Both thermoregulatory behavior and exhaustive exercise play important roles in the life history of lizards. As an ectotherm, a lizard must use behavioral means to maintain its body temperature within its preferred activity range (Bennett, 1980; Bartholomew, 1982; Huey, 1982). Ectothermy also appears to place limits on an animal’s ability to utilize aerobic metabolism to support vigorous activity (Bennett, 1978; Else and Hulbert, 1985). Escape from predators, foraging for food and agonistic encounters frequently result in exhaustion in lizards (Gleeson, 1980; Bennett et al., 1981; Pough and Andrews, 1985). Because exhaustion is a frequent consequence of activity in these animals, and because activity metabolism is thermally sensitive (Bennett, 1980; John-Alder and Bennett, 1981; Bennett and John-Alder, 1984; Autumn et al., 1994), the interactions between exercise recovery and thermoregulatory behavior are potentially of great significance. Previous work has indicated that Dipsosaurus dorsalis will select a body temperature that is significantly cooler than their
preferred activity temperature range of 38.5–40 °C while they are recovering from exhaustive exercise (Wagner and Gleeson, 1997a). There is no evidence that this change in thermoregulatory behavior serves to enhance any aspect of exercise recovery and, in fact, it might have deleterious effects on some aspects of recovery (Wagner and Gleeson, 1997a,b). The results of our previous studies (Wagner and Gleeson, 1997a,b) raise the question of why exhausted D. dorsalis in a thermal gradient do not maintain their preferred activity body temperature. One possible explanation is that one or more of the physiological changes that occur as a consequence of exhaustive exercise serves as a signal for these animals to alter their thermoregulatory behavior. Although such a change in behavior may not be adaptive in the context of exercise recovery, it may be part of a suite of adaptations to physiological stresses that share some of the same characteristics as exhaustion.
Work carried out in other laboratories suggests that elevated blood lactate concentrations and decreased arterial pH will alter Printed in Great Britain © The Company of Biologists Limited 1999
JEB1787
We conducted this study to determine whether two of the physiological changes associated with non-sustainable exercise, elevated blood lactate levels and decreased arterial pH, contribute to the behavioral hypothermia exhibited by exhausted lizards. Dipsosaurus dorsalis were placed in a thermal gradient and their body temperatures were recorded from 08:00 to 14:00 h. At 14:00 h, animals were subjected to different experimental regimens. In the exercise (E) regimen, animals at 40 °C were forced to exercise maximally for 5 min on a treadmill. In the lactate (L) regimen, animals were infused with 11.5 ml kg−1 of 250–500 mmol l−1sodium lactate. In the osmolarity control (O) regimen, animals were injected with 11.5 ml kg−1 of 500 mmol l−1NaCl, and in the injection control (I) regimen, animals were injected with 11.5 ml kg−1 of 150 mmol l−1 NaCl. In the hypercapnia (H) regimen, the thermal gradient was flushed with a gas mixture containing 10 % CO2, 21 % O2 and 69 % N2, a treatment that lowers the arterial pH of D. dorsalis to a value comparable with that imposed by exhaustive exercise. A group of control (C)
animals was left undisturbed in the thermal gradient for 24 h. Animals in all experimental groups were returned to the thermal gradient, and their cloacal temperatures were monitored until 08:00 h the following morning. The mean cloacal temperature of E animals underwent a significant decrease of 4–7 °C, relative to control animals, which persisted for 7 h. The mean cloacal temperatures of animals subjected to 2 h of regimen H also decreased by 3.5–9 °C and remained depressed for 12 h following the beginning of the treatment. L, O and I animals did not undergo a significant change in body temperature following treatment, and their mean body temperatures did not differ from those of C animals at any time during the experiment. The results of this study suggest that the metabolic acidosis, but not the elevated blood lactate level, that follows exhausting exercise might play a role in the behavioral hypothermia that follows exhausting exercise in D. dorsalis.
Key words: lizard, Dipsosaurus dorsalis, acidosis, lactate, behavior, hypothermia, exercise.
Summary
Introduction
THE ROLES OF ACIDOSIS AND LACTATE IN THE BEHAVIORAL HYPOTHERMIA
OF EXHAUSTED LIZARDS
ERICA L. WAGNER*,‡, DAVID A. SCHOLNICK ANDTODD T. GLEESON
Department of Environmental, Population and Organismic Biology, University of Colorado at Boulder, Boulder, CO 80309-0334, USA
*Present address: Department of Biology, SUNY College at Potsdam, Potsdam, NY 13676, USA (e-mail: wagnerel@potsdam.edu)
the thermoregulatory behavior of toads Bufo marinus and of crabs
Carcinus maenas (Branco and Wood, 1994; Pörtner et al., 1994;
De Wachter et al., 1997). It has been known for some time that hypoxia will cause ectothermic vertebrates, including lizards, to lower temporarily their preferred body temperature (Wood, 1991). Branco and Wood (1994) and Pörtner et al. (1994) hypothesized that elevated blood lactate levels and depressed arterial pH might serve as a sort of metabolic alarm for hypoxic animals to lower their energetic demands by lowering their body temperatures. It is possible that elevated blood lactate levels and depressed arterial pH could trigger a similar change in thermoregulatory behavior in animals that are not hypoxic.
The experiment described here was designed to test the hypothesis that elevated blood lactate levels and depressed arterial pH are capable of triggering a decrease in an animal’s preferred body temperature. We artificially elevated the blood lactate concentrations of non-exercised D. dorsalis by infusing sodium lactate or depressed the blood pH by elevating blood
PCO∑ so that blood lactate concentrations or arterial pH were
similar to those of exhausted animals. We allowed animals to ‘recover’ from these manipulations in a thermal gradient in the laboratory while we monitored their body temperatures. The results of this experiment suggest that, although the effect of an artificially elevated blood lactate level is small in D. dorsalis, acidosis appears to play a greater role in the altered thermoregulatory behavior observed in exhausted D. dorsalis.
Materials and methods Research animals
We collected Dipsosaurus dorsalis (Biard and Giard) from a site near Cathedral City, California, USA, under a California Fish and Game Permit. Animals were segregated by gender and housed in groups of 4–12 in galvanized steel tubs (0.75 m×1.5 m×7.5 m). Animals had access to heat lamps for 24 h per day, and water was provided ad libitum. Lettuce sprinkled with powdered rat chow was provided twice a week, but food was always withheld for 5–7 days prior to experimentation. This study utilized adult male animals, ranging in mass from 45 to 85 g. Any animals that lost weight or otherwise appeared ill were excluded from the study. A University Animal Care and Use Committee and a campus veterinarian approved and supervised all animal research protocols.
Thermal gradient
The thermal gradient was constructed in a fiberglass tank (1.5 m×0.5 m×0.5 m) and placed in a quiet room in the animal care facility of the department. The bottom of the tank was covered with 5 cm of sand, and two or three thermostatted aquarium heaters were buried 1 cm beneath the sand surface to provide a thermal gradient ranging from 25 to 50 °C. A 75 W incandescent bulb, suspended 1 m above the middle of the gradient, provided light for 24 h per day for the duration of the experiment. Water was available in the gradient ad libitum.
Body temperature measurement
An 18 gauge, siliconized, lubricated copper–constantan
thermocouple was inserted 1 cm into the cloaca of each experimental animal at least 12 h prior to the beginning of data collection. The probe was held in place by two thin bands of tape around the animal’s tail. A Macintosh computer was interfaced with the thermocouple probe via Maclab data-acquisition hardware and software to sample body temperature data every 30 s. The thermocouple leads were suspended above the gradient by a ring and allowed to slide back and forth on a wire as the animal moved around.
Experimental manipulations
Data collection always started at 08:00 h on the day of experimentation. To provide data on the normal thermoregulatory pattern of undisturbed animals, control (C) animals (N=6) were placed singly in the thermal gradient and left undisturbed for 24 h. Previous work performed in our laboratory (Wagner and Gleeson, 1997a and unpublished observations) indicates that simply removing an animal from the thermal gradient and handling it does not result in a significant change in body temperature, so all experimental body temperature data were compared with those of group C animals in the present study. All experimental manipulations took place between 14:00 and 14:30 h. At exactly 14:00 h, the thermocouple was gently removed from the animals in each of the other experimental groups. The animals were quickly transported to our laboratory (transit time less than 1 min) and placed in a darkened box in a temperature-controlled cabinet at 40 °C for 20 min. Following this 20 min acclimation period, experimental animals were subjected to one of four experimental regimens.
For the exhaustive exercise experiment (group E), the animals (N=6) were forced to exercise to exhaustion on the treadmill surface between 14:20 and 14:25 h (for details of this exercise protocol, see Wagner and Gleeson, 1996). Following exercise, each E animal was quickly transported back to the thermal gradient and monitored until 08:00 h the following morning.
For the lactate experiment (group L), each animal (N=6) received an infusion of 11.5 ml kg−1body mass of a
250–500 mmol l−1sodium lactate solution (mean 375 mmol l−1)
into its abdominal vein. This infusion regimen resulted in blood lactate concentrations ranging from 9.3 to 36.5 mmol l−1.
These values effectively bracketed the range of blood lactate concentrations measured in D. dorsalis following 5 min of exhaustive exercise at 40 °C (Gleeson and Dalessio, 1989; Wagner and Gleeson, 1996).
To control for the effects of handling stress and infusion with a relatively large volume of experimental solution, the third experimental group of animals (N=6), designated as the injection controls (I), was removed from the thermal gradient, as described above, and injected with 11.5 ml kg−1of an
iso-osmotic saline solution (150 mmol l−1). Following this
treatment, each animal was replaced in the thermal gradient and its body temperature was monitored until 08:00 h the following morning.
hyperosmotic saline infusion (11.5 ml kg−1 of 500 mmol l−1 NaCl). These animals are referred to in the text as the osmotic (O) control animals. Following this treatment, each animal was returned to the gradient and its temperature was monitored as described above.
We initially attempted to lower the arterial pH of our experimental animals by infusing them with lactic acid solution, in the manner described by Mitchell and Gleeson (1985) for
Varanus salvator. D. dorsalis, however, respond to this
treatment by undergoing severe, and often fatal, convulsions. Variations in the volume, concentration and duration of the lactic acid injection did not alter this response, so we lowered the arterial pH of our animals by forcing them to breathe normoxic, hypercapnic air. In the hypercapnia experiment (H), the animals (N=7) were removed from the thermal gradient at 14:00 h and brought to our laboratory so that they would experience a similar level of disturbance to that of animals in the E group. These animals remained in a temperature-controlled cabinet at 40 °C until 14:25 h. For this portion of the experiment, the thermal gradient was covered with clear plastic sheeting (1 MIL) and flooded with hypercapnic gas at a flow rate of 7 l min−1. This gas was mixed so that it was composed of 10 % CO2, 21 % O2and
69 % N2. This procedure took approximately 10 min. At 14:35 h,
these animals were returned to the thermal gradient and their body temperatures were monitored until 08:00 h the next morning. Because preliminary data suggested that exhaustively exercised animals alter their thermoregulatory behavior most dramatically for the first 2–4 h of recovery, we changed the gas mixture flowing into the thermal gradient so that room air (21 % O2, 0.04 % CO2) would begin to replace the hypercapnic mixture
in the gradient at 16:30 h.
Effects of experimental treatments on blood pH
To compare the effects of hypercapnic acidosis with those of metabolic acidosis resulting from exercise, we anaesthetized four animals with Halothane and surgically implanted cannulas into their left carotid artery. Following a recovery period of at least 24 h, each animal was placed in a temperature-controlled cabinet at 40 °C and left there undisturbed for at least 4 h. Following this acclimation period, each animal was placed on the surface of a motor-driven treadmill at 40 °C and forced to run for 5 min as described previously (Wagner and Gleeson, 1996). A blood sample was then obtained from the carotid cannula and placed into a 70µl heparinized microcap tube. Each animal was replaced in the temperature-controlled cabinet at 40 °C and allowed to recover for 60 min. A 20 cm extension was placed on the end of the cannula and threaded through a hole in the animal’s chamber so that additional samples could be obtained after 30 min of recovery without disturbing the animal further. The pH of each arterial blood sample was immediately measured using a BMS3 Mk2 Blood Micro System radiometer.
To assess the effects of prolonged exposure to 10 % CO2on
arterial blood pH, four animals were surgically cannulated, as described above, and placed in a 600 ml flow-through tube in a temperature-controlled cabinet at 40 °C. An air mixture containing 10 % CO2was forced through the tube at a rate of
approximately 600 ml min−1. Blood was collected from each animal following 30 min of this treatment. Following 30 min, room air was forced through the tube at the same flow rate for 30 min, and an additional sample of blood was obtained. The arterial pH of each of these samples was determined as described above.
Four cannulated animals were injected with 11.5 ml kg−1of 500 mmol l−1 sodium lactate and placed in a temperature-controlled cabinet at 40 °C. Following 30 min of recovery from this regimen, arterial blood samples were obtained, as described above, and blood pH data were obtained.
Statistical analysis
A repeated-measures analysis of variance (ANOVA) was performed on each experimental group to determine whether the mean body temperature of animals subjected to each regimen changed relative to that of control animals before or after experimental treatment. If the mean body temperature of an experimental group differed significantly from that of the control group following treatment, a factorial ANOVA (Fisher’s PLSD for multiple comparisons) was performed to determine the specific post-treatment time increments in which experimentals and controls differed.
Results
Animals subjected to the undisturbed control regimen (C) did not exhibit a significant alteration in their thermoregulatory behavior at any time during the 24 h monitoring period (P=0.945, repeated-measures ANOVA). The mean pre-exercise body temperature did not differ significantly from that of the control (C) animals for any of the experimental treatment groups (P values ranged from 0.415 to 0.873).
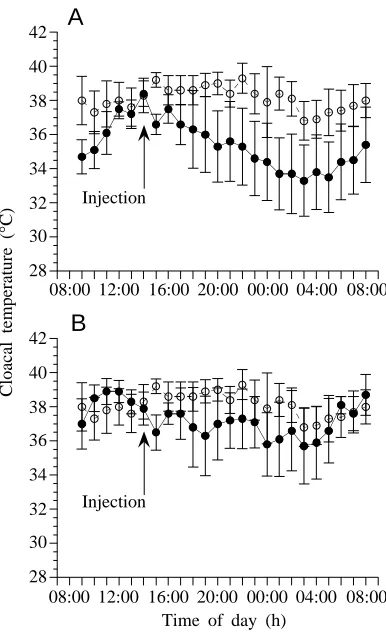
The mean body temperature of the E animals differed significantly from that of the C animals following treatment (P=0.009). The mean body temperature of animals forced to exercise to exhaustion decreased by more than 5 °C after the first 30 min of recovery and remained 4–7 °C lower than that of control animals for 4.5 h following exercise (Fig. 1). The mean body temperature of E animals was significantly lower than that of C animals between 13:00 and 17:00 h (0.5–4.5 h of recovery), and between 18:00 and 20:00 h (5.5–7.5 h of recovery) (Table 1). These results confirmed that exhaustive exercise results in a temporary, but significant, decrease in preferred body temperature, as has been demonstrated previously (Wagner and Gleeson, 1997a).
Injection with lactate (L) did not result in a change in mean post-treatment body temperature that differed significantly from corresponding control values (Fig. 2; P=0.133, repeated-measures ANOVA). Injection with iso-osmotic (I) and hyperosmotic (O) NaCl solutions also failed have a significant effect on the mean post-treatment body temperature of D.
dorsalis relative to that of the C animals (Fig. 3; P=0.437 for
I animals and P=0.168 for O animals, repeated-measures ANOVA).
and 69 % N2) for 2 h caused the mean post-treatment body
temperature of H animals to differ significantly from that of C animals (P=0.033, repeated-measures ANOVA). The H animals began to cool immediately upon the commencement of the H treatment, and their body temperature ultimately dropped to a value that was 3.5–9 °C cooler than the corresponding control temperature (Fig. 4). The mean body temperature of H animals differed significantly from that of C animals from 14:30 to 21:00 h and from 22:00 to 23:00 h (Table 1). These times corresponded to the periods 0.5–8.5 h and 9.5–10.5 h following the beginning of the 2 h treatment with the hypercapnic gas mixture.
Resting D. dorsalis at 40 °C had a mean arterial pH of 7.45, a value similar to that observed by Snyder et al. (1995). Both exercise and exposure to hypercapnia resulted in a significant reduction in the arterial pH of our animals (to a pH of 6.84 after exercise and to a pH of 7.02 after exposure to hypercapnia; Table 2). Arterial pH returned to resting values within 30 min of the cessation of exposure to the hypercapnic gas mixture, but blood pH was still depressed in animals following 30 min of recovery from exercise. Infusion with a 500 mmol l−1 sodium lactate solution had no effect on the arterial pH of D. dorsalis (Table 2).
Discussion
The results of this study suggest that the lactate anion does not trigger significant behavioral hypothermia in D. dorsalis when it is present in the blood at concentrations similar to those
produced by exercise. Thus, it is unlikely that lactate by itself serves as the metabolic ‘trigger’ that causes the behavioral hypothermia occurring in exhausted D. dorsalis. The
28 30 32 34 36 38 40 42
Cloacal temperature (
°
C)
Time of day (h)
08:00 12:00 16:00 20:00 00:00 04:00 08:00 Exercise
[image:4.609.319.543.71.249.2]** * * *
*
[image:4.609.53.277.76.249.2]Fig. 1. Mean body temperatures of Dipsosaurus dorsalis (N=6) forced to exercise maximally (group E) (filled circles) for 5 min between 14:00 and 14:30 h. The body temperature of E animals decreased significantly following exercise (P=0.011, repeated-measures ANOVA), while the mean body temperature of control animals (N=6, open circles) did not change significantly at any point during the 24 h observation period (P=0.367, repeated-measures ANOVA). Asterisks indicate the times at which the mean body temperature of E animals differed significantly from those of control animals (group C; P⭐0.05, factorial ANOVA). Values are means ±S.E.M.
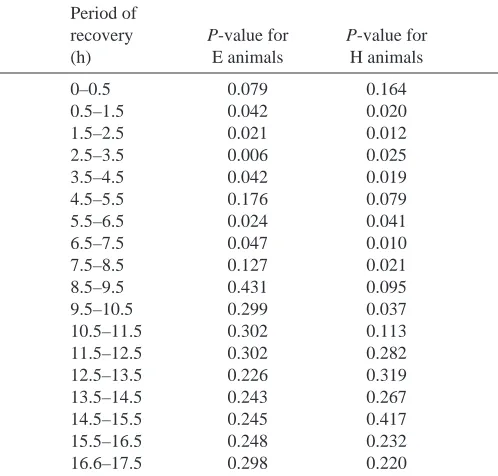
Table 1. Statistical comparisons of post-treatment body temperatures of E and H animals with C animals at each 1 h
time increment during recovery
Period of
recovery P-value for P-value for
(h) E animals H animals
0–0.5 0.079 0.164
0.5–1.5 0.042 0.020
1.5–2.5 0.021 0.012
2.5–3.5 0.006 0.025
3.5–4.5 0.042 0.019
4.5–5.5 0.176 0.079
5.5–6.5 0.024 0.041
6.5–7.5 0.047 0.010
7.5–8.5 0.127 0.021
8.5–9.5 0.431 0.095
9.5–10.5 0.299 0.037
10.5–11.5 0.302 0.113
11.5–12.5 0.302 0.282
12.5–13.5 0.226 0.319
13.5–14.5 0.243 0.267
14.5–15.5 0.245 0.417
15.5–16.5 0.248 0.232
16.6–17.5 0.298 0.220
Comparisons are via Fisher’s PLSD for multiple comparisons. Recovery begins at 14:30 h.
28 30 32 34 36 38 40 42
Injection
Cloacal temperature (
°
C)
Time of day (h)
[image:4.609.311.560.469.707.2]08:00 12:00 16:00 20:00 00:00 04:00 08:00
Fig. 2. The effects of injection with 11.5 ml kg−1 of 375 mmol l−1
observation that hypercapnia-induced acidosis produces behavioral hypothermia in normoxic, non-exhausted lizards provides indirect evidence that the decline in arterial pH that results from exercise does play a role in the altered thermoregulatory pattern of exhausted D. dorsalis.
The thermoregulatory behavior of the exhausted animals examined in this study was similar to that of animals examined previously, although the behavioral hypothermia persisted for longer in the present study (Wagner and Gleeson, 1997a). Some specific details of the construction of the thermal gradient and temperature monitoring were conducted differently in the current study, the most notable difference being that the animals in the previous study were subjected to surgical manipulation (Wagner and Gleeson, 1997a), while the animals in the present study were not. These differences in experimental technique and/or seasonal variation in thermoregulatory behavior may account for the longer duration
of the behavioral thermoregulation observed in the present study. Apparently, the temporary change in thermal setpoint that is brought on by exhaustive exercise serves to override any generalized ‘fever response’ that can be elicited by handling, surgery and light exercise itself (Cannon and Kluger, 1985; Cabanac and Gosselin, 1993).
The response of D. dorsalis to infusion of lactate appears to be less marked than that observed by Pörtner et al. (1994), who found that hypoxic toads (Bufo marinus) decreased their mean body temperature for several hours when injected with sodium lactate, and by De Wachter et al. (1997), who found that normoxic shore crabs (Carcinus maenus) also selected relatively cool microclimates when they were injected with a lactate solution. Our animals showed a brief, but non-significant, reduction in their body temperature in response to
28 30 32 34 36 38 40 42
Injection
A
28 30 32 34 36 38 40 42
Injection
B
Cloacal temperature (
°
C)
08:00 12:00 16:00 20:00 00:00 04:00 08:00
[image:5.609.69.262.71.388.2]08:00 12:00 16:00 20:00 00:00 04:00 08:00 Time of day (h)
Fig. 3. The effects of (A) 11.5 ml kg−1hyperosmotic (375 mmol l−1)
NaCl injection (group O) and (B) iso-osmotic (150 mmol l−1) NaCl
injection (group I) on the thermoregulatory behavior of Dipsosaurus dorsalis. The body temperature of animals subjected to the O (N=7) and I (N=6) injections (filled circles in A and B) did not change significantly following treatment (P=0.0903 for the O animals and P=0.5655 for the I animals, repeated-measures ANOVA). The body temperatures of O and I animals did not differ significantly from those of control animals (group C; open circles in A and B) at any time point before or after treatment (P>0.05, factorial ANOVA). Values are means ±S.E.M.
Table 2. The effects of exhaustive exercise, 10 % CO2and
lactate injection on arterial pH in Dipsosaurus dorsalis
Regimen Arterial pH
Rest 7.45±0.028a
Post-exercise 6.84±0.047b
30 min of recovery from exercise 6.8b
30 min of exposure to hypercapnia 7.02±0.074b,c
30 min of recovery from hypercapnia 7.41±0.028a
30 min post-lactate injection 7.438*
Values are mean ± 1 S.E.M.; N=4, unless indicated otherwise. *Denotes that N=2.
Different superscripts identify values that are significantly different (P艋0.05, factorial ANOVA).
26 28 30 32 34 36 38 40 42
Hypercapnia
* *** * *
*
*
*
Cloacal temperature (
°
C)
Time of day (h)
[image:5.609.326.551.78.251.2]08:00 12:00 16:00 20:00 00:00 04:00 08:00
Fig. 4. The effect of exposure to a hypercapnic gas mixture (10 % CO2) on the thermoregulatory behavior of Dipsosaurus dorsalis
[image:5.609.326.567.600.687.2]infusion of lactate, but this reduction was similar to that resulting from infusion of concentrated NaCl. It is possible that a larger sample size would have yielded significant results for at least some of the post-injection times, but it is still clear that the effect of the lactate anion on thermoregulatory behavior is less marked than that of hypercapnia-induced acidosis and exercise in D. dorsalis. Pörtner et al. (1994) and De Wachter et al. (1997) both cite their results as evidence that the lactate anion itself serves as a metabolic ‘alarm’ for hypoxic animals to lower their body temperatures in order to lower their metabolic demand for O2. Why D. dorsalis do not respond to
lactate infusion as strongly as do toads and crabs is not clear, although it is important to note that the toads used by Pörtner et al. (1994) were mildly hypoxic, while our D. dorsalis were normoxic. The mechanism by which different species of vertebrate and invertebrate detect elevations in blood lactate levels are not well understood, although the existence of both peripheral and central chemoreceptors has been postulated (Pörtner et al., 1994; De Wachter et al., 1997). It is possible that different species of vertebrate possess different sensitivities to elevated lactate levels or that elevated lactate levels serve only as a metabolic ‘alarm’ in a synergistic fashion in D. dorsalis.
The results of our infusion of NaCl solutions into D. dorsalis indicated that handling these animals does not cause this species to alter its thermoregulatory behavior significantly. Nor did infusion with a lactate result in a change in arterial pH (Table 2).
In addition to elevating blood lactate concentrations, the exercise regimen resulted in the blood pH of our D. dorsalis becoming significantly lower than resting values (Table 2). Following 30 min of recovery from exercise, the arterial pH of these animals was still only 6.82±0.138 (mean ±S.E.M., N=4), a value significantly different from resting arterial pH (P<0.001, Fisher’s PLSD) but not from the immediate post-exercise arterial pH of D. dorsalis (P=0.884). This change in arterial pH is both larger and more persistent than the change that results from exhaustive exercise in two species of Varanus and in Iguana iguana as a consequence of exhaustive exercise (Gleeson and Bennett, 1982). D. dorsalis appears to be less competent than at least some other lizard species in buffering the change in blood pH that results from vigorous exercise. It therefore appears likely that D. dorsalis would be particularly vulnerable to any behavioral changes brought about by a reduction of pH in their arterial blood.
The H regimen resulted in animals altering their thermoregulatory pattern in a manner that was initially similar to that of exhausted animals but which persisted for longer. Both exercise and exposure to a hypercapnic gas mixture resulted in a significant decrease in the arterial pH (Table 2). Branco and Wood (1994) found that toads (Bufo marinus) that were forced to breathe air mixtures containing 8 % or more CO2selected a
cooler body temperature than control animals. They also found that this treatment resulted in the arterial pH of these toads decreasing to approximately 7.1 and that the thermoregulatory response to hypercapnia appears to be mediated by both central
H+receptors and peripheral H+receptors, although the locations
of the peripheral ion receptors have not yet been determined (Branco and Wood, 1994). If peripheral H+ receptors are
involved in the responses of these animals to metabolic acidosis, we would expect that an intravenous infusion of lactic acid would result in a behavioral hypothermia indistinguishable from that brought on by hypercapnia. Results of such an experiment are not reported in the present manuscript because, unlike at least two other lizard species (Mitchell and Gleeson, 1985), D.
dorsalis will not tolerate infusion with acidic solutions (E. L.
Wagner and T. T. Gleeson, unpublished observations), so hypercapnia was the only means available of altering the arterial pH of our animals in a manner that was similar to the effects of exercise. It is important to note that the arterial pH of the animals recovered much more rapidly from hypercapnia-induced acidosis than from exercise-induced acidosis, but that the hypothermic responses persisted for longer in the post-hypercapnic animals. This is interesting, because it suggests either that the cerebral spinal acidosis may persist much longer than the arterial acidosis or that the duration of the acidosis of these animals does not necessarily affect the duration of the behavioral response.
The present study has provided evidence that metabolic acidosis is likely to play a major role in the altered thermoregulatory behavior observed in exhausted D. dorsalis. The effect of the lactate anion appears to be much weaker in this species. Other researchers have found that both hypoxia and anemia cause lizards to select body temperatures that are cool relative to their normal activity temperature (Hicks and Wood, 1985). Anemic and hypoxic animals stand to benefit from lowering their demand for O2(Hicks and Wood, 1985),
that mediate exercise-induced hypothermia so that we can elucidate how and why the changes associated with exhaustive exercise essentially serve to ‘override’ the usual hyperthermia that results from stress in vertebrates.
We wish to thank G. Snyder for his loan of the blood pH analyzer, C. Carey for her loan of the temperature-monitoring hardware and D. Bowers for her advice on statistical analyses. This research was supported in part by an EPOB research fellowship awarded to E.W. and by NIH grant DK46428 awarded to T.G.
References
Autumn, K., Weinstein, R. B. and Full, R. J. (1994). Low cost of
locomotion increases performance at low temperature in a nocturnal lizard. Physiol. Zool. 67, 238–262.
Bartholomew, G. A. (1982). Physiological control of body
temperature. In Biology of the Reptilia, Physiology (C), vol. 12 (ed. C. Gans and F. H. Pough), pp. 167–213. New York: Academic Press.
Bennett, A. F. (1978). Activity metabolism of the lower vertebrates.
Annu. Rev. Physiol. 400, 447–469.
Bennett, A. F. (1980). The thermal dependence of lizard behavior.
Anim. Behav. 28, 752–762.
Bennett, A. F. and John-Alder, H. B. (1984). The effect of body
temperature on the locomotory energetics of lizards. J. Comp. Physiol. B 155, 21–27.
Bennett, A. F., Gleeson, T. T. and Gorman, G. C. (1981).
Anaerobic metabolism in a lizard (Anolis bonairensis) under natural conditions. Physiol. Zool. 54, 237–241.
Branco, L. G. S. and Wood, S. C. (1994). Role of central
chemoreceptors in behavioral thermoregulation of the toad, Bufo marinus. Am. J. Physiol. 266, R1483–R1487.
Cabanac, M. and Gosselin, F. (1993). Emotional fever in the lizard
Callopistes maculatus (Teiidae). Anim. Behav. 46, 200–202.
Cannon, J. G. and Kluger, M. J. (1985). Altered thermoregulation
in the iguana Dipsosaurus dorsalis following exercise. J. Therm. Biol. 10, 41–45.
De Wachter, B., Sartoris, F. J. and Pörtner, H. O. (1997). The
anaerobic endproduct lactate has a behavioural and metabolic signalling function in the shore crab Carinus maenas. J Exp. Biol.
200, 1015–1024.
Else, P. L. and Hulbert, A. J. (1985). An allometric comparison of
the mitochondria of mammalian and reptilian tissues: the implications for the evolution of endothermy. J. Comp. Physiol. B
156, 3–11.
Gleeson, T. T. (1980). Lactic acid production during field activity in
the Galapagos marine iguana, Amblyrhynchus cristatus. Physiol. Zool. 53, 157–162.
Gleeson, T. T. and Bennett, A. F. (1982). Acid–base imbalance in
lizards during activity and recovery. J. Exp. Biol. 98, 439–453.
Gleeson, T. T. and Dalessio, P. M. (1989). Lactate and glycogen
metabolism in the lizard Dipsosaurus dorsalis following exhaustive exercise. J. Exp. Biol. 144, 377–393.
Hicks, J. W. and S. C. Wood. (1985). Temperature regulation in
lizards: effects of hypoxia. Am. J. Physiol. 17, R595–R600.
Huey, R. B. (1982). Temperature, physiology and the ecology of
reptiles. In Biology of the Reptilia, Physiology (C), vol. 12 (ed. C. Gans and F. H. Pough), pp. 24–91. New York: Academic Press.
John-Alder, H. B. and Bennett, A. F. (1981). Thermal dependence
of endurance and locomotory energetics in a lizard. Am. J. Physiol.
241, R342–R349.
Kluger, M. J. (1991). Fever: role of pyrogens and cryogens. Physiol.
Rev. 71, 93–125.
Mitchell, G. S. and Gleeson, T. T. (1985). Acid–base balance during
lactic acid infusion in the lizard Varanus salvator. Respir. Physiol.
60, 253–266.
Morrow, L. E., McClellan, J. L., Conn, C. A. and Kluger, M. J.
(1993). Glucocorticoids alter fever and IL-6 responses to psychological stress and to lipopolysaccharide. Am. J. Physiol. 264, R1010–R1016.
Pörtner, H. O., Branco, L. G. S., Malvin, G. M. and Wood, S. C.
(1994). A new function for lactate in the toad Bufo marinus. J. Appl. Physiol. 76, 2405–2410.
Pough, F. H. and Andrews, R. M. (1985). Use of anaerobic
metabolism by free-ranging lizards. Physiol. Zool. 58, 205–213.
Snyder, G. K., Nestler, J. R., Shapiro, J. I. and Huntley, J. (1995).
Intracellular pH in lizards after hypercapnia. Am. J. Physiol. 268, R889–R895.
Soszynski, D., Kozak, W., Conn, C. A., Rudolph, K. and Kluger, M. J. (1996). Beta-adrenoreceptor antagonists suppress elevation
in body temperature and increase in plasma IL-6 in rats exposed to open field. Neuroendocr. 63, 459–467.
Wagner, E. L. and Gleeson, T. T. (1996). Low temperature and
exercise recovery in the desert iguana. Physiol. Zool. 69, 168–190.
Wagner, E. L. and Gleeson, T. T. (1997a). Post-exercise
thermoregulatory behavior and recovery from exercise in desert iguanas. Physiol. Behav. 61, 175–180.
Wagner, E. L. and Gleeson, T. T. (1997b). The influence of
thermoregulation on behavioral recovery from exercise in a lizard. Funct. Ecol. 11, 723–728.
Wood, S. C. (1991). Interactions between hypoxia and hypothermia.