disease
progression [3]. This would have major financial benefits for our national
health service (NHS), but more importantly, disease management has the
potential to benefit the patient in terms of morbidity and mortality. For
example, a patient’s long-term survival may be improved by revascularisation
Page | 4
that there are viable heart muscle cells in the cardiac territory affected by
the occluded vessel [4, 5]
1.1 Diagnostic Imaging
Diagnostic imaging plays a vital role in the identification of cardiac
disease-related changes. A number of well-established techniques are available for this
purpose, all of which obtain images by measuring the interaction between
energy and a biological tissue. This thesis focuses on the application of cardiac
magnetic resonance imaging (CMRI), which is a comparatively new imaging
modality. In particular, we investigate the ability of CMRI to detect age and
disease related changes in the human heart. This chapter begins with a brief
introduction to magnetic resonance imaging (MRI); however the interested
reader is directed to the following books for a more thorough discussion of the
science involved in this technique [6, 7].
1.2 Magnetic Resonance Imaging
Paul Lauterbur produced the first primitive magnetic resonance image in 1973.
Subsequent rapid developments in hardware and software lead to the
introduction of the first clinical whole-body scanners less than 10 years later.
Today MRI is established as an essential radiological tool with an estimated 60
Page | 5
1.3 Nuclear Magnetic Resonance
Magnetic resonance imaging is based on the principles of proton nuclear
magnetic resonance (NMR). NMR is used to induce and detect a very weak
radio frequency signal that is a manifestation of nuclear magnetism. NMR can
only be performed on isotopes with a net spin angular momentum whose
natural abundance is high enough to be detected.
1.4 Spin Angular Momentum
Nuclear spin angular momentum (J) is a fundamental property of nature that
makes the nucleus a continuously rotating positive charge, and as a moving
charge it has an associated magnetic field and magnetic dipole moment ()
(Figure 1.1).
The magnetic dipole moment of a nucleus is directly proportional to the spin
angular momentum:
J
(1.1)Where is a proportionality constant, known as the gyromagnetic ratio.
Page | 6
1.4.1 Gyromagnetic Ratio
The gyromagnetic ratio () relates the nuclear rotating frequency to the strength
of the magnetic field and is commonly expressed in terms of Mega-Hertz per
Tesla (MHz/T) in MRI. Every nucleus suitable for MRI has its own specific
gyromagnetic value. For example, hydrogen nuclei (1H) have a gyromagnetic
ratio of:
The magnitude of the nuclear spin angular momentum is given by:
)
1
(
I
I
J
(1.3)Where I is the spin angular momentum quantum number and
is Planck’sconstant. The magnitude of the angular momentum along a chosen axis is given
by convention as:
I
z
m
J
(1.4)Where
m
Iis the magnetic quantum number and generally = -I, -I+1,…I-1, I.There are 2I+1 allowed orientations or quantum states of the nucleus. All of
which have the same energy in the absence of a magnetic field.
T
MHz
/
6
.
42
Page | 7
1.5 Hydrogen Nuclei
Protons, neutron and electrons are spin one-half particles. An atom with one
proton, one neutron and one electron has a net nuclear spin of 1 and a net
electronic spin of ½. Where two spins of opposite signs are paired, the
observable manifestation of spin is eliminated.
Hydrogen (1H) nuclei only contain a single proton, giving them a net nuclear
spin of ½. This, coupled with their relatively high gyromagnetic ratio and
biological abundance of ~63% in the human body, makes them ideal for
magnetic resonance imaging. Water-based tissues, such as the myocardium,
have an even higher biological abundance of ~80%.
1.6 The Zeeman Effect
In the absence of an external magnetic field (B0), the individual magnetic dipole
moments of the protons within a sample have no preferred orientation, and
therefore precess incoherently. However, once exposed to an external field, the
spin one-half nuclei experience a torque that causes their magnetic moments to
begin precessing in one of two allowed quantum states. The lower energy, spin
up or spin +½ state, is aligned parallel with the applied magnetic field whilst
the higher energy, spin down or spin -½ state, is aligned anti-parallel to the
Page | 8
1.7 The Boltzmann Distribution
At room temperature, the population of spins in the lower energy level (N+) will
slightly outnumber the population of spins in the upper energy level (N-)
(Figure 1.2). This spin excess would equate to 1 in 106 at 1.5 Tesla.
This division of spins is predicted by the classical Boltzmann distribution:
kT E
e
N
N
(1.5)
Where E = the energy difference between the spin states (1.4); k = Boltzmann’s
constant, (1.3805x10-23J/K); and T = the temperature in Kelvin.
0 1
2
E
2
B
E
E
(1.6)0
2
m
B
E
I
(1.7)Figure 1.2 The Zeeman energy levels for a spin one-half system.
0 B 0 E 0 B 2 1 S 0 B 2 1
s N--
Page | 9
1.8 Larmor Equation
For the spin ½ proton,
m
I= +/- ½, hence the energy difference between the twostates is:
0
B
E
(1.8)By applying Planck’s Law to equation (1.6), we obtain:
0
B
hv
(1.9)thus, allowing us to determine a value for the precessional frequency of the
protons using the most fundamental equation in MRI physics – the Larmor
Equation:
0
B
(1.10)1.9 Net Magnetisation Vector
Although individual spins obey the laws of quantum mechanics, the average
behaviour of a group of spins (called spin packets) experiencing the same
magnetic field strength, is best described using classical mechanics. Hence, the
vector sum of the magnetisation vectors from all spin packets is represented by
Page | 10
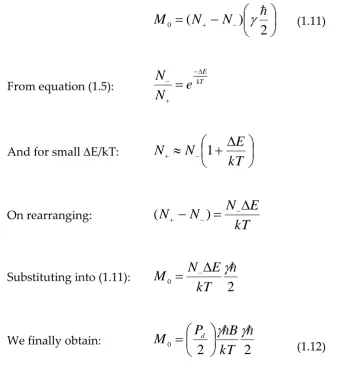
2
)
(
0
N
N
M
(1.11)From equation (1.5): kT
E
e
N
N
And for small E/kT:
kT
E
N
N
1
On rearranging:kT
E
N
N
N
)
(
Substituting into (1.11):
2
0
kT
E
N
M
We finally obtain:
2
2
0
kT
B
P
M
d
(1.12)Where Pd represents the density of protons per unit volume (Pd/2 = N-). Y
Z
B0
M0
[image:26.595.78.425.322.695.2]X
Page | 11
The signal in MRI comes predominantly from M0 and its magnitude is
dependent on a number of factors, which include the magnitude of B0, the
proton density of the tissue, and the proton’s magnetic moment component
(ħ/2).
1.10 The Main Magnetic Field
Magnetic resonance imaging involves the interaction of three types of magnetic
field: the main (static) magnetic field, an oscillating radio frequency (RF) field,
and gradient magnetic fields. The primary job of the main magnetic field in
MRI is to align the spins to form the net magnetisation vector. This is achieved
using superconducting magnets as they provide a range of desirable attributes
including field strengths in the Tesla range that have excellent homogeniety
and temporal stability.
1.10.1 Superconducting Magnets
Superconducting magnets are a form of electromagnet and consist of a solenoid
(a coil of superconducting multifilament wire made of niobium-titanium alloy
embedded as fine filaments in a copper matrix). The solenoid is cryogenically
cooled to a very low temperature of around 4.2K using liquid helium. This
reduces the resistance in the wires to zero, permitting the very strong electrical
0 2 2
0
4
kT
B
P
Page | 12
currents required to create high magnetic fields to be passed through the wires
without generating significant heat. A superconducting magnetic field stores a
substantial amount of energy (Es), which can be calculated using:
2
2
1
LI
Es
(1.14)Where L is the inductance of the coil windings and I is the current flowing
through them. So for a 1.5T magnet with 150 Henrys of inductance and 200
amperes of current, the stored energy would equate to 3.6MJ.
1.11 The Radio Frequency Field
In equilibrium, the net magnetisation vector aligns with the main magnetic field
along the z-axis. M0 is several magnitudes smaller than B0 (T v 1.5T). In
addition to this, M0 is not an oscillating function, and hence cannot be detected
by a receiver coil. To resolve this issue, a radio frequency (RF) pulse (B1) is
applied in a plane perpendicular to B0 (Figure 1.4).
Figure 1.4 An RF pulse is applied in a plane perpendicular to B0
Y Z
X B0
Mxy
Page | 13
1.12 Resonance
If the precessional frequency of the RF pulse matches that of the Larmor
frequency of the protons then energy is added to the system and resonance can
occur.During resonance, spins are encouraged to align with the B1field. This
phase coherence results in the formation of a net transverse magnetisation
vector in the x–y plane (Mxy) (Figure 1.4) that precesses simultaneously about B0
and B1 in a process known as nutation.
1.12.1 Rotating Frame of Reference
The concept of nutation is simplified in MRI physics by considering a rotating
frame of reference (rotating at the Larmor frequency about the z-axis). In a
rotating frame, spins rotating at the Larmor frequency appear stationary whilst
those rotating at higher or lower frequencies are seen to gain or lose phase in
comparison.
Figure 1.5 Using a rotating frame of reference simplifies the concept of flipping.
Y’
B0
M0
B1
X’
Page | 14
An observer within the rotating frame of reference will witness a simple arc
motion as the magnetisation vector is flipped from the z-axis into the x–y plane
(Figure 1.5). An observer standing outside the rotating frame of reference
would witness the more complicated nutational motion of the spins.
1.12.2 Flip Angles
The angle () through which the spins are flipped from the z-axis into the
transverse plane is referred to as the flip angle. It is the choice of amplitude, and
duration (τ) of the applied field (B1) that determines the flip angle of the
magnetisation.
B
1 (1.15)It is possible to obtain the same angle by applying a strong pulse for a short
duration of time, or by applying a weak pulse for a longer period of time. If the
entire M0 vector is flipped into the x–y plane by the RF pulse, then the pulse is
referred to as a 90 RF pulse and the angle as a 90 flip angle. At this point:
Mxy = M0.
A 180 pulse would have twice the amplitude or twice the duration of a 90
pulse. The application of a 180 RF pulse inverts the net magnetisation vector by
exciting the excess spins in the lower energy level into the upper energy level,
Page | 15
1.12.3 Partial Flip Angles
It is possible to flip the net magnetisation vector into the x–y plane by less
than 90 simply by reducing the power and/or duration of the RF pulse.
This results in the magnitude of Mxy being less than the original magnitude
of M0:
Sin
M
M
xy
0 (1.16)1.12.4 Free Induction Decay
Once in the x–y plane, the precessing net magnetisation induces a voltage and
current in a receiver coil which is sensitive only to magnetic fields in the
transverse plane. This induced current is the source of the signal for all MRI
imaging.
A large signal is induced and detected immediately after a 90 pulse, as all the
spins are in phase. This signal is known as the Free Induction Decay (FID)
(Figure 1.6).
Figure 1.6 Left to its own devices the FID would quickly decay away
Amplitude
Time (ms)
Page | 16
Left to its own devices, the FID would rapidly decay away to zero as described
by the Bloch Equations (Appendix A). These macroscopic equations describe
nuclear magnetisation as a function of time when T1 (1.10.8) and T2 (1.10.9)
relaxation processes (Figures 1.7 and 1.8) are present.
1.12.5 T1 Relaxation
The T1 relaxation process is also known as the longitudinal or spin-lattice
relaxation time and describes the recovery of the Mz component of the
net magnetisation vector as it returns to its equilibrium position along the
z-axis after the application of the RF pulse. To relax back into equilibrium,
the spins have to transfer the energy gained from the RF pulse to the
environment. This transfer of energy is not spontaneous and can only happen
if the spins experience an oscillating magnetic field at or near the
Larmor frequency. The rate of energy transfer depends on a molecule’s natural
motions (rotation, vibration and translation). Hydrogen, in the form of water, is
a small molecule and therefore, moves quite rapidly. Larger molecules,
such as fat molecules move more slowly. The T1 relaxation time reflects
the relationship between the natural frequency of these molecules and
the resonant or Larmor frequency. When these frequencies are similar, the
T1 recovery of M0 is rapid, when they are very different, the T1
recovery is slow. Hence different tissues exhibit different T1 recover curves
Page | 17
The equation that describes the T1 relaxation process as a function of time (t) is:
)
1
(
)
(
/ 10
T t
z
t
M
e
M
(1.17)The T1 relaxation process also governs the recovery of the net magnetisation
vector after a 180 pulse.
)
2
1
(
)
(
/ 10
T t
z
t
M
e
M
(1.18)1.12.6 T2 Decay
The phase coherence witnessed immediately after the application of the RF
pulse is short lived as the spins interact with each other. This loss of coherence
results in the decay of the transverse magnetisation and ultimately, the loss of
[image:33.595.157.438.72.304.2]signal. Again, this process varies between tissue types with larger molecules,
Page | 18
such as fat, experiencing many static internal magnetic fields in the presence of
B0due to their chemical structure. The rate of decay of Mxy is governed by the
spin-spin relaxation time (T2) of each tissue (Figure 1.8). The equation that
describes T2 relaxation as a function of time is:
) 2 / (
)
0
(
t Txy
xy
M
e
[image:34.595.169.448.298.550.2]M
(1.19)Figure 1.8 The T2 relaxation curves of the myocardial tissue and the blood pool of the heart at 1.5T.
T1 and T2 are exponential processes. In general, T2 decay is five to ten times
more rapid than T1 recovery.
1.12.7 T2* Relaxation
T2* (t-two star) is a time constant that includes the additional dephasing effects
Page | 19
inhomogeneities and magnetic susceptibility. T2* therefore, describes a quicker
loss of signal than T2 and as a result, T2* is always smaller than T2:
B
T
T
2
*
1
/
2
1
/
2
/
1
(1.20)The dephasing effects that are exclusive to T2* are reversible under certain
circumstances. This reversal can be exploited in MR imaging methods [9].
1.13 Image Formation
Image formation in MRI works by defining the signal intensity in an array of
pixels so that it corresponds to a three-dimensional co-ordinate system (x, y and
z) within the patient. This is a challenging process in MRI as the signal
originates from the entire object rather than a point source and is therefore, not
simply a case of collimating the receiver coils.
In a highly homogenous magnetic field such as B0, identical spins in different
locations will precess at the same frequency and as a result, the detected signal
will contain no spatial information regarding their distribution. However, by
distorting B0with gradient fields, in a precise and controlled way, protons at
different locations will precess at different resonant frequencies. This permits
the signal amplitude to be measured as a function of frequency and phase
whilst the density of protons in the tissue allows an image of spatial structure to
Page | 20
1.13.1 Gradient Magnetic Fields
Gradients fields are small perturbations that have magnitude and direction and
are represented as vectors. They are normally applied as pulses in any chosen
direction or orientation using gradient coils. These gradient fields provide MRI
with its three-dimensional capabilities. When applied in the x, y and z direction
the gradient fields are represented by the symbols Gx, Gy, and Gz respectively.
The isocentre of the magnet is the point where x, y, and z = (0, 0, 0). The
magnetic field at this point is B0 and the resonant frequency is
0.1.13.2 Slice Select Gradient
The strength of the gradient characterises the slope of the field as a function of
its position along the axis. For a field gradient in the z direction:
z
G
grad
B
z
z (1.21)The Larmor frequency of each spin is therefore dependent on its position along
the axis, effectively dividing the patient into 2D slices Larmor frequencies
(Figure 1.9):
z
G
z
G
B
z
z
z
(
)
(
0
)
0(
0
)
Page | 21
The centre frequency of the RF pulse controls the location of the slice whilst the
range of frequencies in the pulse controls the width. Protons either side of the
selected slice will be resonating at higher or lower frequencies. However, the
required frequency has to be present within the RF pulse’s transmit bandwidth
for resonance to occur, thus excitation only takes place close to the isocentre.
This allows the position, orientation and thickness of the slice to be
manipulated simply by adjusting the gradient or RF waveform properties.
1.13.3 Frequency Encoding
During imaging, a frequency encoding gradient is applied perpendicular to the
slice select gradient. Once again the centre of the slice remains unaltered but the
resonant frequency of the spins reduces to the left of the central point and
increases to the right, thus creating columns of varying Larmor frequency
within the slice.
Figure 1.9 A slice select gradient is applied that effectively divides the patient into slices of varying Larmor frequencies.
0
0
0
Page | 22
1.13.4 Phase Encoding
The application of these two gradients is still not enough to ascribe a unique
frequency to each column and row of protons. For this reason, the signal is
encoded in terms of phase in the third direction. The phase encoding gradient
induces a change in phase that is proportional to distance, effectively dividing
the columns of spins into voxels, each with varying precessional speeds (Figure
1.10). When this gradient is switched off the spins revert to their original speeds
but keep their phase encoding until either another gradient is applied or the MR
signal decays.
Gradients are applied with or after an RF pulse. They can be applied
individually or in combination to create transverse, sagittal or coronal, oblique
or double oblique slices. During imaging, spatial localisation in the phase
encoding direction requires many steps. Each step is performed with an
The selected imaging slice is divided into voxels of spins
Apply Gx Gy
Gradients
G
zG
zG
x [image:38.595.138.487.133.228.2]Apply Gz Gradient
Page | 23
incremental change in gradient amplitude so that the protons in the same row
have the same phase but the protons in the same column have different phases.
The gradient system of a modern scanner comprises of a gradient amplifier and
several gradient coils, each of which is positioned around the bore of the
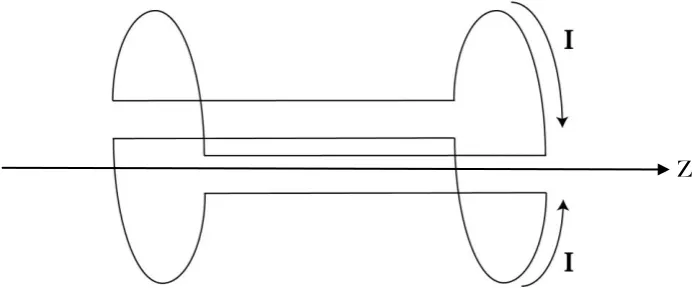
magnet. The z gradient (Gz) is provided by a Maxwell pair (Figure 1.11), whilst
[image:39.595.130.478.521.673.2]the x and y gradients are provided by a Golay pair
Figure 1.11 A Maxwell coil pair is the most efficient choice for producing a z-gradient (Gz).
Figure 1.12 The preferred design for a transverse gradient (Gx or Gy) is the Golay pair.
Page | 24
Modern scanners use shielded gradient coils where secondary coils are used to
cancel out undesired fields from the primary coils.
Gradient strength is a measure of the change in field strength over distance and
is directly proportional to the applied current in the coil. Gradient rise time is a
measure of the rate of change of field generated when the gradients are
switched on and off. The gradient strength divided by the rise time gives the
slew rate of the gradient coils. Slew rates refer to the speed with which the
gradients can be turned on and off. Higher gradients are desirable as they allow
thinner image slices or smaller fields of view (FOV) to be obtained without
changing any other measurement parameters.
Gradient linearity is another important factor in today’s high end scanners as
gradient non-linearity can lead to image distortion and signal loss. Thus,
manufacturers strive to restrict gradient deviations to within 5% of their desired
value.
1.13.5 Repetition Time
Typical MR experiments use a series of pulsed RF energy. The repetition time
(TR) between successive RF pulses should be long enough to allow additional
absorption during the next RF pulse and to prevent the spin system from
Page | 25
1.13.6 Echo Time
Fundamental limitations in the electronics of an MR system prevent a
measurement of the signal immediate after the application of the RF pulse.
Hence, the signal is measured after a short time period, known as the time to
echo (TE). TR and TE are intimately related to T1 and T2 respectively. However,
unlike T1 and T2, TR and TE can be adjusted and controlled by the operator.
1.13.7 Biological Parameters
T1, T2, T2* and proton density values are inherent properties of biological
tissue. Generally, T1 lengthens with increasing field strength as the energy
exchange between the spins and their surroundings is less efficient at higher
frequencies. Field strength also impacts on T2* values as higher fields have an
increased influence on intrinsic susceptibility changes in tissues.
1.14 Pulse sequences
A pulse sequence is basically an MRI software program that has the timing
parameters TR and TE embedded within it. A pulse sequence diagram
(Figure 1.11) is a timing diagram that illustrates the timings of the RF
pulses, gradients and echoes. Pulse sequences control all hardware aspects of
an MRI imaging session. Many of the advances in CMRI have resulted from
pulse sequence developments rather than hardware updates or alterations.
Pulse sequences can be used to accentuate or suppress different tissues, reduce
Page | 26
Sequences can be selected that are sensitive or insensitive to dynamic
parameters such as flow, contrast uptake or perfusion, offering exceptional
versatility.
There are two main sequence types in MRI, known as Spin Echo (SE) and
Gradient Echo (GE).
1.14.1 Spin Echo
The spin echo (SE) technique uses a 90 excitation pulse to flip the
net magnetisation vector into the x–y plane where the spins start to precess
and de-phase. A short time later (TE/2), a 180 refocusing pulse is applied
that rotates the magnetisation about the axis (Figure 1.15). Spins continue to
de-phase; however, as the magnetisation has been rotated, the spins are now
refocusing. This rotation eliminates the de-phasing effects caused by magnetic
field inhomogeneities resulting in the creation of an echo that is dependent on
T2 decay. Multiple RF refocusing pulses can be applied after the 90 RF
excitation pulse, as long as there is sufficient transverse magnetisation. This
creates a train of spin echoes.
Figure 1.15 The spin echo pulse sequence has a 90 excitation pulse followed by 180 refocusing
[image:42.595.193.393.619.736.2]Page | 27
1.14.2 Spin Echo – T1 Weighted Images
Image contrast can be manipulated in SE imaging simply by varying the TR and
TE values. The selection of a long TR (e.g. 2000ms) will allow the complete (or
almost complete) recovery of the T1 curves of the different tissues. Conversely a
short TR (e.g. 300ms) will limit the time allowed for the longitudinal
magnetisation vector to fully recover and result in the incomplete T1 recovery
for some or all tissues. This results in the contrast in such images being
dependent on the T1 characteristics of the different tissue types (Table 1.1).
Thus, these images are described as being T1 weighted.
1.14.3 Spin Echo – T2 Weighted Image
Similarly, the relaxation (dephasing) rates of the transverse magnetisation
vector can provide T2 weighted images in SE if a long TR and a long TE (e.g.
80-140ms) are selected (Table 1.1). The long TR allows the complete recovery of the
longitudinal magnetisation vector and the long TE provides sufficient time for
T2 relaxation to happen. These T2 weighted images depend on the T2 relaxation
rates of the different tissues only as the application of the 180 refocusing pulse
at TE/2 refocuses the dephasing spins and cancels the dephasing effects of static
magnetic field inhomogeneities. Tissues with a long T2 provide a stronger
Page | 28 Table 1.1 The contrast in SE images is determined by the choice of TR and TE values
Spin Echo Imaging Short T2 Long T2
Short TR T1 Weighted X
Long TR Proton density T2 Weighted
1.14.4 Spin Echo – Proton Density Image
Where the operator selects a long TR and a short TE, the T1 and T2 dependence
of these images is insubstantial. For this reason, image contrast is dictated by
the density of protons in the different tissue types. Tissues with a high
Hydrogen content will appear bright whilst tissues with a low Hydrogen
content will appear dark.
1.14.5 Gradient Echo
Gradient echo (GE) pulse sequences differ from SE pulse sequences in that they
can use an excitation pulse of < 90 to produce an echo. This partial flip angle
means that at the time point TE/2, a large component of the net magnetisation
vector is lying in the z-axis. Applying a 180 pulse at this point would invert
this magnetisation (Mz) into the negative z-axis. This is not desirable as a long
TR would be required for Mzto fully recover. Alternatively, measuring the FID
would not provide the time interval essential to spatially encoding the signal.
Hence, in GE the FID is de-phased and then re-phased at a later time using a
refocusing gradient (Figure 1.16). The gradient reversal in GE sequences
Page | 29
itself and not those de-phased by magnetic field inhomogeneities. Hence, GE
image contrast is dictated by T2*.
As only one RF pulse is applied in GE, it is possible to record the signal more
quickly than in SE, resulting in a shorter TE. Partial flip angles also allow the
use of shorter TRs. Consequently, GE images are ideal for cardiac imaging as
their short TRs and reduced flip angles allow faster imaging times, significantly
reducing motion related artefacts. GE sequences are commonly referred to as
white-blood imaging, as generally blood and fat appear white in these images.
The signal weighting in a GE image depends on the TR, TE and flip angle. The
higher the flip angle, the more T1 weighted the image will be (Table 1.2). The
shorter the TE, the less T2* weighted the image will be. In addition to this, there
is an optimal combination between TR and flip angle for maximum MR signal.
The optimal angle is known as the Ernst angle (E) and is calculated from the
TR and T1 values:
Page | 30
1
exp
cos
T
TR
E
(1.22)As the TR is always assumed to be short for a GE sequence, it has much less of
an effect on image contrast. By selecting a small flip angle the magnetisation
will be almost completely recovered, so there will be very little difference in T1
[image:46.595.201.395.521.717.2]recovery curves (Table 1.2).
Table 1.2 The contrast in GE images is determined by the choice of flip angle and TE
Gradient Echo Imaging Short TE Long TE
Small flip (< 40) PD weighted T2* weighted
Larger flip (>50) T1 weighted X
1.14.6 Anatomy Scans
T1 weighted images usually have excellent contrast making it easy to
differentiate between fluids, water-based tissue and fat. For this reason, they are
commonly referred to as anatomy scans.
Page | 31
1.14.7 Pathology Scans
Fluids have the highest intensity in T2 weighted images, showing up very
brightly against the darker soft tissue. It is possible to differentiate between
normal and abnormal fluids (such as oedema) in an image as they have
different T2 relaxation rates and hence different signals. For this reason, T2
scans are considered pathology scans. Most cardiac images are acquired using a
[image:47.595.199.396.307.501.2]ratio of T2 to T1 (Chapter 5).
Figure 1.14 A T2 image of the same brain highlights abnormal fluid
1.14.8 Proton Density Scans
Proton density images are dependent primarily on the concentration of mobile
hydrogen atoms within the imaging volume. The variation of proton densities
in different tissues provides the range of signal intensities and hence, image
Page | 32
1.15 Coils
In addition to the solenoid coil and gradient coils mentioned previously in this
chapter, a body coil and shim coil sets are also built in to the bore of the magnet.
1.15.1 Body Coil
The body coil surrounds the patient in the magnet bore. The main function of
this coil is to transmit the RF pulse for all scans and to receive the MRI signal
when large parts of the body are being imaged.
1.15.2 Shim Coil Sets
Shim coil sets are built into all state-of-the-art MRI systems. These coils are used
to compensate for undesirable field distortions in a passive or active manner.
Passive coils consist of shim plates (pieces of metal) that correct for field
distortions. Active coils are comprised of loops of wire that a current is passed
through to produce supplementary magnetic fields. Shim coils are essential for
cardiac imaging at 1.5T and 3.0T as they provide improved field homogeniety
across the heart. This will be discussed in Chapter 7.
Various other radio frequency coils are used in MRI to transmit energy and to
receive signals. These coils are placed on or around the region of tissue to be
scanned. They comprise of transmit receive coils, receive only coils and transmit
Page | 33
1.15.3 Surface Coils
Insulated surface coils are placed directly on top of the patient’s clothing to
ensure that the receiver is as close as possible to the MR signal. Surface coils are
commonly used in MRI as their close proximity to the patient limits the volume
from which noise is detected thus providing good SNR for superficial tissue.
1.15.4 Phased Array Coils
Phased array coils consist of an array of surface receiver coil elements with
known sensitivity profiles whose signals are combined to provide a uniform
signal intensity over a volume that is in excess of each of the smaller individual
coils (Figure 1.17). Phased array coils have a superior SNR to larger coils
covering the same area, as the design of these coils ensures that the noise from
coil to coil is largely uncorrelated.
Page | 34
This technology is ideal suited for cardiac imaging as the array can be wrapped
around the patient’s torso in a bid to obtain optimal images of the heart (Figure
1.18).
1.16 K-Space
Applying gradients in MRI changes the frequency across the patient as a
function of position effectively taking the patient from a physical space into a
frequency space. Hence, the signal we obtain is a sum of sine waves that add to
create a rapidly changing continuous voltage in our receiver coil. This complex
[image:50.595.137.425.72.288.2]signal originates from every voxel in the image.
Page | 35
The signal is digitised by sampling the voltage at each point across the
echo using an analogue to digital converter (ADC). This allows the amplitude
and phase of the signal to be determined as a function of time. Each of
these points is represented by a complex number that includes a real
and `imaginary part. Rather than directly expressing position, the points
indicate the amount of spatial encoding that has taken place at that point,
with each point being identified by its value and location, which are equivalent
to the amplitude and the frequency of a sinusoid respectively.
In conventional imaging, each excitation pulse provides a single echo that
is sampled to fill a single line in k-space (Figure 1.19), with the number
of samples acquired across the echo in the x direction determining the number
of pixels in the x direction. The number of echoes sampled determines
the number of lines in the y direction, which in turn determines the number of
Figure 1.19 The signal is digitised by sampling the voltage across the echo. Each echo fills a single line in the
k-space array.
Ky
Page | 36
pixels in the y direction. Hence, filling k-space conventionally is a time
consuming process as an image with 128 pixels in the y direction would require
128 echoes each with a different phase encoding step.
It would be impossible to create an image before the gradient fields are
applied as the uniform precession of all the spins would simply create a
uniform sine wave that would superimpose as one dot in K-space. The
effective position in k-space is determined by the gradient amplitude and
duration as well as the gyromagnetic ratio.
Applying a gradient to de-phase the spins creates a temporary shift in
frequency across the sample, making the phases of the protons change as a
function of position. The spins at the centre of k-space effectively do not
experience a gradient and therefore, they do not experience a frequency shift
(
0)
, whereas those over towards the edges of k-space experience the greatestPage | 37 Figure 1.20 Each point in k-space is represented by a complex number that contains a real and an
imaginary part and represents the spatial encoding that has taken place at that point.
As the middle point of k-space does not experience a gradient, every proton is
in phase, providing a very strong constant signal across the image space. When
a mathematical operation [known as a Fourier transform (FT)] is performed on
the k-space array to produce the final image, this one point in k-space creates a
sheet of uniform intensity that determines the intensity of every pixel in the
image. The next point along the x-axis in k-space creates a second sheet of
intensity that has an oscillation along the x-axis but is constant in y. This
additional sheet is added to the first sheet to increasing the intensity across the
whole image, improving the spatial resolution.
The centre of k-space provides the image with contrast and the edges of k-space
provide the image with high frequency definition. Frequency is proportional to
Increasing Phase Encoding
Increasing Phase Encoding
Ky
Variation in Amplitude
[image:53.595.142.410.63.289.2]Page | 38
distance from the centre of k-space. Removing high frequency data would
result in a blurred image with normal contrast.
The desired spatial resolution of the final image (Figure 1.21) is specified by
selecting the field of view (FOV) and the number of phase and frequency
encoding steps. This in turn determines the extent of the k-space array. The
more tightly packed k-space is, the larger the field of view (FOV). Conversely, if
the echo is loosely sampled a small field of view is obtained; however the pixel
size, contrast and resolution are the same.
1.17 SAR limitation
The specific absorption rate (SAR) is a measure of the rate at which energy is
absorbed by tissue when exposed to a radiofrequency electromagnetic field.
More precisely, it is a measure of the energy deposited by a radiofrequency
field in a given mass of tissue. The SAR is limited by the Medicines and
[image:54.595.106.474.359.523.2]Healthcare Regulatory Agency (MHRA) guidelines [10], which state that the
FT
Page | 39
SAR must not exceed 10 W/kg of tissue for the head and trunk or 20 W/kg for
the limbs over any 6-minute period. The SAR value is affected by many
parameters such as the flip angle, amplitude of the RF pulse, selected protocol
parameters, the subject’s weight and region of exposure, and the
radiofrequency coil. To prevent limits being exceeded, the scanner will slow
imaging or suggest that a parameter is reduced, such as increasing the TR or
reducing the number of slices to be acquired.
1.18 Cardiac Magnetic Resonance Imaging
Initially, CMRI failed to gain widespread acceptance due to the challenges
associated with imaging the beating heart using a complicated technique that is
inherently sensitive to patient/organ motion. Despite this, CMRI remained an
area of intense interest within the cardiac community due to its unique
potential to provide an accurate, and reproducible, full cardiac assessment of
morphology and function, perfusion and viability, valvular disease and
coronary artery stenosis, all within a single imaging session. The sustained
development of CMRI hardware and software techniques discussed in
Chapter 3 of this thesis have resulted in a paradigm shift in CMRI’s clinical
potential, allowing it to fulfil its promise beyond the limitations of other well
Page | 40
1.19 Other Techniques and their Limitations
Although cardiac magnetic resonance imaging is now well-established and has
growing popularity within the radiology and cardiology communities, other
imaging modalities are presently utilised more frequently in clinical practice.
1.19.1 SPECT and PET
Single photon emission computed tomography (SPECT) and positron emission
computed tomography (PET) are imaging techniques that work on the same
basic principles. Patients are injected intravenously with a labelled
radioisotope, which travels through the coronary arteries to the myocardium
where it is absorbed. The decay rate of the isotope is then measured using either
a gamma camera or PET scanner, providing a functional picture of the blood
[image:56.595.186.409.462.678.2]supply to the myocardium.
Figure 1.22 A series of SPECT images in three planes showing the uptake of a radioisotope in the heart
Page | 41
Single Photon Emitted Computed Tomography has become the most popular
imaging technique for the diagnostic work-up of patients with coronary artery
disease. Conversely, cardiac PET imaging initially failed to become widely
established in the United Kingdom because of its requirement for isotopes from
restricted sites with a cyclotron. However, this situation is now changing as the
required isotopes become more available.
In comparison to CMRI, SPECT and PET are immediately limited by their use
of ionising radiation. A further limitation is the difficulty in assigning cardiac
wall boundaries when coronary arteries are narrowed or occluded due to the
reduced blood flow and hence reduced isotope count in the area fed by the
diseased vessel (Figure 1.22).
1.19.2 Computed Tomography
Computed tomography (CT) is an imaging modality that is known to
provide accurate and reproducible images [11, 12] of the heart and coronary
arteries (Figure 1.23). It does this using a fan-shaped beam of x-rays that pass
through the patient to reach detectors. Subsequently, the radiation exposure
to the patient is non-trivial. This naturally restricts the use of CT for
longitudinal studies designed to chart the progression of cardiac disease-related
Page | 42
A further restriction of CT is that images can only be obtained in sagittal
or coronal planes.
1.19.3 Echocardiography
Echocardiography (echo) is the modality of choice for the detection of
heart-wall motion abnormalities that are often the earliest manifestations of coronary
blood flow restrictions. Echo is a non-ionising technique, which uses a
transducer to create a beam of very high frequency sound (ultrasound) waves
that are transmitted into the patient and reflected back. The shape, size, density
and motion of all objects lying in the path of the beam are then reconstructed on
[image:58.595.172.421.150.363.2]a screen as an image (Figure 1.24).