ARTICLE
Acellular Pertussis Vaccine Booster Combined With
Diphtheria and Tetanus Toxoids for Adolescents
Michael E. Pichichero, MDa, Mark M. Blatter, MDb, William A. Kennedy, MDc, James Hedrick, MDd, Dominique Descamps, MDe, Leonard R. Friedland, MDfaUniversity of Rochester Medical Center, Rochester, New York;bPrimary Physicians Research, Pittsburgh, Pennsylvania;cUniversity of California Los Angeles Center for
Vaccine Research, Los Angeles Biomedical Research Institute at Harbor-University of California Los Angeles Medical Center, Torrance, California;dKentucky Pediatric
Research, Inc, Bardstown, Kentucky;eGlaxoSmithKline, Rixensart, Belgium;fGlaxoSmithKline, King of Prussia, Pennsylvania
Financial Disclosure: Drs Pichichero and Blatter report having served as a paid consultant or speaker for GlaxoSmithKline and Sanofi-Aventis. Drs Descamps and Friedland are employees of GlaxoSmithKline and report ownership of equity or stock options.
ABSTRACT
BACKGROUND.The incidence of pertussis is increasing, especially in adolescents,
at-tributed in part to waning of immunity after childhood immunization. Recently licensed in the United States for use in adolescents, acellular pertussis vaccines will provide an immunogenic and safe option for booster immunization against per-tussis.
METHODS.This prospective, randomized, observer-blinded, multicenter, comparative
study evaluated the safety and immunogenicity of a vaccine formulated with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis antigens (Tdap) compared with tetanus and diphtheria toxoids vaccine (Td) for booster immuni-zation in adolescents. There were 4114 healthy adolescents aged 10 to 18 years who completed childhood vaccination against diphtheria, tetanus, and pertussis who were enrolled, randomized, and received study vaccine.
RESULTS.Local and general symptoms were comparable between the Tdap and Td
groups. The immune response of Tdap was comparable with Td vaccine for tetanus and diphtheria seroprotection and booster responses. In addition, geometric mean concentrations of antibody to pertussis antigens, pertussis toxoid, filamentous hemagglutinin, and pertactin exceeded the antibody response elicited after infant immunization with diphtheria and tetanus toxoids and acellular pertussis antigens (DTaP) that had proven efficacy against pertussis.
CONCLUSIONS.In adolescents, the studied Tdap was safe and immunogenic and
in-duced pertussis antibodies that were higher than those associated with efficacy in infants.
www.pediatrics.org/cgi/doi/10.1542/ peds.2005-1759
doi:10.1542/peds.2005-1759
All of the authors had input into study design and participated in the trial, had access to study data, and were involved with article preparation. An independent analysis of the complete clinical study report was performed by the biometrics group at Kendle International Inc, Munich, Germany. This clinical trial was registered at ClinicalTrials.gov under registration number NCT00109330.
Key Words
pertussis, diphtheria, tetanus, vaccine, adolescents
Abbreviations
Tdap—tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine DTaP— diphtheria and tetanus toxoids and acellular pertussis vaccine DTP— diphtheria and tetanus toxoid and pertussis vaccine
Td—tetanus and diphtheria toxoids vaccine
Lf—limit of flocculation PT—pertussis toxoid
FHA—filamentous hemagglutinin PRN—pertactin
ELISA— enzyme-linked immunosorbent assay
EL.U.— enzyme-linked immunosorbent assay units
CI— confidence interval ATP—according to protocol GMC— geometric mean concentration DTwP— diphtheria and tetanus toxoids and whole-cell pertussis vaccine
Accepted for publication Sep 14, 2005
Address correspondence to Michael E. Pichichero, MD, University of Rochester Medical Center, 601 Elmwood Ave, Box 672, Rochester, NY 14642. E-mail:
R
EPORTED PERTUSSIS INCIDENCEin the United States increased from 1010 cases in 1976 to 25 827 cases in 2004.1,2 The increase primarily occurred in infants aged⬍6 months and thoseⱖ10 years, with most cases in adolescents.2–5 Recent prospective, population-based studies of cough illness in adolescents and adults esti-mated the incidence of pertussis in the United States to be much higher than reported, possibly⬎1 million cases per year.6,7Increased disease recognition, improved diagnostic techniques, and active surveillance have contributed to the reported increase in pertussis among adolescents and adults. There is less opportunity for natural pertussis boosting because of widespread childhood immuniza-tion and waning vaccine-induced immunity within 5 to 10 years.1,4,8,9 Pertussis can cause significant morbidity, impact quality of life, and incur substantial costs in time and money.9–14Health economists and public health of-ficials indicate that pertussis immunization of adoles-cents would be beneficial and cost-effective.15–18
Acellular pertussis vaccines are already licensed out-side the United States for older individuals, and several countries recommend routine adolescent vaccination.19 In May 2005, a vaccine consisting of acellular pertussis antigens combined with tetanus toxoid and reduced diphtheria toxoid (Tdap; Boostrix, GlaxoSmithKline Bio-logicals, Rixensart, Belgium) was licensed in the United States for use in adolescents. In June 2005, a second vaccine (Aventis Pasteur Limited, Toronto, Ontario, Canada) also consisting of acellular pertussis antigens combined with tetanus toxoid and reduced diphtheria toxoid was licensed for use in the United States. This article describes the pivotal US safety and immunogenic-ity trial that led to licensure of the Boostrix Tdap vaccine for booster immunization in adolescents. The studied adolescent Tdap vaccine composition was based on a US-licensed pediatric diphtheria, tetanus, and acellular pertussis vaccine (DTaP)20,21licensed in the United States in 1997, except with reduced amounts of diphtheria, tetanus, and pertussis antigens to minimize reactogenic-ity.
METHODS
Population
The study occurred from November 2002 to December 2003 at 45 US centers after institutional review board approval. Healthy adolescents 10 to 18 years of age who completed routine childhood vaccination against diph-theria, tetanus, and pertussis were enrolled. Written in-formed assent and/or consent was obtained. Adolescents were ineligible if any of the following were present: diphtheria and tetanus toxoid and pertussis vaccine (DTP) or tetanus and diphtheria toxoids vaccine (Td) within 5 or 10 years, respectively, pertussis disease or household exposure to pertussis within 5 years, immune
dysfunction, hypersensitivity to vaccine components, or DTP contraindication.
Study Design
The study was prospective, randomized, observer-blinded, multicenter, and comparative with eligible adolescents receiving 1 of 3 lots of Tdap (to test for manufacturing consistency) or Td in a 1:1:1:1 ratio. A sample size of 3600 adolescents was estimated to be needed for statis-tical comparisons. Adolescents were stratified such that 75% were 10 to 14 years old, and 25% were 15 to 18 years old. Investigators used a central randomization call-in system on the Internet followed by a randomiza-tion blocking scheme (1:1:1:1 ratio) to ensure balance among treatment groups. The primary objectives were evaluated by predefined noninferiority criteria, a statis-tical method commonly used when a standard of care exists and when the objective is to show that the new treatment is clinically as good as the standard. The power to meet evaluation criteria for each primary ob-jective of the study was⬎99%. Because of vaccine prep-aration differences (ie, single versus multiple dose vials), the individual responsible for vaccine preparation and administration was unblinded. All of the other partici-pants were blinded throughout. Adherence to protocol requirements and verification of accurate data genera-tion were achieved through monitoring visits to all of the investigator sites by the sponsor.
Study Vaccines
A single 0.5-mL dose was administered by intramuscular injection in the deltoid muscle of the nondominant arm. Tdap was supplied as single-dose vials containing 2.5 limit of flocculation (Lf) of diphtheria toxoid, 5 Lf of tetanus toxoid, 8 g pertussis toxoid (PT), 8g of fila-mentous hemagglutinin (FHA), 2.5 g of pertactin (PRN), and 0.3 mg of aluminum and was free of thimer-osal and other preservatives. The US-licensed Td vaccine (Massachusetts Public Health Biological Laboratories, Ja-maica Plain, MA) was supplied in multidose vials and contained 2.0 Lf of diphtheria toxoid, 2.0 Lf of tetanus toxoid, 0.45 mg of aluminum, and thimerosal as preser-vative.
Safety Evaluation
Data on solicited local and general adverse events were collected by the adolescents and their parents/guardians using standardized diaries for 15 consecutive days after vaccination. Local adverse events included pain, redness, and swelling at the injection site and measurement of mid upper–arm circumference. The adolescents were observed for 30 minutes after vaccination and instructed to contact study personnel immediately if they experi-enced a large injection-site swelling reaction (swelling
in-terfering with or preventing normal activities). General symptoms evaluated included temperature, headache, fatigue, and nonspecific gastrointestinal events (see Ta-ble 2 for intensity grading scales). Unsolicited adverse events occurring within 1 month of vaccination were recorded. The adolescents also were monitored for an additional 5-month period for nonroutine medical visits, visits to an emergency department, onset of new chronic illness, and serious adverse events.
Serologic Evaluations
Blood samples were obtained before and 1 month after vaccination. Standardized enzyme-linked immunosor-bent assays (ELISAs) were used to assess antibody con-centrations to diphtheria and tetanus toxoids, PT, FHA, and PRN. For both anti-diphtheria and anti-tetanus ELISA assays, antibody concentration of 0.1 IU/mL was the lowest quantifiable protective concentration.22,23 Antibody concentrations to each pertussis antigen of
ⱖ5 ELISA units (EL.U.)/mL were prespecified to indicate seropositivity. Booster response definitions (antibody con-centrations 1 month after vaccination relative to pre-vaccination concentrations) to diphtheria, tetanus, and pertussis antigens are provided in Table 3.
Statistical Analysis
Primary safety analyses were based on the vaccinated cohort. The percentage of adolescents experiencing so-licited symptoms within 72 hours or 15 days after vac-cination or unsolicited symptoms during 31 days of fol-low-up, and 2-sided exact 95% confidence intervals (CIs) were computed by symptom and intensity. Differ-ences between groups were compared using 2-sided Fisher’s exact test and quantified using standardized asymptotic 95% CIs. A 2-sidedP⬍.05 was considered significant. According to prespecified criteria, Tdap was noninferior to Td if the upper limit of the 2-sided 95% CI on the difference in incidence of grade 3 pain for Tdap⫺ Td wasⱕ4%.
Primary immunogenicity analyses were based on the according-to-protocol (ATP) cohort, which included ad-olescents who met eligibility criteria, complied with the protocol, and had antibody results for ⱖ1 antigen. Pre-specified exclusions from the ATP cohort were made by individuals blinded to randomization. Geometric mean concentrations (GMCs) of antibody were calculated for each antigen. The percentages of adolescents with anti-diphtheria and anti-tetanus concentrationsⱖ0.1 IU/mL (ie, seroprotection rate) andⱖ1.0 IU/mL; seropositivity rates to each pertussis antigen; and booster responses to diphtheria, tetanus, and pertussis antigens were calcu-lated with 95% CI. Standardized asymptotic 95% CIs for group differences in anti-diphtheria and anti-tetanus se-roprotection rates, antibody concentrationsⱖ1.0 IU/mL, and booster response rates were calculated. According to prespecified criteria, Tdap was noninferior to Td if the
upper limit of the 2-sided 95% CI on the difference for Td⫺ Tdap wasⱕ10% for both diphtheria and tetanus seroprotection rates and booster responses.
Given the absence of recognized serologic correlates of protection against pertussis, the efficacy of a vaccine against pertussis as demonstrated in infants can be extrapolated to a booster pertussis vaccine in an older age group.24 Therefore, GMCs to PT, FHA, and PRN in the Tdap group were compared with GMCs from a study in which infants received 3 doses of DTaP vaccine (Infanrix, GlaxoSmithKline Biologicals, Rixensart, Bel-gium)25 with similar composition, albeit higher antigen concentrations, and in which efficacy against World Health Organization-defined pertussis was 88.7%.21 Comparison was made through computation of the 95% CI of the GMC ratio between adolescents who received Tdap and infants who received DTaP. According to prespecified cri-teria, noninferiority was met if the upper limit of the 95% CI for the GMC ratio of DTaP over Tdap for each antigen was⬍1.5.
Limited immunogenicity data were available before trial initiation on the control Td vaccine. Therefore, 1 planned interim analysis was performed after the first 400 adolescents were enrolled to confirm the feasibility of the projected sample size to demonstrate noninferior-ity of Tdap to Td. The analysis was conducted by indi-viduals blinded to randomization, and “stopping rules” were prespecified.
All of the analyses and summaries were performed by using SAS 8 (SAS Institute, Inc, Cary, NC). The study was designed and data collected and analyzed by Glaxo-SmithKline in coordination with the principal investiga-tor who had access to the data and attests to the accuracy of the data and data analysis.
RESULTS
Study Population
Safety
Solicited Local Symptoms
Injection-site pain was the most frequently reported lo-cal symptom in both groups at both reporting time frames (Table 2). Any pain was reported significantly more often with Tdap than with Td within 72 hours (75.0% vs 71.4%; P ⫽ .02) and 15 days (75.3% vs 71.7%;P⫽.02) after vaccination, respectively. In addi-tion, grade 2 or 3 pain was reported significantly more often with Tdap than with Td within 72 hours (50.7% vs 42.2%; P⬍ .001) and 15 days (51.2% vs 42.5%;P ⬍ .001) after vaccination. The incidence of the primary safety end point of grade 3 pain was low (⬍5%) and not significantly different between groups (within the limits for noninferiority) at both time frames. There were no significant differences between groups with respect to injection-site redness, swelling, or increase in mid up-per–arm circumference. One adolescent in each group reported an episode of large injection-site swelling 3 days after vaccination, which did not involve a large increase in mid upper–arm circumference or the elbow or shoulder and resolved without sequelae.
Solicited General Symptoms
In both groups, the most frequently reported solicited general symptoms within 72 hours and 15 days after vaccination were headache and fatigue (Table 2). There was no significant difference between groups for general symptoms during both follow-up periods, with the
ex-ception of a significantly greater rate of grade 2 or 3 headache in the Tdap group within 15 days (15.7% vs 12.7%; P ⫽ .02). The incidence of grade 3 solicited general symptoms was low (⬍4% for each group).
The incidence of adverse events in both groups when analyzed by age (10 –14 and 15–18 years), gender, or race was consistent with those of the vaccinated cohort. In adolescents who previously received multiple consec-utive DTaP doses, there was no consistent trend toward increased reactogenicity after Tdap administration.
Unsolicited Symptoms
There were 771 of 3034 adolescents (25.4%) in the Tdap group and 248 of 1013 adolescents (24.5%) in the Td group who reportedⱖ1 unsolicited symptom within 1 month postvaccination. The most commonly reported events were pharyngitis and upper respiratory tract infec-tion. No serious adverse events were reported in either group during this period. At least 97% of the 4114 vac-cinated adolescents completed the additional 5-month safety follow-up evaluation (Tdap, n ⫽ 3005; Td, n ⫽ 1003). During the extended safety follow-up period, the percentages of adolescents reporting a serious adverse event, new onset of a chronic illness, or an adverse event that led to an emergency department or nonroutine office visit were similar and not statistically significantly different in the Tdap and Td groups. Fourteen of the 3005 (0.5%) adolescents in the Tdap group and 2 of the 1003 (0.2%) adolescents in the Td group reported a
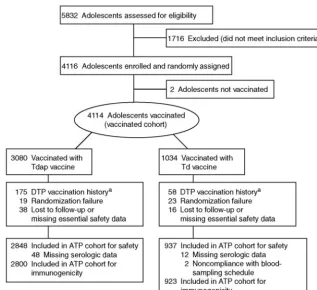
FIGURE 1
Disposition of adolescents enrolled.aIncorrect number of doses (⬍4 or⬎5),⬍5-year interval between previous DTP vaccination and study vaccine, or adolescent was
serious adverse event during the extended safety fol-low-up period (P⫽.4). All of the serious adverse events were judged by the investigators to be unrelated to vac-cination, and none were of potential autoimmune origin or new onset and chronic in nature.
Immunogenicity
Consistency of the 3 manufacturing lots of Tdap in terms of immunogenicity for all 5 of the antigens was demon-strated (data not shown).
Response to Diphtheria and Tetanus Toxoids
Noninferiority of Tdap compared with Td was demon-strated for anti-diphtheria and anti-tetanus seropro-tective rates and booster responses. GMC responses to
diphtheria and tetanus were statistically significantly greater in the Td group because the 95% CI of the GMC ratios for Td over Tdap were above and excluded 1.0 (Table 3). One month after vaccination, seroprotective antibody concentrations (ⱖ0.1 IU/mL) against diphthe-ria and tetanus were achieved by 99.9% and 100% of adolescents in both groups, respectively. Booster response rates to diphtheria were 90.6% with Tdap and 95.9% with Td. Among adolescents seronegative before vaccination, 97.2% and 100%, respectively, in the Tdap and Td groups demonstrated a booster response to diphtheria. Booster response rates to tetanus were 89.7% with Tdap and 92.5% with Td. In both groups, all of the adolescents seronegative before vaccination had a booster response to tetanus.
Response to Pertussis
For all 3 of the pertussis antigens, seropositivity rates 1 month after Tdap vaccination wereⱖ98.9% (Table 3). Tdap elicited large increases in anti-PT, anti-FHA, and anti-PRN GMCs. The lower limit of the exact 2-sided 95% CI in the percentage of adolescents with a booster response was ⱖ83.0% for each pertussis antigen, ex-ceeding the predefined criterion of a lower limit of 80% needed to demonstrate a booster response.
Antibody concentrations achieved after Tdap vaccina-tion were higher than those achieved after a 3-dose primary series with infant DTaP where efficacy was demonstrated previously (Table 4).21,25Noninferiority of Tdap compared with DTaP was demonstrated for the DTaP over Tdap GMC ratio for each antigen.
DISCUSSION
An adolescent Tdap vaccine administered as a single-dose booster to 10- to 18-year-olds was demonstrated to be safe and immunogenic compared with Td vaccine. The Tdap vaccine had a comparable safety profile to that of Td vaccine when administered to adolescents. A higher incidence of any and grade 2 or 3 injection-site pain was reported in the Tdap group compared with Td, which may relate in part to the 3 additional antigens contained in Tdap versus Td. A significant difference in grade 2 or 3 headache was observed within 15 days, but not within 4 days, after vaccination with Tdap versus Td. The significant difference in headache incidence within 15 days after vaccination is only likely to be because of chance alone and not to be clinically relevant. Signifi-cant differences between groups for other solicited local or general symptoms were absent. No serious adverse events were reported with either vaccine within 1 month after vaccination. The Tdap sample size allowed for the detection of any adverse event occurring at a rate of⬎0.1% (␣⫽5%).
Large injection-site swelling reactions have been de-scribed after booster doses of DTaP, diphtheria and
tet-TABLE 1 Baseline Characteristics of Adolescents in the Vaccinated Cohort
Characteristic Tdap Vaccine
(n⫽3080)
Td Vaccine (n⫽1034)
Age
Mean, y 12.9 12.9
9–14 y, % 75.9 72.9
15–18 y, % 24.1 27.1
Gender, %
Male 51.6 53.6
Female 48.4 46.4
Race, %
White 85.8 85.4
Black 5.7 5.4
Hispanic 5.6 6.0
Other 2.9 3.2
Time since last DT-containing vaccine, %
⬍5 y 1.4 1.7
5–10 y 86.3 85.3
⬎10 y 11.7 12.6
Unknown 0.6 0.4
Number of previous DT-containing doses, %
⬍4 0.6 0.6
4 6.1 6.0
5 91.8 92.1
⬎5 0.8 0.9
Unspecified 0.6 0.4
Type of previous DTP vaccine, %
0 DTaP, 5 DTwP 13.9 15.7
1 DTaP, 4 DTwP 9.9 9.4
2 DTaP, 3 DTwP 3.5 3.9
Unspecified 72.8 71.0
Prevaccination antibody concentration Anti-D
N 2466 814
ⱖ0.1 IU/mL, % 85.8 84.8
ⱖ1.0 IU/mL, % 17.1 19.5
GMC, IU/mL 0.34 0.34
Anti-T
N 2471 817
ⱖ0.1 IU/mL, % 97.7 96.8
ⱖ1.0 IU/mL, % 36.8 39.9
GMC, IU/mL 0.74 0.73
anus toxoid vaccine, DTwP, and Td vaccines.26–28In this study, 1 adolescent in each group reported large injec-tion-site swelling. Most adolescents in this study are assumed to have received DTwP for their primary im-munization series, because DTaP was not available until 1996. More data are needed on Tdap safety in adoles-cents who received only DTaP, although a sizable cohort will be not available in the United States until at least 2007.
Noninferiority of Tdap compared with Td was dem-onstrated for anti-diphtheria and anti-tetanus seropro-tective rates and booster responses. There were no pre-defined criteria for noninferiority of diphtheria and tetanus GMCs. Although the GMC responses were sig-nificantly greater in the Td group, the differences are
unlikely to be clinically relevant because the antibody concentrations with Tdap exceeded the cutoff for sero-protection by 10-fold (ⱖ1.0 IU/mL) in 97.3% of adoles-cents for diphtheria and in 99.5% for tetanus, and diph-theria and tetanus antibody concentrationsⱖ1.0 IU/mL are associated with long-term protection (ⱖ5–10 years) against diphtheria and tetanus disease.22,23,29,30Both the Tdap and Td vaccines investigated in this study will provide adolescents with long-term protection against diphtheria and tetanus disease.
The pertussis antibody response after Tdap was not inferior to, and in fact exceeded, that after DTaP in infants.21,25Therefore, 1 booster dose of Tdap should be at least as efficacious as infant DTaP in preventing per-tussis.
TABLE 2 Overall Incidence of Solicited Local and General Symptoms Reported Within the 72-Hour and 15-Day Postvaccination Periods With Tdap or Td Vaccine (Vaccinated Cohort)
Symptom and Intensity 72-h Postvaccination 15-d Postvaccination
Tdap Vaccine,
n(%)
Td Vaccine,
n(%)
P Tdap Vaccine,
n(%)
Td Vaccine,
n(%)
P
Local symptoms (n⫽3032) (n⫽1013) (n⫽3032) (n⫽1013),
Paina
Any 2273 (75.0) 723 (71.4) .03 2284 (75.3) 726 (71.7) .02
Grade 2 or 3 1538 (50.7) 427 (42.2) ⬍.001 1553 (51.2) 431 (42.5) ⬍.001
Grade 3b 135 (4.5) 37 (3.7) .32 139 (4.6) 41 (4.0) .54
Redness
Any 664 (21.9) 198 (19.5) .12 681 (22.5) 201 (19.8) .09
⬎20 mm 122 (4.0) 38 (3.8) .78 125 (4.1) 40 (3.9) .86
⬎50 mm 49 (1.6) 15 (1.5) .89 51 (1.7) 16 (1.6) .89
Swelling
Any 613 (20.2) 201 (19.8) .82 641 (21.1) 204 (20.1) .53
⬎20 mm 153 (5.0) 49 (4.8) .87 160 (5.3) 50 (4.9) .74
⬎50 mm 72 (2.4) 32 (3.2) .17 75 (2.5) 32 (3.2) .26
Vaccinated mid upper–arm circumference increase
⬎5 mm 648 (21.4) 235 (23.2) .24 858 (28.3) 299 (29.5) .47
⬎20 mm 47 (1.6) 15 (1.5) 1.0 60 (2.0) 22 (2.2) .70
⬎40 mm 10 (0.3) 3 (0.3) 1.0 14 (0.5) 3 (0.3) .59
General symptoms (n⫽3030) (n⫽1013) (n⫽3030) (n⫽1013)
Fever, °Fc
⬎99.5 196 (6.5) 55 (5.4) .26 406 (13.4) 133 (13.1) .87
⬎100.4 54 (1.8) 15 (1.5) .58 153 (5.0) 48 (4.7) .74
⬎102.2 11 (0.4) 3 (0.3) 1.0 41 (1.4) 10 (1.0) .42
Headachea
Any 943 (31.1) 313 (30.9) .91 1305 (43.1) 420 (41.5) .38
Grade 2 or 3 235 (7.8) 69 (6.8) .34 477 (15.7) 129 (12.7) .02
Grade 3 42 (1.4) 12 (1.2) .75 112 (3.7) 27 (2.7) .14
Fatiguea
Any 906 (29.9) 310 (30.6) .69 1121 (37.0) 371 (36.7) .85
Grade 2 or 3 267 (8.8) 87 (8.6) .89 437 (14.4) 131 (12.9) .25
Grade 3 48 (1.6) 19 (1.9) .57 111 (3.7) 32 (3.2) .49
Gastrointestinal symptomsa,d
Any 516 (17.0) 176 (17.4) .81 789 (26.0) 261 (25.8) .90
Grade 2 or 3 148 (4.9) 50 (4.9) .93 298 (9.8) 98 (9.7) .90
Grade 3 40 (1.3) 16 (1.6) .54 90 (3.0) 32 (3.2) .75
“Any” indicates any report of the specified event regardless of intensity.
aGrade 2⫽local: painful when limb was moved; general: interfering with normal activity; grade 3⫽local: spontaneously painful and/or preventing normal activity; general: preventing normal
activity.
bGrade 3 injection-site pain within the 15-day postvaccination period after Tdap was noninferior to Td (upper limit of 2-sided 95% CI for the difference in the percentage of subjectsⱕ4%). cOral or axillary temperature.
A limitation of this study relates to persistence of antibody and longer-term protection (eg, 5–10 years) against diphtheria, tetanus, and pertussis. Although such data are not available with this Tdap vaccine, 1 study demonstrated sustained pertussis efficacy toⱖ6 years of age after primary immunization with DTaP.31Although a serologic correlate of protection for pertussis has not been established, the pertussis antibody responses in the adolescents after a single dose of Tdap exceeded those in infants after a 3-dose primary vaccination series with DTaP. The protective efficacy for pertussis infection of Tdap given to adolescents will only really be known
based on postmarketing studies. A similar Tdap vaccine with the identical antigens and antigen quantities as the Tdap studied in this clinical trial (marketed outside the United States) given to adolescents and adults sustained seroprotection against diphtheria and tetanus and sero-positivity of PT, FHA, and PRN antibodies for 36 months, and all of the antibody concentrations remained higher than prevaccination levels.32,33
Acellular pertussis vaccines without tetanus and diphtheria have been studied in clinical trials in the United States.7 The pertussis vaccine evaluated in this trial will only be available as a combination Tdap booster
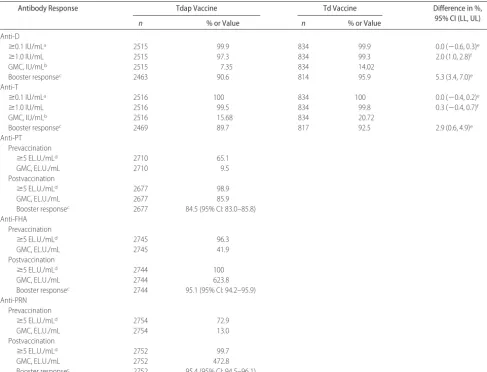
TABLE 3 Antibody Responses to Diphtheria and Tetanus Toxoids and Pertussis Antigens After Vaccination With Tdap or Td (ATP Cohort for Immunogenicity)
Antibody Response Tdap Vaccine Td Vaccine Difference in %,
95% CI (LL, UL)
n % or Value n % or Value
Anti-D
ⱖ0.1 IU/mLa 2515 99.9 834 99.9 0.0 (⫺0.6, 0.3)e
ⱖ1.0 IU/mL 2515 97.3 834 99.3 2.0 (1.0, 2.8)f
GMC, IU/mLb 2515 7.35 834 14.02
Booster responsec 2463 90.6 814 95.9 5.3 (3.4, 7.0)e
Anti-T
ⱖ0.1 IU/mLa 2516 100 834 100 0.0 (⫺0.4, 0.2)e
ⱖ1.0 IU/mL 2516 99.5 834 99.8 0.3 (⫺0.4, 0.7)f
GMC, IU/mLb 2516 15.68 834 20.72
Booster responsec 2469 89.7 817 92.5 2.9 (0.6, 4.9)e
Anti-PT Prevaccination
ⱖ5 EL.U./mLd 2710 65.1
GMC, EL.U./mL 2710 9.5
Postvaccination
ⱖ5 EL.U./mLd 2677 98.9
GMC, EL.U./mL 2677 85.9
Booster responsec 2677 84.5 (95% CI: 83.0–85.8)
Anti-FHA Prevaccination
ⱖ5 EL.U./mLd 2745 96.3
GMC, EL.U./mL 2745 41.9
Postvaccination
ⱖ5 EL.U./mLd 2744 100
GMC, EL.U./mL 2744 623.8
Booster responsec 2744 95.1 (95% CI: 94.2–95.9)
Anti-PRN Prevaccination
ⱖ5 EL.U./mLd 2754 72.9
GMC, EL.U./mL 2754 13.0
Postvaccination
ⱖ5 EL.U./mLd 2752 99.7
GMC, EL.U./mL 2752 472.8
Booster responsec 2752 95.4 (95% CI: 94.5–96.1)
Anti-D indicates anti-diphtheria toxoid; anti-FHA, anti-filamentous hemagglutinin; anti-PT, anti-pertussis toxoid; anti-T, anti-tetanus toxoid; LL, lower limit; UL, upper limit.
aAnti-D and anti-T antibody concentrationsⱖ0.1 IU/mL indicated seroprotection.
bGMC ratio (Td GMC divided by Tdap GMC), 95% CI (LL, UL) for anti-D was 1.9 (1.8, 2.0) and for anti-T was 1.3 (1.2, 1.4).
cBooster response for diphtheria and tetanus defined as a postvaccination antibody concentrationⱖ0.4 IU/mL in initially seronegative adolescents (antibody concentrations⬍0.1 IU/mL) or a
postvaccination increase ofⱖ4 times the prevaccination antibody concentration for initially seropositive adolescents. Booster response for PT, FHA, and PRN defined as an antibody concentration
ⱖ20 EL.U./mL in adolescents who were seronegative (antibody concentrations⬍5.0 EL.U./mL) before vaccination or at least a fourfold increased antibody concentration in adolescents who were seropositive with prevaccination antibody concentrationsⱖ5.0 EL.U./mL and⬍20 EL.U./mL or at least a twofold increased antibody concentration in adolescents who were seropositive with prevaccination antibody concentrationsⱖ20 EL.U./mL.
dAnti-PT, anti-FHA, and anti-PRN antibody concentrationsⱖ5.0 EL.U./mL indicated seropositivity.
for the benefit of protection against diphtheria, tetanus, and pertussis in a single injection. In the United States, the current focus of vaccine manufacturers is on combi-nation Tdap vaccines, which, if administered at the rec-ommended routine 11- to 12-year-old adolescent assess-ment,34 will not require an additional office visit. Furthermore, use of Tdap vaccine may reduce circulat-ingBordetella pertussisin the general population and re-duce the likelihood that susceptible persons in the com-munity will become infected.35The Tdap vaccine studied in this clinical trial, as well as a different Tdap vaccine were both recently licensed in the United States for use in adolescents. Although there is no head-to-head clin-ical trial comparing the 2 vaccines, both appear to be safe and immunogenic.36 In June 2005, the Advisory Com-mittee on Immunization Practices recommended that adolescents 11 or 12 years of age receive Tdap in place of the Td booster vaccine currently being given to adoles-cents. The committee also recommended that Tdap be given to adolescents 13 to 18 years of age who did not receive a Td booster at 11 or 12 years of age and encour-aged provision of Tdap to adolescents 11 to 18 years old who have already been vaccinated with Td to further protect them against pertussis.37The Boostrix Tdap vac-cine studied in this clinical trial will help reduce the number of cases of pertussis among adolescents.
ACKNOWLEDGMENTS
The clinical trial was funded by GlaxoSmithKline (iden-tifying code is 776423/001), including design and con-duct of the study, collection, management, analysis, and interpretation of the data, and preparation and review of the article. Dr Pichichero received research grant support from GlaxoSmithKline and Sanofi-Aventis. Dr Kennedy received grant support from the National Institutes of Health, the International Vaccine Institute, and Glaxo-SmithKline.
In addition to the authors, the following investigators participated in this clinical trial: Southern California Kai-ser Permanente Medical Group, Los Angeles, CA: V. Wong, M. Quan, E. Curry; Pediatric Health Associates/
Hunnewell Ground Children’s Hospital, Boston, MA: H. Bernstein; Gray Station Primary Care, Gray, TN: S. Combs; Children’s Hospital Medical Center of Akron, Akron, OH: B. Congeni; DiscoveResearch, Inc, Bryan, TX: A. Damian; Arkansas Children’s Hospital, Little Rock, AR: J. Elser; Tempe Primary Care Associates, PC, Tempe, AZ: T. Fiel; Scott and White Clinic, Temple, TX: M. Gaglani; Norwich Pediatric Group, PC, Norwich, CT: R. Geller; St Joseph Heritage Healthcare, Fullerton, CA: J. Gilbert, Jr; Middlesex Family Physicians/Pro Health Physicians PC, Middletown, CT: M. Good; Boulder Clin-ical Research, Inc, Boulder, CO: K. Graff; Children’s Hospital of Pittsburgh, Pittsburgh, PA (at time of study): D. Greenberg; New West Physicians, Golden, CO: D. Grosser; Radiant Research, Austin, TX: J. Guerrero; Foothill Family Clinic, Salt Lake City, UT: D. Henry; Whitehouse Station Family Medicine, Whitehouse Sta-tion, NJ: A. Kelsey; Olentangy Pediatrics, Columbus, OH: K. Koranyi; MetroHealth Medical Center, Cleveland, OH: M. Kumar; Woburn Pediatric Associates, Woburn, MA: J. Leader; 1stAllergy and Clinical Research Center, Centennial, CO : I. Melamed; Center for Pediatric Re-search, Norfolk, VA: D. Mitchell; Baylor College of Med-icine and Affiliated Hospitals, Houston, TX: F. Mun˜oz; University Hospital, Stony Brook, NY: S. Nachman; Cap-itol Pediatric and Adolescent Center, Raleigh, NC: M. Ogle; Milford Emergency Associates, Milford, MA: A. Puopolo; Shands Jacksonville, Jacksonville, FL: M. Rathore; Creighton University, Omaha, NE (at time of study): J. Romero; Pennridge Pediatric Associates, Sell-ersville, PA: E. Rothstein; The Children’s Clinic of Jones-boro, JonesJones-boro, AR: K. Rouse; Dr Senders and Associ-ates, University Heights, OH: S. Senders; St Jude Heritage Medical Group, Yorba Linda, CA: T. Schmidt; Peninsula Research Associates, Rolling Hills Estates, CA: L. Sher; Clinic of Physicians and Surgeons, Ltd, Mesa, AZ: G. Shockey; Edinger Medical Group, Fountain Val-ley, CA: M. Sperling; Roslindale Pediatric Associates, PC, Boston, MA: R. Stacks; Sylva Pediatrics, Sylva, NC: C. Toledo; Duke Children’s Primary Care, Durham, NC: E. Walter; J. Lewis Research, Inc, West Jordan, UT: R.
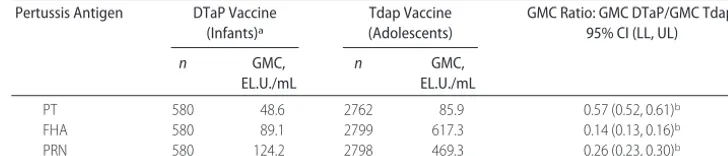
TABLE 4 Comparison of Pertussis Immune Responses 1 Month After a Single Dose of Tdap Vaccine in Adolescents With Responses 1 Month After Completion of a 3-Dose Primary Immunization Series With DTaP Vaccine in Infants (ATP Cohort for Immunogenicity)
Pertussis Antigen DTaP Vaccine
(Infants)a
Tdap Vaccine (Adolescents)
GMC Ratio: GMC DTaP/GMC Tdap, 95% CI (LL, UL)
n GMC,
EL.U./mL
n GMC,
EL.U./mL
PT 580 48.6 2762 85.9 0.57 (0.52, 0.61)b
FHA 580 89.1 2799 617.3 0.14 (0.13, 0.16)b
PRN 580 124.2 2798 469.3 0.26 (0.23, 0.30)b
LL indicates lower limit; UL, upper limit.
aSchmitt et al.25
Watson; Children’s Memorial Hospital, Chicago, IL: R. Yogev.
Assistance with the article was provided by Una Kist-ner, RPh (Scientific Therapeutics Information, Inc, Springfield, NJ), and Merry Saba, PharmD (Sparta, NJ). Assistance with data collection, analysis, and reporting was provided by Alix Collard (GlaxoSmithKline, Rixen-sart, Belgium), Isabelle Maviglia (GlaxoSmithKline, Rix-ensart, Belgium), Yaela Baine (GlaxoSmithKline, King of Prussia, PA), and Diane Sullivan (GlaxoSmithKline, King of Prussia, PA).
REFERENCES
1. Centers for Disease Control and Prevention. Pertussis: United States, 1997–2000. MMWR Morb Mortal Wkly Rep. 2002;51: 73–76
2. Centers for Disease Control and Prevention.Pertussis Surveil-lance Report: 8/12/05(final data). Atlanta, GA: Bacterial Vaccine Preventable Diseases Branch, National Immunization Program, Centers for Disease Control and Prevention; 2005
3. Gu¨ris˛ D, Strebel PM, Bardenheier B, et al. Changing epidemi-ology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990 –1996.Clin Infect Dis.1999;28:1230 –1237
4. Yih WK, Lett SM, des Vignes FN, Garrison KM, Sipe PL, March-ant CD. The increasing incidence of pertussis in Massachusetts adolescents and adults, 1989 –1998. J Infect Dis. 2000;182: 1409 –1416
5. Sotir MJ, Cappazzo DL, Schmidt CE, et al. A resource and labor intensive county-wide outbreak of pertussis, Wisconsin, 2003: high impact on the adolescent population (abstract). Presented at: the 39th National Immunization Conference; March 21–24, 2005; Washington, DC
6. Strebel P, Nordin J, Edwards K, et al. Population-based inci-dence of pertussis among adolescents and adults, Minnesota, 1995–1996.J Infect Dis.2001;183:1353–1359
7. Ward JI, Cherry JD, Chang S-J, et al, for the APERT Study Group. Efficacy of acellular pertussis vaccine among adoles-cents and adults.N Engl J Med. 2005;353:1555–1563 8. Wirsing von Ko¨nig CH, Postels-Multani S, Bogaerts H, et al.
Factors influencing the spread of pertussis in households.Eur J Pediatr.1998;157:391–394
9. Aoyama T, Harashima M, Nishimura K, Saito Y. Outbreak of pertussis in highly immunized adolescents and its secondary spread to their families.Acta Paediatr Jpn.1995;37:321–324 10. Senzilet LD, Halperin SA, Spika JS, Alagaratnam M, Morris A,
Smith B, and the Sentinel Health Unit Surveillance System Pertussis Working Group. Pertussis is a frequent cause of pro-longed cough illness in adults and adolescents.Clin Infect Dis.
2001;32:1691–1697
11. Hewlett EL, Edwards KM. Pertussis: not just for kids.N Engl J Med.2005;352:1215–1222
12. Lee GM, Lett S, Schauer S, et al. Societal costs and morbidity of pertussis in adolescents and adults. Clin Infect Dis. 2004;39: 1572–1580
13. Lee LH, Pichichero ME. Costs of illness due toBordetella pertussis
in families.Arch Fam Med.2000;9:989 –996
14. Pichichero ME, Treanor J. Economic impact of pertussis.Arch Pediatr Adolesc Med.1997;151:35– 40
15. Caro JJ, Getsios D, El-Hadi W, Payne K, O’Brien JA. Pertussis immunization of adolescents in the United States: an economic evaluation.Pediatr Infect Dis J.2005;24:S75–S82
16. Purdy KW, Hay JW, Botteman MF, Ward JI. Evaluation
strat-egies for use of acellular pertussis vaccine in adolescents and adults: a cost-benefit analysis.Clin Infect Dis.2004;39:20 –28 17. Wharton M. Prevention of pertussis among adolescents by
vaccination: taking action on what we know and acknowledg-ing what we do not know [editorial].Clin Infect Dis.2004;39: 29 –30
18. Lee GM, LeBaron C, Murphy TV, Lett S, Schauer S, Lieu TA. Pertussis in adolescents and adults: should we vaccinate? Pedi-atrics.2005;115:1675–1684
19. Forsyth KD, Campins-Marti M, Caro J, et al, for the Global Pertussis Initiative. New pertussis vaccination strategies be-yond infancy: recommendations by the Global Pertussis Initia-tive.Clin Infect Dis.2004;39:1802–1809
20. Greco D, Salmaso S, Mastrantonio P, et al, and the Progetto Pertosse Working Group. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis.N Engl J Med.1996;334:341–348
21. Schmitt HJ, Wirsing von Ko¨nig CH, Neiss A, et al. Efficacy of acellular pertussis vaccine in early childhood after household exposure.JAMA.1996;275:37– 41
22. Wassilak SGF, Roper MH, Murphy TV, Orenstein WA. Tetanus toxoid. In: Plotkin SA, Orenstein WA, Offit PA, eds.Vaccines. 4th ed. Philadelphia, PA: Saunders; 2004:745–781
23. Wharton M, Vitek CR. Diphtheria toxoid. In: Plotkin SA, Oren-stein WA, Offit PA, eds. Vaccines. 4th ed. Philadelphia, PA: Saunders; 2004:211–228
24. Food and Drug Administration Center for Biologics Evaluation and Research.Vaccines and Related Biological Products Advisory Committee (day one). 1997. Available at: www.fda.gov/ohrms/ dockets/ac/97/transcpt/3300t1.pdf. Accessed April 5, 2005 25. Schmitt HJ, Schuind A, Knuf M, et al. Clinical experience of a
tricomponent acellular pertussis vaccine combined with diph-theria and tetanus toxoids for primary vaccination in 22,505 infants.J Pediatr.1996;129:695–701
26. Rennels MB. Extensive swelling reactions occurring after booster doses of diphtheria-tetanus-acellular pertussis vac-cines.Semin Pediatr Infect Dis.2003;14:196 –198
27. Woo EJ, Burwen DR, Gatumu SNM, Ball R, and the Vaccine Adverse Event Reporting System (VAERS) Working Group. Extensive limb swelling after immunization: reports to the vaccine adverse event reporting system.Clin Infect Dis.2003; 37:351–358
28. Pichichero ME, Deloria MA, Rennels MB, et al. A safety and immunogenicity comparison of 12 acellular pertussis vaccines and one whole-cell pertussis vaccine given as a fourth dose in 15- to 20-month-old children.Pediatrics.1997;100:772–786 29. Atkinson WL, Pickering LK, Schwartz B, et al. General
recom-mendations on Immunization. Recomrecom-mendations of the Advi-sory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAP). MMWR Recomm Rep.2002;51(RR-2):1–35
30. Centers for Disease Control and Prevention. Diphtheria, teta-nus, and pertussis: recommendations for vaccine use and other preventive measures. Recommendations of the immunization practices advisory committee (ACIP). MMWR Recomm Rep.
1991;40(RR-10):1–28
31. Salmaso S, Mastrantonio P, Tozzi AE, et al, and the Stage III Working Group. Sustained efficacy during the first 6 years of life of 3-component acellular pertussis vaccines administered in infancy: the Italian experience. Pediatrics. 2001;108(5). Available at: www.pediatrics.org/cgi/content/full/108/5/e81 32. McIntyre PB, Turnbull FM, Egan A-M, Burgess MA, Wolter
33. Edelman KJ, He Q, Makinen JP, et al. Pertussis-specific cell-mediated and humoral immunity in adolescents 3 years after booster immunization with acellular pertussis vaccine. Clin Infect Dis.2004;39:179 –185
34. Centers for Disease Control and Prevention. Recommended childhood and adolescent immunization schedule: United States, 2005.MMWR Morb Mortal Wkly Rep.2005;53:Q1–Q3 35. Kandola K, Lea A, Santos M. Pertussis rates in
North-west Territories after introducing adult formulation acellular
vaccine [abstract]. Can J Infect Dis Med Microbiol. 2004;15: 351.
36. Pichichero ME, Rennels MB, Edwards KM, et al. Combined tetanus, diphtheria, and 5-component pertussis vaccine for use in adolescents and adults.JAMA.2005;293:3003–3011 37. Advisory Committee on Immunization Practices. ACIP
recom-mends adolescent vaccination for tetanus, diphtheria and pertussis vaccine. Available at: www.cdc.gov/nip/pr/pr㛭tdap㛭jun2005. htm. Accessed July 5, 2005
COMPARISON OF THE INSTRUCTIONAL EFFICACY OF INTERNET-BASED CME WITH LIVE INTERACTIVE CME WORKSHOPS: A RANDOMIZED CONTROLLED TRIAL
“Context:Despite evidence that a variety of continuing medical education (CME) techniques can foster physician behavioral change, there have been no randomized trials comparing performance outcomes for physicians par-ticipating in Internet-based CME with physicians parpar-ticipating in a live CME intervention using approaches documented to be effective.
Objective: To determine if Internet-based CME can produce changes com-parable to those produced via live, small-group, interactive CME with respect to physician knowledge and behaviors that have an impact on patient care. Design, Setting, and Participants:Randomized controlled trial conducted from August 2001 to July 2002. Participants were 97 primary care physicians drawn from 21 practice sites in Houston, TX, including 7 community health centers and 14 private group practices. A control group of 18 physicians from these same sites received no intervention.
Conclusions:Appropriately designed, evidence-based online CME can pro-duce objectively measured changes in behavior as well as sustained gains in knowledge that are comparable or superior to those realized from effective live activities.”
DOI: 10.1542/peds.2005-1759
2006;117;1084
Pediatrics
Dominique Descamps and Leonard R. Friedland
Michael E. Pichichero, Mark M. Blatter, William A. Kennedy, James Hedrick,
Toxoids for Adolescents
Acellular Pertussis Vaccine Booster Combined With Diphtheria and Tetanus
Services
Updated Information &
http://pediatrics.aappublications.org/content/117/4/1084
including high resolution figures, can be found at:
References
http://pediatrics.aappublications.org/content/117/4/1084#BIBL
This article cites 30 articles, 2 of which you can access for free at:
Subspecialty Collections
_sub
http://www.aappublications.org/cgi/collection/vaccine:immunization
Vaccine/Immunization
b
http://www.aappublications.org/cgi/collection/infectious_diseases_su
Infectious Disease
orders_sub
http://www.aappublications.org/cgi/collection/ear_nose_-_throat_dis
Ear, Nose & Throat Disorders following collection(s):
This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtml
in its entirety can be found online at:
Information about reproducing this article in parts (figures, tables) or
Reprints
http://www.aappublications.org/site/misc/reprints.xhtml
DOI: 10.1542/peds.2005-1759
2006;117;1084
Pediatrics
Dominique Descamps and Leonard R. Friedland
Michael E. Pichichero, Mark M. Blatter, William A. Kennedy, James Hedrick,
Toxoids for Adolescents
Acellular Pertussis Vaccine Booster Combined With Diphtheria and Tetanus
http://pediatrics.aappublications.org/content/117/4/1084
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397.