ARTICLE
Genetic Susceptibility to Retinopathy of Prematurity
Matthew J. Bizzarro, MDa, Naveed Hussain, MDb, Baldvin Jonsson, MDc, Rui Feng, PhDd, Laura R. Ment, MDa, Jeffrey R. Gruen, MDa,e,f, Heping Zhang, PhDd, Vineet Bhandari, MD, DMa
Departments ofaPediatrics,dEpidemiology and Public Health, andeGenetics, and thefYale Child Health Research Center, Yale University School of Medicine, New Haven,
Connecticut;bDivision of Neonatology, University of Connecticut Health Center, Farmington, Connecticut;cDepartment of Neonatology, Karolinska University Hospital,
Karolinska Institute, Stockholm, Sweden
The authors have indicated they have no financial relationships relevant to this article to disclose.
ABSTRACT
OBJECTIVES.The goals were to isolate and to estimate the genetic susceptibility to
retinopathy of prematurity.
METHODS.A retrospective study (1994 –2004) from 3 centers was performed with
zygosity data for premature twins who were born at a gestational age of ⱕ32 weeks and survived beyond a postmenstrual age of 36 weeks. Retinopathy of prematurity was diagnosed and staged by pediatric ophthalmologists at each center. Data analyses were performed with mixed-effects logistic regression anal-ysis and latent variable probit modeling.
RESULTS.A total of 63 monozygotic and 137 dizygotic twin pairs were identified and
analyzed. Data on gestational age, birth weight, gender, respiratory distress syn-drome, retinopathy of prematurity, bronchopulmonary dysplasia, duration of ventilation and supplemental oxygen use, and length of stay were comparable between monozygotic and dizygotic twins. In the mixed-effects logistic regression analysis for retinopathy of prematurity, gestational age and duration of supple-mental oxygen use were significant covariates. After controlling for known and unknown nongenetic factors, genetic factors accounted for 70.1% of the variance in liability for retinopathy of prematurity.
CONCLUSION.In addition to prematurity and environmental factors, there is a strong
genetic predisposition to retinopathy of prematurity.
www.pediatrics.org/cgi/doi/10.1542/ peds.2006-1088
doi:10.1542/peds.2006-1088
Dr Feng’s current address is Section on Statistical Genetics, Department of Biostatistics, Ryals Public Health Building 420B, University of Alabama at Birmingham, Birmingham, AL 35294.
Key Words
neonate, retinopathy of prematurity, twins, genetic
Abbreviations
ROP—retinopathy of prematurity RDS—respiratory distress syndrome BPD— bronchopulmonary dysplasia MELR—mixed-effects logistic regression GA— gestational age
BW— birth weight OR— odds ratio CI— confidence interval
R
ETINOPATHY OF PREMATURITY(ROP) is a disease pro-cess that results from disruption of the normal vas-cularization of the developing retina. The retinal blood supply is derived from both the choroidal and retinal circulations. The choroidal circulation is completed by⬃20 weeks of gestation, when the development of the retinal circulation is only beginning.1Vasculogenesis in
the fetus is regulated by several factors, including vas-cular endothelial growth factor and various regulatory cytokines.1–4In prematurely delivered neonates,
fluctu-ations in oxygen tension and other undetermined vari-ables may affect these factors adversely and may result in incomplete and abnormal neovascularization of the ret-ina.5The outcome, despite early detection and
interven-tion, may be retinal detachment and blindness.6–8
ROP is most prevalent and severe in neonates with extremely low birth weight (BW),9–11with overall
inci-dence rates estimated to be as high as 68% among infants born at⬍1251 g and 93% among infants born at
⬍750 g.12Given its prevalence and considerable
morbid-ity, investigators have attempted to identify and to target significant contributory factors (eg, supplemental oxy-gen) in efforts to prevent and to treat this disease.5,9,12–16
Despite these measures, ROP remains a common prob-lem in the NICU.9This suggests the possible involvement
of other nonenvironmental influences and the need to identify them. We hypothesized that genetic factors play a major role in predisposing neonates toward developing ROP. The goal of our present study was to analyze a cohort of preterm monozygotic and dizygotic twin pairs to determine and to estimate the genetic susceptibility.
METHODS
Subjects
Data on premature twins born atⱕ32 weeks of gestation between January 1, 1994, and December 31, 2004, in-cluding zygosity information, were collected from 3 cen-ters (ie, the Karolinska Institute, the University of Con-necticut, and Yale University). We included only infants who survived beyond a postmenstrual age of 36 weeks, with complete data on the variables necessary for anal-ysis. The institutional review boards of all participating centers approved the contribution of data to this study.
Definitions
The zygosity of each twin pair was determined through histopathologic examination of the placenta, with con-firmation from gender concordance or discordance. ROP was defined as the incomplete or abnormal vascular proliferation of the retina, as determined by experienced pediatric ophthalmologists at each institution, and was staged according to the criteria established by the Inter-national Committee for Classification of ROP.17All stages
of ROP were included in the analysis. Respiratory dis-tress syndrome (RDS) was defined as the presence of
respiratory distress with an oxygen requirement in the first 6 hours of life, accompanied by a characteristic chest radiograph. Bronchopulmonary dysplasia (BPD) was de-fined as the need for supplemental oxygen at postmen-strual age of 36 weeks, in association with characteristic radiographic changes.18All levels of severity of BPD were
included in the analysis. The duration of mechanical ventilation was defined as the total number of days that the infant, while hospitalized, required positive pressure ventilation. Positive pressure ventilation included high-frequency ventilation, synchronized intermittent man-datory ventilation, synchronized nasal intermittent pos-itive pressure ventilation, and/or nasal continuous positive airway pressure. The duration of oxygen use was defined as the total number of days that the infant, while hospitalized, required the use of supplemental oxygen (⬎21%).
Statistical Analyses
Demographic data were analyzed by using Student’s t
test, Wilcoxon rank-sum test, or2analysis, where
ap-propriate. Mixed-effects logistic regression (MELR) anal-ysis was performed to identify the impact of putative risk factors on ROP. The covariates used in the model in-cluded male gender, gestational age (GA), BW, RDS, duration of oxygen use, treating institution, and BPD. The status of the outcomes from twin pairs was treated as a correlated event. The treating institution was eval-uated as an overall variable and as individual institutions in comparison with a reference institution chosen at random. A MELR model was fitted to assess the relation-ship between the covariates listed and the outcome of interest (ROP) and to incorporate the correlation be-tween twin pairs.
Latent variable probit modeling for twin data was then used to estimate the variance in liability for ROP.19–21 A mixed-effects probit model was fitted to
es-timate the genetic contribution to ROP by adjusting for all covariates used in the MELR analysis. A liability variable underlying the respective outcome was esti-mated. This variable was assumed to follow a normal distribution with the mean dependent on the MELR covariates. The variance was partitioned into a genetic component, a shared nongenetic component, and a ran-dom component. The sum of the first 2 components constituted the overall sharing between twins and was determined from the correlation between monozygotic twins and dizygotic twins. We estimated this heritability (ie, the ratio of the genetic variance to the total variance in liability) on the basis of a model that assumed that the correlation among twins resulted from both genetic and nongenetic components.
(PROC GLIMMIX and PROC NLMIXED; SAS Institute, Cary, NC), SPSS 13.0 for Windows and Macintosh (SPSS, Chicago, IL), and GraphPad Prism 3.0 (GraphPad Software, San Diego, CA). P values of⬍.05 were con-sidered statistically significant.
RESULTS
ROP was diagnosed in 86 (21.5%) of 400 infants from our cohort. The incidence of ROP was inversely propor-tional to BW, with the majority of disease occurring in the population with BW of⬍1000 g (Table 1); of those infants, 67 (66%) of 101 were diagnosed as having ROP, compared with 17 (11%) of 156 infants with BW of 1000 to 1500 g and 2 (2%) of 125 infants with BW of 1501 to 2000 g (Table 1). No ROP was diagnosed in the subpopulation with BW of⬎2000 g.
Zygosity data for 63 monozygotic and 137 dizygotic twin pairs from 3 institutions were used for analysis. These 200 twin pairs had mean GA and BW of 29 weeks and 1332 g, respectively. Despite a discrepancy in the overall number of twin pairs in each group, no statisti-cally significant differences were observed between monozygotic and dizygotic twins, with respect to GA, BW, gender, 5-minute Apgar scores, incidences of RDS, ROP, and BPD, duration of mechanical ventilation, du-ration of supplemental oxygen use, and length of hos-pital stay (Table 2).
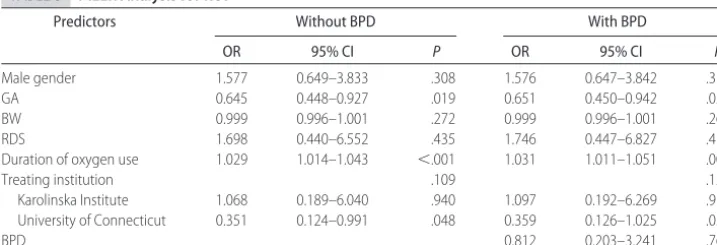
MELR analysis was performed using ROP as the de-pendent variable in an attempt to identify significant factors in our cohort that might contribute to the out-come of interest (Table 3). The analysis determined GA (odds ratio [OR]: 0.65; 95% confidence interval [CI]: 0.45– 0.94; P⫽.024) and duration of oxygen use (OR: 1.03; 95% CI: 1.01–1.05; P ⫽ .003) to be significant predictors for ROP (Table 3). The data presented dem-onstrate the results of analyses with and without BPD as a covariate. Previous investigators demonstrated an as-sociation between ROP and BPD in preterm infants, suggesting the possibility of shared nongenetic and/or genetic components.22–26 Because we demonstrated a
significant genetic susceptibility to BPD previously,21
we asked whether BPD and ROP might share genetic factors. To analyze this, we performed MELR analyses using BPD as the dependent variable with ROP as a covariate (data not shown) and then ROP as the depen-dent variable with BPD as a covariate (Table 3). The data showed that ROP and BPD were not independent
pre-dictors of each other (BPD as dependent variable:P ⫽
.090; ROP as dependent variable:P⫽.764).
After significant nongenetic cofactors for ROP were identified in the MELR analysis, a latent variable probit model was used to estimate the genetic susceptibility to ROP. Our model assumed that genetic and shared and unshared nongenetic factors contributed to the correla-tion among twins. Using this model, we determined that 70.1% (95% CI: 9%–100%;P⫽.026) of the variance in liability to ROP was the result of genetic factors alone.
DISCUSSION
ROP remains a significant and prevalent cause of mor-bidity among preterm infants worldwide.27In the more
highly developed countries of the world, with screening programs and interventions aimed at prevention and treatment, ROP still accounts for 3% to 11% of blindness in children.27Although the onset and progression of ROP
may be influenced strongly by the GA of the newborn, factors that are unrelated to prematurity are likely in-volved. Previous investigators attempted to show an as-sociation between genetic factors and susceptibility to ROP.28–34Potential candidate genes that have been
eval-uated involve known pathophysiologic mediators in-volved in this disease processes. These have included primarily mediators of angiogenesis in the developing retina, with particular focus on vascular endothelial growth factor,28,29the Norrie disease gene (NDP),30–33and
angiotensin-converting enzyme.34 These studies should
be interpreted with some degree of caution. Variations of candidate genes were present in small subsets of subjects and/or were limited geographically to specific ethnic populations, making the generalizability of the results uncertain. Although a significant amount of information regarding these genetic polymorphisms has been pub-lished,28–34 no previous definitive study has confirmed
that a genetic susceptibility to ROP exists. Also, informa-tion regarding the extent of that genetic contribuinforma-tion has not been identified previously.
Our cohort consisted of 200 monozygotic and dizy-gotic twin pairs sampled from 2 centers in the United States and 1 in Sweden. By comparing concordance within monozygotic twin pairs, which share 100% of their genetic information, and concordance within dizy-gotic twin pairs, which share at least 50%, and control-ling for known and unknown nongenetic covariates, we were able to estimate the genetic contribution to ROP.
The statistical model to estimate the genetic suscepti-bility to ROP separated variance into 3 major compo-nents, namely, additive genetic effects, unshared or re-sidual nongenetic effects, and unidentified common nongenetic effects.19,35To determine the effect of additive
genetic effects, we identified and estimated the contri-butions from unshared or residual nongenetic effects and unidentified common nongenetic effects. Residual nongenetic effects were represented only by variables
TABLE 1 Incidence of ROP According to BW
BW, g Incidence of ROP,n/N(%)
⬍1000 67/101 (66)
1000–1500 17/156 (11)
1501–2000 2/125 (2)
included in the MELR; these included, among other factors, the effects of prematurity,22,23,25 use of
supple-mental oxygen,22,25RDS,22,23and BPD,22–26all previously
determined risk factors for ROP. A second component included in our model, unidentified common nonge-netic effects, factored in the potential effects of uniden-tified factors not incorporated into the MELR analysis (ie, variables not available from our data set); these included the potential influences of race,16,36
intraven-tricular hemorrhage,22,23,25 periventricular
leukomala-cia,22necrotizing enterocolitis,37and septicemia,22,23,26in
addition to unknown unidentified factors. By modeling the effects of these nongenetic components, we were able to determine that 70.1% of the variance in liability to ROP was attributable to genetic factors alone.
Although the statistical analyses of data for our cohort determined a significant genetic susceptibility to ROP, we recognize that there are some limitations to our data. Although criteria for the diagnosis and staging of ROP are standardized, there is still the potential for error and disagreement, even among the most experienced exam-iners.11In our cohort, however, the same individual, or
group of individuals, from each site performed all retinal examinations over the entire study period. In addition, our cohort was restricted to twin pairs with available zygosity information and was therefore limited in num-ber. Specific subgroup analyses to address potential dif-ferences in genetic susceptibility according to BW and/or ROP stage could not be performed. Despite this
limita-tion, no differences in the percentages of infants with severe ROP and/or BW of ⬍1000 g were observed be-tween monozygotic and dizygotic twin pairs. Lastly, the covariates collected in our data set did not include all of the potential contributing factors for ROP. We did at-tempt to control for these unknown variables in our statistical model, however.
Using our statistical model, we have identified a sig-nificant genetic susceptibility to ROP. The extent to which genetic factors contribute to this major cause of infant morbidity accentuates the need for a shift in the paradigm used to identify and to treat disease processes. Similar models using data from twin pairs revealed sub-stantial genetic heritability for several disorders, includ-ing BPD (53% heritability),21 schizophrenia (82%),38
schizoaffective disorders (85%),38type I bipolar disorder
(93%),39Alzheimer disease (79%),40and corneal
thick-ness, which is an important factor in glaucoma (95%).41
Significant genetic effects have also been shown to in-fluence reading and mathematics performance (82% heritability).42 The identification of a genetic influence
has led to the isolation of specific genes associated with some of these disorders, including Alzheimer disease, schizophrenia, and dyslexia.43–47It is possible that similar
discoveries may be achievable for ROP, and dual ther-apy, aimed at limiting potential environmental risk fac-tors and identifying and targeting specific genetic facfac-tors, may become the model for future interventions.
TABLE 2 Comparison of Demographic Data for Monozygotic and Dizygotic Twin Pairs Monozygotic
(n⫽126)
Dizygotic (n⫽274)
P
GA, mean⫾SD, wk 29.39⫾2.29 29.22⫾2.35 .495
BW, mean⫾SD, g 1334.67⫾460.13 1331.25⫾444.81 .944
Male gender,n(%) 63 (50.00) 162 (59.12) .088
Apgar score at 5 min, median 8 8 .743
RDS,n(%) 77 (61.11) 181 (66.06) .337
ROP,n(%) 21 (18.75) 65 (26.00) .134
BPD,n(%) 36 (28.57) 59 (21.53) .124
Duration of mechanical ventilation, mean⫾SD, d 7.64⫾12.85 9.67⫾19.29 .215 Duration of oxygen use, mean⫾SD, d 29.17⫾35.69 30.06⫾39.76 .824 Length of stay, mean⫾SD, d 54.12⫾34.06 51.99⫾31.75 .564
TABLE 3 MELR Analysis for ROP
Predictors Without BPD With BPD
OR 95% CI P OR 95% CI P
Male gender 1.577 0.649–3.833 .308 1.576 0.647–3.842 .310
GA 0.645 0.448–0.927 .019 0.651 0.450–0.942 .024
BW 0.999 0.996–1.001 .272 0.999 0.996–1.001 .263
RDS 1.698 0.440–6.552 .435 1.746 0.447–6.827 .416
Duration of oxygen use 1.029 1.014–1.043 ⬍.001 1.031 1.011–1.051 .003
Treating institution .109 .120
Karolinska Institute 1.068 0.189–6.040 .940 1.097 0.192–6.269 .916 University of Connecticut 0.351 0.124–0.991 .048 0.359 0.126–1.025 .056
ACKNOWLEDGMENTS
Dr Bizzaro was supported in part by National Institute of Child Health and Human Development training grant T32 HD07094. Dr Jonsson was supported by Sallskapet Barnavard and Stiftelsen Frimurare Barnhuset in Stock-holm. Dr Ment was supported by National Institute of Neurological Disorders and Stroke grants NS27116, NS35476, and NS42027. Dr Gruen was supported by National Institute of Neurological Disorders and Stroke grant R01 NS43530. Dr Zhang was supported by Na-tional Institute on Drug Abuse grants K02 DA017713 and R01 DA016750. Dr Bhandari was supported by Na-tional Heart, Lung, and Blood Institute grant K08 HL074195.
REFERENCES
1. Reynolds JD. Retinopathy of prematurity. In: Nelson LB, Olitsky SE, eds.Harley’s Pediatric Ophthalmology. 4th ed. Phila-delphia, PA: Lippincott Williams & Wilkins; 2005:77–78 2. Stone J, Itin A, Alon T, et al. Development of retinal
vascula-ture is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia.J Neurosci.1995; 15:4738 – 4747
3. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors.FASEB J. 1999;13:9 –22
4. Ferrara N, Bunting S. Vascular endothelial growth factor, a specific regulator of angiogenesis.Curr Opin Nephrol Hypertens. 1996;5:35– 44
5. Recchia FM, Capone A. Contemporary understanding and management of retinopathy of prematurity.Retina. 2004;24: 283–292
6. Cryotherapy for Retinopathy of Prematurity Cooperative Group. The natural outcome of premature birth and retinopathy: status at 1 year. Arch Ophthalmol. 1994;112: 903–912
7. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: Snellen visual acuity and structural outcome at 51⁄2 years after randomization. Arch Ophthalmol. 1996;114:
417– 424
8. Quinn GE, Dobson V, Kivlin J, et al. Prevalence of myopia between 3 months and 51⁄2years in preterm infants with and
without retinopathy of prematurity: Cryotherapy for Retinop-athy of Prematurity Cooperative Group.Ophthalmology.1998; 105:1292–1300
9. STOP-ROP Multicenter Study Group. Supplemental therapeu-tic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial, part I: primary outcomes. Pediatrics.2000;105:295–310
10. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121: 1684 –1694
11. Reynolds JD, Dobson V, Quinn GE, et al. CRYO-ROP and LIGHT-ROP Cooperative Study Groups: evidence-based screening criteria for retinopathy of prematurity: natural his-tory data from the CRYO-ROP and LIGHT-ROP studies.Arch Ophthalmol.2002;120:1470 –1476
12. Good WV, Hardy RJ, Dobson V, et al. The incidence and course of retinopathy of prematurity: findings from the early treat-ment for retinopathy of prematurity study. Pediatrics. 2005; 116:15–23
13. Kinsey VE, Jacobus JT, Hemphill F. Retrolental fibroplasias: cooperative study of retrolental fibroplasias and the use of oxygen.Arch Ophthalmol.1956;56:481–547
14. Kinsey VE, Arnold HJ, Kalina RE, et al. PaO2levels and retro-lental fibroplasia: a report of the cooperative study.Pediatrics. 1977;60:655– 668
15. Dani C, Cecchi A, Bertini G. Role of oxidative stress as phys-iopathologic factor in the preterm infant.Minerva Pediatr.2004; 56:381–394
16. Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity: the Cryotherapy for Ret-inopathy of Prematurity Cooperative Group. Ophthalmology. 1991;98:1628 –1640
17. International Committee for Classification of ROP. An interna-tional classification of retinopathy of prematurity. Pediatrics. 1984;74:127–133
18. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: predic-tion from oxygen requirement in the neonatal period. Pediat-rics.1988;82:527–532
19. Neale MC, Cardon LR.Methodology for Genetic Studies of Twins and Families. Dordrecht, Netherlands: Kluwer Academic Publishers; 1992
20. Lynskey MT, Heath AC, Nelson EC, et al. Genetic and envi-ronmental contributions to cannabis dependence in a national young adult twin sample.Psychol Med.2002;32:195–207 21. Bhandari V, Bizzarro MJ, Shetty A, et al. Familial and genetic
susceptibility to major neonatal morbidities in preterm twins. Pediatrics.2006;117:1901–1906
22. Hussain N, Clive J, Bhandari V. Current incidence of retinop-athy of prematurity, 1989 –1997.Pediatrics.1999;104(3). Avail-able at: www.pediatrics.org/cgi/content/full/104/3/e26 23. Holmstro¨m G, Broberger U, Thomassen P. Neonatal risk factors
for retinopathy of prematurity: a population-based study.Acta Ophthalmol Scand.1998;76:204 –207
24. Ajayi OA, Raval D, Lucheese N, Pildes RS. Ophthalmological morbidity in very-low-birthweight infants with bronchopul-monary dysplasia.J Natl Med Assoc.1997;89:679 – 683 25. Brown DR, Biglan AW, Stretavsky MM. Retinopathy of
prematurity: the relationship with intraventricular hemor-rhage and bronchopulmonary dysplasia.J Pediatr Ophthalmol Strabismus.1990;27:268 –271
26. Purohit DM, Ellison RC, Zierler S, Miettinen OS, Nadas AS. Risk factors for retrolental fibroplasia: experience with 3,025 premature infants: National Collaborative Study on Patent Ductus Arteriosus in Premature Infants. Pediatrics. 1985;76: 339 –344
27. Gilbert C, Fielder A, Gordillo L, et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115(5). Available at: www.pediatrics.org/cgi/content/full/115/5/e518
28. Vannay A, Dunai G, Banyasz I, et al. Association of genetic polymorphisms of vascular endothelial growth factor and risk for proliferative retinopathy of prematurity.Pediatr Res.2005; 57:396 –398
29. Cooke RW, Drury JA, Mountford R, Clark D. Genetic polymor-phisms and retinopathy of prematurity.Invest Ophthalmol Vis Sci.2004;45:1712–1715
30. Shastry BS, Pendergrast SD, Hartner MK, Liu X, Trese MT. Identification of missense mutations in the Norrie disease gene associated with advanced retinopathy of prematurity. Arch Ophthalmol.1997;115:651– 656
polymorphism in the Norrie disease gene is associated with advanced retinopathy of prematurity in premature Kuwaiti infants.J Biomed Sci.2002;9:365–370
33. Kim JH, Yu YS, Kim J, Park SS. Mutations of the Norrie gene in Korean ROP infants.Korean J Ophthalmol.2002;16:93–96 34. Haider MZ, Devarajan LV, Al-Essa M, Kumar H.
Angiotensin-converting enzyme gene insertion/deletion polymorphism in Kuwaiti children with retinopathy of prematurity.Biol Neonate. 2002;82:84 – 88
35. Williams CJ. On the covariance between parameter estimates in models of twin data.Biometrics.1993;49:557–568
36. Lang DM, Blackledge J, Arnold RW. Is Pacific race a retinop-athy of prematurity risk factor?Arch Pediatr Adolesc Med.2005; 159:771–773
37. McGinnity FG, Halliday HL. Perinatal predictors of ocular mor-bidity in school children who were very low birthweight. Pae-diatr Perinat Epidemiol.1993;7:417– 425
38. Cardno AG, Marshall EJ, Coid B, et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series.Arch Gen Psychiatry.1999;56:162–168
39. Kieseppa T, Partonen T, Haukka J, Kaprio J, Lonnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins.Am J Psychiatry.2004;161:1814 –1821
40. Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and
environments for explaining Alzheimer disease.Arch Gen Psy-chiatry.2006;63:168 –174
41. Toh TY, Liew SHM, MacKinnon JR, et al. Central corneal thickness is highly heritable: the twin eye studies.Invest Oph-thalmol Vis Sci.2005;46:3718 –3722
42. Light JG, DeFries JC, Olson RK. Multivariate behavioral ge-netic analysis of achievement and cognitive measures in read-ing-disabled and control twin pairs. Hum Biol. 1998;70: 215–237
43. Holmans P, Hamshere M, Hollingsworth P, et al. Genome screen for loci influencing age at onset and rate of decline in late onset Alzheimer’s disease.Am J Med Genet B Neuropsychiatr Genet.2005;135:24 –32
44. Van Gassen G, Van Broeckhoven C. Molecular genetics of Alzheimer’s disease: what have we learned?Acta Neurol Belg. 2000;100:65–76
45. Tosato S, Dazzan P, Collier D. Association between the neu-regulin 1 gene and schizophrenia: a systematic review. Schizo-phr Bull.2005;31:613– 617
46. Williams NM, O’Donovan MC, Owen MJ. Is the dysbindin gene (DTNBP1) a susceptibility gene for schizophrenia? Schizo-phr Bull.2005;31:800 – 805
47. Meng H, Smith SD, Hager K, et al.DCDC2is associated with reading disability and modulates neuronal development in the brain.Proc Natl Acad Sci USA.2005;102:17053–17058
MMR DOCTOR ‘TO FACE GMC CHARGES’
“The doctor who first suggested a link between the MMR vaccine and autism is to be charged with serious professional misconduct, it is reported. Vacci-nation rates fell sharply after Dr. Wakefield questioned the safety of MMR, raising fears of a measles epidemic. His initial Lancet paper has since been disowned by the journal. . . . The Independent reports that Dr.Wakefield will face four charges: that he published inadequately founded research, failed to obtain ethical committee approval for the work, obtained funding for it improperly, and subjected children to ‘unnecessary and invasive investiga-tions.’”
BBC News. http://news.bbc.co.uk/go/pr/fr/-/2/hi/health/5070670.stm
UK ‘IN GRIP OF MEASLES OUTBREAK’
“Numbers of measles cases in the UK have risen to their highest level in nearly 20 years, experts said. Surrey and Sussex could have up to 156 cases and South Yorkshire may have 180. Last year there were just 77 cases across England and Wales. The south east’s health agency blames low uptake of the MMR vaccine. It comes as a doctor who linked MMR with autism faces possible misconduct charges. A boy’s death from the disease in April was the first UK fatality in 14 years.“
BBC News.http://news.bbc.co.uk/go/pr/fr/-/2/hi/health/5081286.stm
DOI: 10.1542/peds.2006-1088
2006;118;1858
Pediatrics
Jeffrey R. Gruen, Heping Zhang and Vineet Bhandari
Matthew J. Bizzarro, Naveed Hussain, Baldvin Jonsson, Rui Feng, Laura R. Ment,
Genetic Susceptibility to Retinopathy of Prematurity
Services
Updated Information &
http://pediatrics.aappublications.org/content/118/5/1858
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/118/5/1858#BIBL
This article cites 43 articles, 11 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/ophthalmology_sub
Ophthalmology following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2006-1088
2006;118;1858
Pediatrics
Jeffrey R. Gruen, Heping Zhang and Vineet Bhandari
Matthew J. Bizzarro, Naveed Hussain, Baldvin Jonsson, Rui Feng, Laura R. Ment,
Genetic Susceptibility to Retinopathy of Prematurity
http://pediatrics.aappublications.org/content/118/5/1858
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.