Agronomic and genetic analysis of Suweon 542, a rice floury mutant line suitable for dry milling
Full text
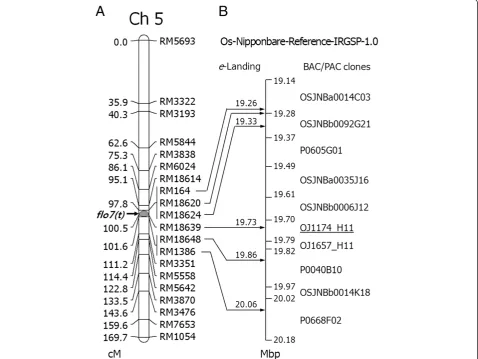
Figure




Related documents
A New Method to Build Gene Regulation Network Based on Fuzzy Hierarchical Clustering Methods..
We investigate the local dynamics in the neighborhood of a steady state in an economy where agents face financial constraints and goods are produced in two sectors with
• Corporate & private parties-events • Traditional Estonian Dinners with folklore Catering for events can be choosed from various menus and will be served either inside the
(2006) found that things such as website design features (e.g., pictures and employee testimonials), organizational policy statements, specific comments related
Chapter 2 presents a numerical scheme for three-dimensional modelling of marine CSEM data, by decomposing the electric field in primary and secondary components, and discretiz- ing
Kouadri and Brézillon [12] described the security context as: “A set of information collected from the user's environment and the application environment and
This rare variant was classified as likely pathogenic due to its frameshift nature in the gene, in which loss-of-function mutations have been previously reported to
A regulatory threshold is a specified concentration of the medication or a derivative thereof in plasma or urine, whereas a withdrawal time guideline is a suggested time before an