D
E
V
E
LO
P
M
E
N
T
INTRODUCTION
The embryonic lung develops from three distinct tissue layers: the outer mesothelium, the mesenchyme and the bronchial epithelium. Following formation of the primary epithelial branches that define the lobular structure of the lung, morphogenesis proceeds to the pseudoglandular stage (E9.5-E16), in which continued mesenchymal growth is coupled with epithelial branching to create the respiratory tree. During this crucial developmental stage, a dynamic network of signaling growth factors interact across the three tissue boundaries to regulate lung growth and branching, orchestrating proportional growth of the proximal conducting airways, the terminal epithelial buds and the surrounding vascular network (Shannon and Hyatt, 2004).
Several studies have identified fibroblast growth factors (FGFs) that signal across epithelial and mesenchymal boundaries to regulate both the pseudoglandular and subsequent stages of lung development (Shannon and Hyatt, 2004). In vitro studies have shown that mesenchymally expressed FGF10 activates the ‘b’ splice form of FGF receptor 2 (FGFR2b) in lung epithelium to direct budding by stimulating epithelial cell migration and proliferation (Bellusci et al., 1997b; Park et al., 1998; Peters et al., 1992; Weaver et al., 2000). Consistent with this activity, loss of function of Fgf10 or Fgfr2bresults in the absence of primary branching in vivo, causing the trachea to terminate as a blind sac (Arman et al., 1999; Celli et al., 1998; De Moerlooze et al., 2000; Min et al., 1998; Peters et al., 1995; Sekine et al., 1999). Fgf7, also a ligand for FGFR2b, is expressed in developing lung mesenchyme, but its function during
normal lung development is not clear (Bellusci et al., 1997b; Cardoso et al., 1997; Guo et al., 1996; Park et al., 1998; Post et al., 1996; Simonet et al., 1995; Tichelaar et al., 2000). Spry2, an inducible inhibitor of FGF and EGF receptor tyrosine kinase signaling, is expressed in the distal epithelial tips and is functionally downstream of the mesenchymal FGF genes, acting to suppress mesenchymal FGF signaling to epithelium (Hanafusa et al., 2002; Mailleux et al., 2001; Tefft et al., 2002; Tefft et al., 1999; Zhang et al., 2001).
FGF9 fulfills the role of a reciprocal epithelial to mesenchymal signal in the lung. At E10.5, Fgf9 is expressed in both the mesothelial lining (future pleura) of the lung bud and in the epithelium of the developing airways. As lung development progresses through the pseudoglandular stage, Fgf9expression persists in the mesothelium but can no longer be detected in the lung epithelium (Colvin et al., 1999). Fgf9–/–mice have severe lung
hypoplasia and die in the perinatal period. The most characteristic feature of Fgf9–/– lungs is a reduced ratio of mesenchyme to
epithelium caused by a reduction in mesenchymal (but not epithelial) proliferation at E10.5-E11.5. In addition, a secondary consequence of Fgf9gene inactivation is reduced branching of the epithelial tubules after ~E12.5. Previous studies suggested that a molecular etiology of reduced branching morphogenesis may be decreased mesenchymal Fgf10at actively branching regions of the lung (Colvin et al., 2001).
Sonic hedgehog (SHH) and bone morphogenic protein 4 (BMP4) also modulate lung mesenchymal and epithelial development. Bmp4 is expressed in the distal epithelium, where it appears to have a primary role in promoting distal epithelial differentiation and antagonizing FGF-mediated epithelial budding, and has a suggested role in specifying smooth muscle precursors (Bellusci et al., 1996; Bitgood and McMahon, 1995; Mailleux et al., 2005; Weaver et al., 2000). Additionally, Bmp4is upregulated by FGF10 in vitro and in vivo (Lebeche et al., 1999; Mailleux et al., 2005; Mailleux et al.,
FGF9 and SHH signaling coordinate lung growth and
development through regulation of distinct mesenchymal
domains
Andrew C. White1, Jingsong Xu2, Yongjun Yin1, Craig Smith1, Gregory Schmid1and David M. Ornitz1,*
Morphogenesis of the lung is regulated by reciprocal signaling between epithelium and mesenchyme. In previous studies, we have shown that FGF9 signals are essential for lung mesenchyme development. Using Fgf9loss-of-function and inducible gain-of-function mouse models, we show that lung mesenchyme can be divided into two distinct regions: the mesothelial and sub-epithelial compartments, which proliferate in response to unique growth factor signals. Fibroblast growth factor (FGF) 9 signals from the mesothelium (the future pleura) to sub-mesothelial mesenchyme through both FGF receptor (FGFR) 1 and FGFR2 to induce proliferation. FGF9 also signals from the epithelium to the sub-epithelial mesenchyme to maintain SHH signaling, which regulates cell proliferation, survival and the expression of mesenchymal to epithelial signals. We further show that FGF9 represses
peribronchiolar smooth muscle differentiation and stimulates vascular development in vivo. We propose a model in which FGF9 and SHH signals cooperate to regulate mesenchymal proliferation in distinct submesothelial and subepithelial regions. These data provide a molecular mechanism by which mesothelial and epithelial FGF9 directs lung development by regulating mesenchymal growth, and the pattern and expression levels of mesenchymal growth factors that signal back to the epithelium.
KEY WORDS: Fibroblast growth factor 9 (FGF9), Sonic hedgehog (SHH), Lung development, Branching morphogenesis, Mesothelium, Epithelium, Mesenchyme, Mouse
Development 133, 1507-1517 (2006) doi:10.1242/dev.02313
1Department of Molecular Biology and Pharmacology, Washington University Medical School, St Louis, MO 63110, USA. 2Brigham and Women’s Hospital, Division of Critical Care and Pulmonary Medicine, Boston, MA 02115, USA.
*Author for correspondence (e-mail: dornitz@wustl.edu)
D
E
V
E
LO
P
M
E
N
T
2001; Weaver et al., 2000). Shhis expressed in the distal epithelium of the lung throughout the pseudoglandular stage and binds to its receptor, patched 1 (Ptch1), in the adjacent sub-epithelial mesenchyme (Bellusci et al., 1997a; Bitgood and McMahon, 1995; Miller et al., 2001; Weaver et al., 2003). Other members of this signaling pathway, such as Gli1and Hip1, colocalize with Ptch1 (Chuang et al., 2003; Grindley et al., 1997). Both Shh gain-of-function and loss-of-gain-of-function mutations, as well as combinatorial Gli loss-of-function mutations, demonstrate that SHH signaling positively affects both mesenchymal and epithelial growth, as well as lobe formation (reviewed by van Tuyl and Post, 2000). SHH is proposed to modulate the epithelial branching pattern by focally suppressing Fgf10in distal mesenchyme and upregulating Fgf7 (Lebeche et al., 1999; Pepicelli et al., 1998). Conversely, FGF10 does not affect Shh, whereas high levels of FGF7 suppresses both Shhexpression and signaling (Lebeche et al., 1999; Park et al., 1998). Although Shhappears unaffected at late stages of branching in Fgf9–/–lungs (Colvin et al., 2001), we hypothesized that FGF9
may directly or indirectly regulate SHH signaling at early stages of branching, which in turn may affect mesenchymal proliferation and the level and pattern of Fgf10.
To investigate further the mechanisms by which FGF9 regulates lung development, we have developed two gain-of-function models: an in vivo method to transiently express ectopic FGF9 in lung epithelium, and in vitro organ culture that mimics FGF9 overexpression in the mesothelium. Combined with the Fgf9 loss-of-function mouse, these two systems facilitated an examination of the role of FGF9 on sub-mesothelial and sub-epithelial mesenchyme.
Here, we identify two distinct mesenchymal zones that are differentially regulated by FGF9 and SHH. We show that FGF9 stimulates proliferation of the sub-mesothelial population of mesenchymal cells, and is an upstream regulator of mesenchymal SHH signaling, which is required for cell proliferation and cell survival in the sub-epithelial mesenchymal compartment. Finally, FGF9 regulates expression of the mesenchymal to epithelial signals that may generate the crucial developmental switch from branching to dilation during lung development.
MATERIALS AND METHODS
In vitro organ culture
Embryonic mouse lungs were dissected and cultured on Transwell filters (Costar, Corning) for 24 or 48 hours. Heparin-coated acrylic beads (Sigma) were loaded with BSA, mouse FGF9, human FGF10 or mouse FGF7 at
100 ng/l (PeproTech). Beads were placed against the central left lobe or
protein was added directly to media at 500 ng/ml. Cyclopamine (Toronto
Research Chemicals) was diluted to a 10 M stock in 95% ethanol and
added to culture media to a final concentration of 10 nM.
Generation of transgenic mice and doxycycline administration
The TRE-Fgf9-IRES-eGfp transgene was constructed by cloning the Fgf9
cDNA (Santos-Ocampo et al., 1996) upstream of the IRES-eGfp-SV40
polyA cassette (pIRES2-eGfp vector, Clontech) in the pTRE2 vector
(Clontech). This TRE-Fgf9-IRES-eGfp transgene was released with NotI and
used to generate five founder lines of transgenic mice.
TRE-Fgf9-IRES-eGfp mice were mated to SPC-rtTAmice (provided by Dr Jeff Whitsett) (Perl et al., 2002; Tichelaar et al., 2000). Two bi-transgenic
TRE-Fgf9-IRES-eGfp;SPC-rtTAlines exhibited a doxycycline-inducible embryonic phenotype. One line was used for these studies.
Doxycycline-containing water (200 g/ml) can activate a TREtransgene within 16 hours
(Perl et al., 2002) and was given to time-mated females 48 hours before
embryo harvest. Mice were genotyped with PCR primers specific for
SPC-rtTA (Tichelaar et al., 2000) and eGfp (primers 5⬘
-CGTAAACG-GCCACAAGTTCAG-3⬘and 5⬘-ATGCCGTTCTTCTGCTTGTCG-3⬘).
Whole mount in situ hybridization, lacZstaining and immunohistochemistry
Whole-mount in situ hybridization was performed as described previously
(Colvin et al., 2001). Tie2LacZ+/–and Noggin-lacZexpression was visualized
by staining whole lobes with 1 mg/ml X-gal, 2 mM MgCl2, 35 mM
Ke3F(CN)6, 35 mM Ke4F(CN)6and 1⫻PBS at room temperature in the
dark. Following adequate color reaction, tissues were washed and dehydrated to methanol. Tissues were then sectioned in paraffin wax at 5
M (Tie2LacZ+/–) or cryosectioned at 12 M (Noggin-lacZ) and counterstained with Eosin. Panel comparisons were paired with littermate controls, ensuring similar developmental time points, tissue processing and staining incubation periods (8-10 hours).
For whole-mount immunohistochemistry, dissected lung tissues were fixed overnight in 4% PFA, dehydrated to 100% methanol and stored at –20°C. All incubations and washes were performed at 4°C while shaking. Tissues were rehydrated, incubated with a blocking solution (2% skim milk, 1% sheep serum, 0.1% Triton X-100) for 2 hours, primary and secondary antibodies, in blocking solution, overnight at 4°C and then washed (2% skim milk, 0.1% Triton X-100) five times at hourly intervals. Visualization of secondary antibody conjugated to HRP was performed using the DAB kit (Vector Laboratories), according to manufacturer’s instructions.
PECAM1-stained tissues were sectioned in paraffin wax at 5 M and counterstained
with Hematoxylin. Rat anti-PECAM1 (BD) was incubated at 1:500, monoclonal TTF1 (DakoCytomation) at 1:200, monoclonal anti-phosphohistone H3 (Sigma) at 1:500 and rabbit anti-active caspase 3 (R&D) at 1:600. Secondary HRP conjugated anti-mouse antibody (Chemicon) was incubated at 1:300, HRP anti-rat at 1:200 and HRP anti-rabbit at 1:400.
Immunohistochemistry on paraffin embedded 5 M sections was performed
using a standard protocol with citrate buffer antigen retrieval. Rat anti-SMA (Sigma) was incubated at 1:500 overnight at 4°C, followed by a secondary HRP-conjugated anti-rat antibody (Chemicon) at 1:200. All expression studies are representative of at least three embryos.
Mesenchyme proliferation analysis
Mitotic cells were identified with anti-phospho histone H3 antibodies and
photographed at 90⫻ magnification in whole-lung quadrants. Distal
mesenchyme was defined as the area between the outermost edge of the distal epithelium and the mesothelium. Sub-mesothelial and sub-epithelial regions were defined by dividing the mesenchyme at the relative halfway mark between epithelium and mesothelium. Labeled cells present in the mesenchyme were counted and averaged over the total or regional area, determined by tracing and calculating the area using Canvas 9 software. Both 24 and 48 hour data represent littermate biological samples. Each proliferation index was averaged over each of the four quadrants per sample.
Error bars represent one s.d., and Student’s t-test values indicate statistical
significance for comparable tissues.
RESULTS
FGF9 induces lung mesenchyme proliferation and airway epithelial expansion in vitro
FGF9 regulates mesenchymal proliferation and the expression or activity of mesenchymal factors that in turn signal to airway epithelium. In this model, we predicted that Fgf9overexpression would primarily affect lung mesenchyme and indirectly affect developing airway epithelium.
D
E
V
E
LO
P
M
E
N
T
hours of incubation with FGF9 in media (see below). After 48 hours in culture, the FGF9-exposed explants developed a single extended epithelial tubule that often completely enveloped the adjacent bead (Fig. 1E). Notably, this luminal enlargement was only readily apparent after ~48 hours in culture, suggesting that a secondary signal is required to mediate this epithelial phenotype.
In contrast to exposure to an FGF9 bead, FGF10-treated explants exhibited no detectable lung mesenchyme expansion (Fig. 1A,C,D,F). However, within 24 hours of FGF10 treatment, airway epithelium showed robust growth towards the bead and luminal expansion that resembled FGF9 stimulated explants at 48 hours. After 48 hours, the epithelium was in very close apposition to the FGF10 bead, whereas in the presence of an FGF9 bead, a band of mesenchyme was visible between the bead and the epithelial duct (Fig. 1E,F). These data are consistent with a primary role for FGF9 in mesenchymal proliferation and a secondary role in epithelial development in vitro.
The mesenchymal receptor(s) that mediates the FGF9 response is not known. Both Fgfr1and Fgfr2are expressed in the developing lung mesenchyme (Arman et al., 1999; Peters et al., 1992) and are thus candidate receptors to transduce the FGF9 signal. To determine if one or both of these receptors are necessary for lung mesenchymal development, we used the Dermo1(Twist2)-Cre allele to target
floxed alleles of Fgfr1and Fgfr2(Trokovic et al., 2003; Yu et al., 2003). Dermo1-Creactivity in the developing lung was assessed by mating to the Rosa26 reporter line (Soriano, 1999). This analysis showed that by E13.5, most lung mesenchymal cells have been exposed to functional Cre activity (Fig. 1G). Conditional knockout mice lacking Fgfr1and one copy of Fgfr2(Fgfr1–/–; Fgfr2+/–), or heterozygous for both receptors (Fgfr1+/–; Fgfr2+/–), did not exhibit a lung phenotype when compared with wild-type and Dermo1-Cre controls (Fig. 1H; data not shown). By contrast, mice lacking both Fgfr1and Fgfr2(Fgfr1–/–; Fgfr2–/–) showed lung hypoplasia at E12.5 through E18.5 (Fig. 1J,K; data not shown). These double conditional knockout lungs failed to fill the thoracic cavity and exhibited variable morphologies. Lungs lacking Fgfr2and one allele of Fgfr1(Fgfr1+/–; Fgfr2–/–) also exhibited a reduction in size, although less severe than double receptor knockout lungs (Fig. 1I). These data demonstrate that Fgfr1and Fgfr2are redundant but that one wild-type allele of Fgfr2is sufficient to transduce an FGF signal to lung mesenchyme.
Induced expression of Fgf9in lung epithelial cells results in lung hyperplasia in vivo
Lung organ culture experiments predict that in vivo overexpression of Fgf9will promote mesenchymal growth but will also alter lung branching, possibly by disrupting the pattern of mesenchymal to epithelial signals. To express Fgf9in developing lung epithelium with temporal and spatial specificity, we constructed transgenic mouse lines that contain the Fgf9cDNA under the control of seven tetracycline-inducible regulatory elements (TREs). To monitor transgenic Fgf9 expression, an IRES-eGfp (enhanced green fluorescent protein) gene was placed 3⬘to the Fgf9cDNA, which allowed for co-expression of eGfpwith Fgf9. The TRE-Fgf9-IRES-eGfpmice were mated to a transgenic line containing the 3.7 kb human surfactant C promoter driving expression of the reverse tetracycline activator (SPC-rtTA). The SPC-rtTA transgene has been used to induce genes efficiently in developing lung epithelium, as early as E10, and in adult type II pneumocytes (Perl et al., 2002; Tichelaar et al., 2000). Two out of five of the transgenic lines demonstrated robust and reproducible doxycycline-inducible Fgf9 and eGfpexpression in lung epithelium. One of these lines was used for the following studies.
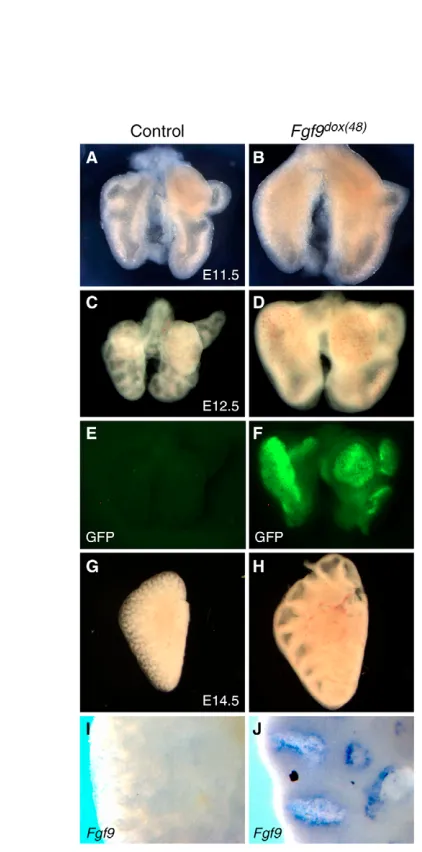
At all time points examined, following 48 hours of doxycycline administration, control embryos containing only the TRE-Fgf9-IRES-eGfpgene were phenotypically normal with no detectable eGFP activity or Fgf9expression (Fig. 2). Transgenic pups that contained both the SPC-rtTAgene and the TRE-Fgf9-IRES-eGfp gene, but which were not exposed to doxycycline, were also phenotypically normal and showed no eGFP activity (data not shown), indicating tight regulation of transgene expression. By contrast, Fgf9 mRNA was detected throughout the lung epithelium of mice positive for both the TRE-Fgf9-IRES-eGfp and SPC-rtTA transgenes and exposed to doxycycline for 48 hours (Fgf9dox(48) mice) (Fig. 2J). Visual inspection of these
lungs showed eGFP fluorescence under UV illumination (Fig. 2F). No additional sites of eGFP fluorescence were observed. Control lungs from the same litter demonstrated no fluorescence (Fig. 2E).
[image:3.612.50.300.59.275.2]Fgf9expression was induced for 48 hours prior to embryo harvest and lungs were examined at E11.5, E12.5 and E14.5 (Fig. 2). Similar to the in vitro response to FGF9, in vivo overexpression of Fgf9 during early lung morphogenesis induced a large mesenchymal expansion and a reduction in epithelial branching (Fig. 2A-D). Beginning at E12.5 (doxycycline at E10.5), the dramatic Fig. 1. FGF9 induces mesenchymal and epithelial expansion in
D
E
V
E
LO
P
M
E
N
T
enlargement in lung size was characterized by an arrest in branching and luminal dilation, with morphology resembling a lung at the onset of doxycycline induction (Fig. 2C,D,G,H; data not shown). These observations suggest that the arrest in branching morphogenesis was initiated soon after doxycycline administration. Littermate control embryos treated with doxycycline and singly positive for SPC-rtTA, TRE-Fgf9-IRES-eGfp, or genotypically wild type, exhibited no difference in epithelial or mesenchymal morphology from non-doxycycline treated wild-type lungs (Fig. 2A,C,G).
At E11.5 and E12.5, histological sections of Fgf9dox(48)lungs showed increased mesenchymal area surrounding an epithelium containing fewer branches (Fig. 3A,B; data not shown). To determine the status of mesenchymal differentiation in Fgf9dox(48)
lungs, vascular development and smooth muscle differentiation were examined. At E11.5, the endothelial cell-specific Tie2-lacZreporter (Fadel et al., 1999) showed an increased number of vessels in Fgf9dox(48);Tie2-lacZlungs (Fig. 3C,D). The pattern of Tie2-lacZ
[image:4.612.51.262.187.613.2]expression demonstrated the formation of a multilayered capillary network in the presence of excess FGF9, in contrast to a single
Fig. 2. FGF9 induces mesenchymal and epithelial expansion in vivo.(A-D) Bi-transgenic TRE-Fgf9-IRES-eGfp;SPC-rtTA lungs from E11.5 and E12.5 embryos induced for 48 hours with doxycycline (Fgf9dox(48)) (B,D) exhibit increased overall size, an extended
mesenchymal area and a reduction in branching compared with controls (A,C). (E,F) eGFP fluorescence in control (E) and Fgf9dox(48)(F)
lung epithelium. (G,H) E14.5, Fgf9dox(48)lungs (H) demonstrated an
increased size and expanded epithelial lumens. (I,J) Whole-mount in situ hybridization demonstrates Fgf9 epithelial expression in E14.5 Fgf9dox(48)lungs (J), consistent with eGFP fluorescence (F). Fgf9
expression and eGFP fluorescence was not seen in single TRE-Fgf9-IRES-eGfplungs (E,I). G-J are left lung lobes.
Fig. 3. Histology and mesenchymal differentiation in Fgf9dox(48)
developing lungs. (A,B) Hematoxylin and Eosin stained sections showed increased size and mesenchymal area in E11.5 Fgf9dox(48)lungs
(B). (C,D) Tie2-lacZstaining for endothelial cells in E12.5 Fgf9dox(48)
lungs showing a multi-layered vascular network extending into the expanded mesenchyme (D), in contrast to a single layer of endothelial cells in wild-type controls (C). (E,F) Anti-PECAM immunohistochemistry confirmed an extended domain of blood vessels in E13.5 Fgf9dox(48)
lungs (F). (G,H) Increased luminal space, fewer epithelial airways and a wide band of mesenchyme separating epithelial ducts was found in E14.5 Fgf9dox(48)lungs (H). (I,J) Smooth muscle actin (SMA) staining in
proximal sub-epithelial mesenchyme adjacent to airways (a) is absent in E14.5 Fgf9dox(48)lungs (arrow, J). SMA was still found adjacent to blood
[image:4.612.305.534.190.589.2]D
E
V
E
LO
P
M
E
N
T
layered vascular domain in control tissue. This pattern was confirmed by using an anti PECAM1 antibody (Fig. 3E,F). E14.5 lungs were characterized by rapidly expanding epithelial airways embedded in wide bands of mesenchyme (Fig. 3H). At this stage, a smooth muscle layer is formed in the sub-epithelial mesenchyme adjacent to proximal airways (Fig. 3I). In Fgf9dox(48)lungs, smooth
muscle is notably absent from the sub-epithelial compartment, although still present surrounding large blood vessels (Fig. 3J). This is consistent with in vitro data showing FGF9 inhibition of smooth muscle actin (SMA) expression in mesenchyme-only explants in the presence of SHH (Weaver et al., 2003), and suggests that the FGF10-expressing lineage that gives rise to sub-epithelial smooth muscle (Mailleux et al., 2005) may be inhibited by epithelial expression of Fgf9.
FGF9 positively regulates mesenchymal SHH signaling
SHH signaling positively regulates both mesenchymal and epithelial proliferation and functions to pattern Fgf10expression to indirectly control the pattern of epithelial branching morphogenesis (Bellusci et al., 1997a; Chuang et al., 2003; Lebeche et al., 1999; Litingtung et al., 1998; Pepicelli et al., 1998; Weaver et al., 2003). Because Fgf9 also regulates mesenchymal proliferation and branching morphogenesis, we hypothesized that early modulation of SHH signaling by FGF9 could account for some or all of these effects. Therefore, Shhand Ptch1expression were examined in Fgf9–/–and Fgf9dox(48)lungs during early stages of lung development. Ptch1was of particular interest, as it acts as transcriptional reporter of SHH signaling activity (Bellusci et al., 1997a; Grindley et al., 1997; Ingham and McMahon, 2001; Litingtung et al., 1998; Pepicelli et al., 1998). At E11.5 and E12.5, the relative expression of epithelial Shh was decreased in Fgf9–/–lungs, but remained unchanged in the
epithelium of the attached esophagus (Fig. 4A,B,I,J). Importantly, the expression of Ptch1was significantly decreased in sub-epithelial
mesenchyme at E11.5 and E12.5, suggesting a decrease in SHH signaling in Fgf9–/– lungs (Fig. 4C,D,K,L). Gli1, another
transcriptional reporter of SHH signaling, was downregulated in distal sub-epithelial mesenchyme in E12.5 Fgf9–/–lungs (see Fig. S1 in the supplementary material). These data indicate that Fgf9is necessary to maintain normal levels of Shhexpression and SHH signaling to sub-epithelial mesenchyme during early lung development.
To determine whether FGF9 is sufficient to enhance SHH signaling, Ptch1expression was examined in response to increased levels of mesothelial or epithelial FGF9. Consistent with the loss-of-function data, expansion of Ptch1expression was observed in the mesenchyme of lung explant cultures exposed to FGF9 containing media (see below, Fig. 6B). Moreover, in E11.5 and E12.5 Fgf9dox(48)
lungs, both Shhand Ptch1 were expressed at significantly higher levels throughout both proximal and distal regions (Fig. 4E-H,M-P). Taken together, these data indicate that FGF9 is both necessary and sufficient to induce Shhexpression and SHH signaling.
FGF9 and SHH promote proliferation in distinct mesenchymal compartments of the developing lung
[image:5.612.53.401.449.741.2]Lung histology at E12.5 revealed two morphologically distinct populations of mesenchyme. The sub-epithelial mesenchymal cells appear condensed and orient circumferentially around epithelial ducts in transverse sections, while sub-mesothelial mesenchymal cells appear as a loose, non-oriented mesenchyme (Fig. 5A). Noggin-lacZ(Brunet et al., 1998) labels sub-epithelial mesenchyme in both distal and proximal regions at E12.5 (see Fig. S2). As both Fgf9or Shhregulate mesenchyme proliferation (Colvin et al., 2001; Litingtung et al., 1998) and FGF9 signaling maintains SHH signaling, we hypothesized that FGF9 promoted sub-mesothelial proliferation directly and sub-epithelial proliferation indirectly through SHH signaling.
Fig. 4. FGF9 positively regulates HH signaling. (A,B) Whole-mount in situ hybridization demonstrates reduced Shh expression in E11.5 Fgf9–/–distal lung
epithelium (box, B) compared with controls (A). Attached esophagus (e), by contrast, retained a similar level of expression in Fgf9–/–and wild-type
controls. (C,D) Ptch1expression was downregulated in E11.5 Fgf9–/–distal
sub-epithelial mesenchyme (D). Esophageal expression of Ptch1, however, was not changed. (E-H) Increased Shhand sub-epithelial Ptch1 expression both distally and proximally in E11.5 Fgf9dox(48)lungs (F,H),
D
E
V
E
LO
P
M
E
N
T
Lung tissue from E12.5 Fgf9–/–embryos lack sub-mesothelial mesenchyme to a greater extent than sub-epithelial mesenchyme (Fig. 5A,B), resulting in an irregularly shaped distal mesenchymal edge (Colvin et al., 2001). By contrast, a wide expanse of loose mesenchyme was evident prior to epithelial expansion in E12.5 Fgf9dox(48) lungs (Fig. 5C). Anti-phosphohistone H3 labeling of
mitotic cells in whole lung explants revealed a 38±8% and 62±7% increase in the distal mesenchyme when treated with FGF9 for 24 and 48 hours, respectively (Fig. 5D,E,L,M). This increased proliferation primarily affected the sub-mesothelial region (57±12% increase) versus the sub-epithelial region (21±12% increase) at 24 hours. Lung explants treated with FGF9 appeared significantly more rounded and epithelial tubules were difficult to visualize beneath the thickened mesenchyme (Fig. 5D,E; data not shown). Examination of these explants at high magnification showed increased labeling of the sub-mesothelial region (Fig. 5D⬘,E⬘).
To determine whether FGF9-mediated mesenchymal proliferation required SHH signaling, the ratio of epithelial to sub-mesothelial mesenchyme proliferation was assessed in response to
[image:6.612.104.565.58.470.2]FGF9 and cyclopamine. Treatment with cyclopamine inhibited Ptch1expression (Fig. 6A,B) and resulted in a 31±4% (24 hours) and 66±19% (48 hours) decrease in distal mesenchyme proliferation (Fig. 5G,L,M). When explant cultures were incubated with both cyclopamine and FGF9, distal mesenchyme proliferation was at an intermediate level between the two conditions (Fig. 5F,L,M). However, when anti-phosphohistone H3-labeled cells were examined at high magnification, the sub-mesothelial region resembled explants treated only with FGF9 (39±10% increase), while proliferation was reduced in the sub-epithelial region (12±10% decrease) (Fig. 5E⬘,F⬘). Furthermore, Ptch1was absent in FGF9/cyclopamine co-incubated explants, similar to cyclopamine alone (data not shown). This suggests that mesothelial FGF9 does not require SHH signaling to stimulate sub-mesothelial mesenchymal proliferation, but that epithelial FGF9 may positively influence sub-epithelial mesenchymal proliferation by stimulating or maintaining SHH signaling (increased Ptch1expression is present in FGF9-treated explants, Fig. 6B). These data indicate that FGF9 secretion from the mesothelium during the pseudoglandular stage Fig. 5. FGF9 induces
sub-mesothelial mesenchymal proliferation independent of SHH signaling.(A-C) Two histologically distinct regions of mesenchymal cells are evident at E13.5. A population of sub-epithelial mesenchymal cells (SEM) wrap around the epithelial ducts, and a loose network of non-oriented sub-mesothelial mesenchymal cells (SMM) inhabit the area between the mesothelium and the SEM (A). In Fgf9–/–lungs, the SMM is largely
absent (B), whereas a large expanse of SMM is present in Fgf9dox(48)
lungs (C). (D-G) Whole-mount immunohistochemistry with anti-phosphohistone H3 (PH3) identified an increase in PH3-labeled cells in E11.5 lung explants incubated with FGF9 for 24 hours (E), compared with BSA-treated controls (D). Cyclopamine-only treated explants showed decreased overall
proliferation (G). Explants incubated with both FGF9 and cyclopamine (F) appeared similar to FGF9-only treated explants (E) at low magnification. At higher
magnification, cyclopamine-treated cultures (F⬘,G⬘) showed reduced cell proliferation in the SEM, whereas FGF9 increased proliferation in the SMM (E⬘,F⬘). Explants treated with both cyclopamine and FGF9 (Cy/F9) have a combined effect, with increased PH3 labeling in the SMM and decreased labeling in the SEM (F9). (H-K) Staining for active caspase 3 demonstrates high levels of apoptosis in cyclopamine-treated
D
E
V
E
LO
P
M
E
N
T
acts to regulate lung volume by inducing the growth of sub-mesothelial mesenchyme, and indirectly regulates sub-epithelial mesenchyme through SHH signaling.
Mesenchyme-only lung explants require SHH for cell survival (Weaver, 2003). To determine if SHH signaling is required for mesenchymal cell survival in whole lung explants, an antibody to active caspase 3 was used to assess cell death in cyclopamine treated lung explant cultures. Anti-caspase 3 staining was increased when cultured with cyclopamine or with FGF9 and cyclopamine, indicating that SHH signaling is necessary for cell survival as well as proliferation, and that FGF9 does not completely rescue sub-epithelial viability (Fig. 5J,K).
Models of lung development suggest that high levels of SHH signaling decrease expression levels of Fgf10 but upregulate Fgf7 (Bellusci et al., 1997a; Chuang et al., 2003; Lebeche et al., 1999; Pepicelli et al., 1998). Consistent with this, at E12.5, the domain of Fgf10expression was expanded in Shh–/–lungs (Pepicelli et al.,
1998). We hypothesized that loss of SHH signaling would affect epithelial branching morphogenesis by enhancing budding through a de-repression of Fgf10, and that FGF9 would result in a decrease in budding through increased repression of Fgf10(through enhanced SHH signaling) and resemble the branching pattern seen in vivo. To test this hypothesis, we examined patterns of epithelial branching in lung explants incubated with FGF9 and cyclopamine.
Cyclopamine-treated E12.5 lung explants incubated for 48 hours exhibited a complete absence of Ptch1expression (Fig. 6A,C), and a statistically significant increase in the number of distal epithelial buds, which also appeared elongated (Fig. 6D,E,G). This phenotype is similar to that observed in Fgf10-treated lung epithelial cultures (Bellusci et al., 1997b). By contrast, FGF9-treated E12.5 lung explants showed increased Ptch1expression (Fig. 6B) and were characterized by increased distal mesenchyme, decreased distal buds and dilated epithelial lumens (Fig. 6D-F).
FGF9 mediated epithelial expansion results from induction of mesenchymal FGF expression
FGF9 signals to mesenchymal splice forms of FGFRs and is, therefore, unlikely to directly signal to lung epithelium. We hypothesized that FGF9 regulates the expression or activity of lung mesenchymal genes in addition to SHH signaling, which in turn act as effectors of epithelial morphogenesis. FGF7 and FGF10 signal to epithelial FGFRs to regulate epithelial development and are likely candidates for this mesenchymal to epithelial signal.
In Fgf9–/–lungs, regional expression of Fgf10was decreased in sub-mesothelial mesenchyme at E13.5-E14.5, resulting in a decrease in branching morphogenesis (Colvin et al., 2001). To further explore the epithelial-mesenchymal relationship between FGF9 and FGF10, and to determine whether FGF10 could contribute to all or part of the Fgf9dox(48)epithelial phenotype, Fgf10expression was examined
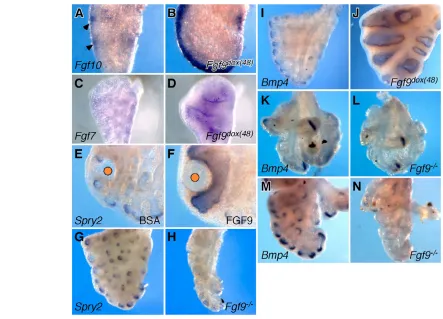
following induced expression of FGF9 from E12.5 to E14.5. In control tissues, the intensity of Fgf10expression varied between distal and lateral locations around epithelial structures, with highest expression between epithelial tubules (Fig. 7A, arrowheads). By contrast, these regional variations in Fgf10 expression were no longer present in tissues overexpressing Fgf9, and Fgf10 was expressed at high levels throughout the distal sub-mesothelial mesenchyme (Fig. 7B). The location of Fgf10expression appeared distal to the domain of Ptch1, consistent with the proposed ability of SHH to repress Fgf10.
Loss of focal Fgf10expression in the distal mesenchyme does not account for the observed epithelial dilation throughout both the proximal and distal epithelium. We hypothesized that another mesenchymal factor must be responsible for the luminal dilation. Fgf7is diffusely expressed at low levels throughout the mesenchyme during the pseudoglandular stage (Fig. 7C) and has a stronger proliferative effect on epithelium than FGF10 (Bellusci et al., 1997b; Park et al., 1998). Furthermore, Fgf7overexpression in vitro and in lung epithelium in vivo resulted in a luminal dilation (Cardoso et al., 1997; Park et al., 1998; Simonet et al., 1995; Tichelaar et al., 2000). Examination of Fgf7expression in Fgf9dox(48)lungs demonstrated upregulation throughout the sub-epithelial mesenchyme (Fig. 7C,D). These observations suggest that bothFgf7and Fgf10are induced by FGF9 and together may mediate the Fgf9dox(48) lung epithelial
phenotype.
To further test this hypothesis, the expression of Spry2and Bmp4 was examined following induction of Fgf9 in vivo or exposure to FGF9 in vitro. Both Spry2and Bmp4are normally expressed in the distal tip epithelium and are upregulated in response to FGF10 signaling to FGFR2b in vitro (Lebeche et al., 1999; Mailleux et al., 2001; Weaver et al., 2000). Consistent with increased mesenchymal to epithelial FGF signaling, both Spry2and Bmp4 expression were significantly upregulated in both proximal and distal epithelium in Fgf9dox(48)lungs and in explants exposed to FGF7, FGF9 or FGF10
beads (Fig. 7F,J; data not shown). The extension of Spry2 and Bmp4 expression in both proximal and distal locations is consistent with mesenchymal to epithelial FGF signals in both proximal (FGF7) and distal (FGF7 and FGF10) locations. We conclude from these data that FGF9 is sufficient to induce Spry2and Bmp4through induction of Fgf7and Fgf10.
[image:7.612.51.299.58.228.2]To determine whether Fgf9 is necessary to maintain Spry2and Bmp4expression in vivo, Spry2and Bmp4expression patterns were examined in Fgf9–/–lungs. Spry2was decreased in lateral epithelial branches at E13.5 (Fig. 7G,H). At E12.5, Bmp4was expressed in locations comparable with controls (Fig. 7K,L). By contrast, at E14.5 Bmp4was dramatically reduced in the distal epithelium (Fig. Fig. 6. Inhibition of HH signaling enhances epithelial budding.
(A,B) Whole-mount in situ hybridization showing enhanced Ptch1 expression in the sub-epithelial mesenchyme following incubation with FGF9 (B), and inhibition of expression when incubated with
D
E
V
E
LO
P
M
E
N
T
7M,N). These data are in agreement with the observed reduced expression of Fgf10 in Fgf9–/– lungs at mid to late stages of
branching morphogenesis (Colvin et al., 2001) and suggest regulation of Spry2and Bmp4by FGF10 in vivo.
DISCUSSION
FGF9 signals to sub-mesothelial mesenchyme to regulate lung growth
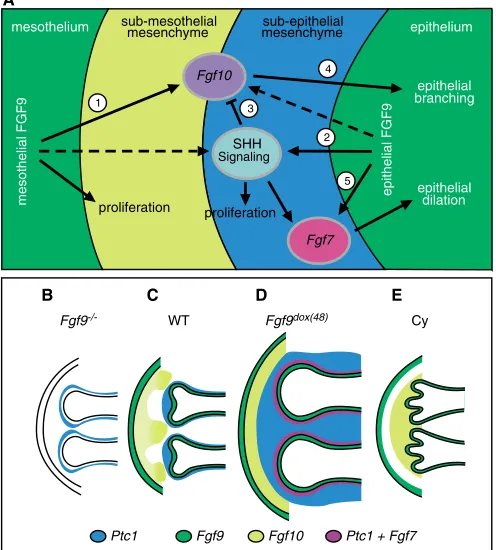
Growth of the lung and thoracic cavity are tightly coordinated developmental processes. During development, lung size must keep pace with growth of the thoracic cavity or, conversely, growth of the thoracic cavity must accommodate an expanding lung. Fgf9, which is expressed in the mesothelial lining of the lung from E9.5 to at least E15.5, and in airway epithelium from E9.5-E10.5 (Colvin et al., 1999), is located in a position where it could fulfill a function in sensing the size and shape of the thoracic cavity. Regulation of the expansion of lung mesenchyme serves at least two roles: to allow the lung to fill the thoracic space and to create a sufficient volume of mesenchyme through which branching morphogenesis can proceed. This model suggests that Fgf9 expression or activity could be spatially regulated (possibly by mechanical forces) to allow the growing lung to contour the growing thoracic cavity.
Using both in vitro organ culture and in vivo gain- and loss-of-function models, we demonstrated that FGF9 is both necessary and sufficient for growth of distal mesenchyme. Because SHH signaling appears to affect both mesenchymal proliferation and epithelial development (Litingtung et al., 1998; Pepicelli et al., 1998), we hypothesized that FGF9 could regulate mesenchymal proliferation both directly and indirectly through upregulation of SHH signaling at early stages. We showed that increased FGF9 signaling enhanced HH signaling and that loss of Fgf9reduced HH signaling in the sub-epithelial compartment. Because both of these effects are modulated by epithelial Shh, we posit that FGF9 should regulate a mesenchymal to epithelial signal that controls Shhexpression. Our preliminary data excludes BMP signaling, and although retinoic acid (RA) has been shown to induce Shhexpression in culture, previous studies indicate that RA signaling is not active in the epithelium at this stage (Bellusci et al., 1997a; Malpel et al., 2000). Additionally, FGF10 does not affect epithelial expression of Shh(Lebeche et al., 1999).
[image:8.612.53.496.57.376.2]Given this relationship between FGF9 and SHH signaling, we asked whether HH signaling contributes to FGF9-induced mesenchymal proliferation. As Fgf9is expressed in mesothelium throughout pseudoglandular development, and Shhis expressed exclusively in the epithelium, we propose a model (Fig. 8A) in Fig. 7. FGF9 signaling regulates mesenchymal FGF gene expression and signaling.(A,B) Whole-mount in situ hybridization demonstrating upregulated and broad expression of Fgf10in the sub-mesothelial mesenchyme of E13.5 Fgf9dox(48)lungs (B) compared with focal expression in
control lungs (arrowhead, A). (C,D) Increased Fgf7expression in both proximal and distal mesenchyme of Fgf9dox(48)lungs (D) compared with low
level mesenchymal expression in control lungs (C). (E,F) Upregulation of Spry2in epithelium of E11.5 whole lung explant cultures incubated for 48 hours with an FGF9 bead (F) compared with distally restricted expression in BSA controls (E). (G,H) Decreased Spry2expression in E14.5 Fgf9–/– lungs (H) compared with controls (G). (I,J) Increased distal and ectopic proximal epithelial expression of Bmp4in E14.5 Fgf9dox(48)lungs (J) compared
D
E
V
E
LO
P
M
E
N
T
which FGF9 primarily functions to stimulate the sub-mesothelial mesenchyme, whereas HH signaling functions to increase proliferation and maintain cell survival in the sub-epithelial compartment. In vitro organ cultures, in which lung explants were treated with FGF9 and cyclopamine, support a model in which SHH acts in an independent, spatially specific manner to regulate mesenchymal proliferation. Additionally, FGF9 may indirectly contribute to sub-epithelial mesenchymal proliferation through regulation of Shh.
Discrepancies in current models of lung mesenchyme development may be due to the previously unrecognized division of mesenchyme compartments. Our model is inconsistent with that of
Weaver et al. (Weaver et al., 2003), which suggests that FGF9 does not induce proliferation in SHH treated mesenchyme-only explants (Weaver et al., 2003). By contrast, we show that FGF9 is both necessary and sufficient to promote mesenchymal proliferation in vivo and in vitro. A possible explanation for the lack of observed FGF9-induced proliferation in the mesenchyme-only explant system may be due to the requirement of SHH for both survival and proliferation, which we suggest will enrich for sub-epithelial cells that are unable to proliferate in response to FGF9. Interestingly, in the Weaver et al. study, some markers in the mesenchyme-only explants demonstrated an unusual distribution, which supports a mixture of distinct mesenchymal cells.
Additionally, the model presented by Weaver et al. (Weaver et al., 2003) suggested that an FGF9-promoted zone of undifferentiated cells is present in the sub-mesothelial mesenchyme. We show that FGF9 is sufficient to expand Tie2-lacZ and PECAM-positive cells into the sub-mesothelial region in vivo, which probably represents increased vascular development. Thus, FGF9 may signal to undifferentiated mesenchyme and/or vascular progenitor cells to control vascular development. Interestingly, a recent study suggests that early lung vascular development occurs through distal angiogenesis (Parera et al., 2005) and would suggest that FGF9 promotes blood vessel formation from pre-existing vessels. Studies are under way to determine the relationship between FGF9 and vascular development.
FGF9 regulates epithelial development through mesenchymal FGF expression
A key requirement for the formation of an epithelial branch is a focal source of a ligand that is capable of stimulating epithelial migration. A mesenchymal FGF10 to epithelial FGFR2b signal fulfills this role (Arman et al., 1999; Bellusci et al., 1997b; Celli et al., 1998; De Moerlooze et al., 2000; Min et al., 1998; Park et al., 1998; Peters et al., 1992; Sekine et al., 1999). Because branching morphogenesis is severely decreased in lungs lacking FGF9 and absent in lungs overexpressing FGF9, we sought to further explore the relationship between FGF9 signaling and Fgf10expression and signaling.
Overexpression of FGF9 caused dramatic epithelial expansion in vitro and in vivo, and loss of FGF9 resulted in mesenchymal depletion, decreased Fgf10 expression and decreased branching morphogenesis (Fig. 8B,D). Because FGF9 is unlikely to signal to epithelial splice forms of FGFR1 and FGFR2 (Ornitz et al., 1996), we hypothesized that FGF9 overexpression induces a secondary mesenchymal signal(s), such as Fgf10, which could mediate epithelial luminal expansion. To characterize this mesenchymal to epithelial signal, we examined the expression of Fgf10and the distal epithelial markers, Spry2andBmp4, both of which are positively regulated by FGF10 in vitro (Lebeche et al., 1999; Mailleux et al., 2001; Weaver et al., 2000).At E11.5, Fgf10expression appeared normal in Fgf9–/–lungs but was greatly reduced at later stages
(Colvin et al., 2001). Consistent with FGF10 being a primary mesenchymal to epithelial signal, expression of Spry2and Bmp4 were downregulated over a similar time course, and ectopic FGF9 enhanced expression of Fgf10, Bmp4and Spry2both in vivo and in vitro. Interestingly, in FGF9 gain-of-function experiments, Bmp4 and Spry2expression were upregulated ectopically throughout the proximal and distal airway epithelium, whereas Fgf10expression was restricted to the distal mesenchyme (Fig. 8A,D). This suggested that the hypothesized FGF9-induced mesenchymal to epithelial signal may originate from both proximal and distal mesenchyme. Consistent with this, ectopic FGF9 upregulated Fgf7throughout the sub-epithelial mesenchyme, making FGF7 a likely candidate to proliferation
proliferation
Fgf7
epithelium
epithelial dilation
Fgf10 sub-mesothelial
mesenchyme mesenchymesub-epithelial
epithelial branching mesothelium
1
2 3
4
5
mesothelial FGF9 epithelial FGF9
Fgf10
Fgf9 Ptc1 + Fgf7
Ptc1
SHH Signaling
A
Fgf9-/- WT Fgf9dox(48) Cy
[image:9.612.51.299.66.341.2]B C D E
D
E
V
E
LO
P
M
E
N
T
mediate FGF9-induced epithelial expansion and ectopicSpry2and Bmp4expression (Fig. 8A,D). These data suggest that during normal lung development, an epithelial to mesenchymal FGF signal, such as FGF9, induces Fgf7following branching morphogenesis. FGF7 may then facilitate the transition from the pseudoglandular to the canalicular phase of lung development.
SHH is expressed at the highest levels in the distal tip of the epithelial ducts and has been shown to suppress Fgf10expression (Lebeche et al., 1999; Pepicelli et al., 1998). Consistent with this, FGF10 expression (Mailleux et al., 2005) and branching (Fig. 6; Fig. 8E) is increased in cyclopamine treated lung explants. In Fgf9 -overexpressing lungs, the uniformity of sub-mesothelial Fgf10 in the distal mesenchyme and the upregulation of SHH signaling represents an apparent paradox as enhanced SHH signaling should repress Fgf10. Additionally, current models predict an increase in branching with increased Fgf10 expression. Instead, branching morphogenesis abruptly stops when Fgf9is overexpressed. This paradox can be resolved by a model in which increased HH signaling in the immediate sub-epithelial mesenchyme (induced by FGF9) represses Fgf10 expression through a HH-dependent pathway, resulting in the termination of branching morphogenesis. In the sub-mesothelial region, distal from the source of SHH, FGF9 induces Fgf10expression. Distal non-focal expression of FGF10 may be insufficient to induce epithelial branching, but sufficient to enhance tubule elongation (Fig. 8D).
We thank H. Kanazawa and K. Yu for providing embryos; and B. Mecham, D. Beebe, F. Long, K. Lavine and X. Zhang for helpful discussions. We thank B. Hogan (Fgf10), H. Umemori (Fgf7), A. McMahon (Bmp4,Shh,Ptch1), G. Martin (Spry2) and F. Long (Gli1) for providing in situ probes; and J. Whitsett (SPC-rtTA), T. Sato (Tie2-LacZ), R. Harland (Noggin-LacZ) and J. Partanen (Fgfr1flox) for providing mice. This work was supported by the March of Dimes
Foundation (FY02-194), NIH DK52574 (microinjection), Training Grant T32 HL07873 and a contribution from the Alice & Julius Kantor Charitable Trust.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/132/8/1507/DC1
References
Arman, E., Haffner-Krausz, R., Gorivodsky, M. and Lonai, P.(1999). Fgfr2 is required for limb outgrowth and lung-branching morphogenesis. Proc. Natl. Acad. Sci. USA 96, 11895-11899.
Bellusci, S., Henderson, R., Winnier, G., Oikawa, T. and Hogan, B. L.(1996). Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development122, 1693-1702.
Bellusci, S., Furuta, Y., Rush, M. G., Henderson, R., Winnier, G. and Hogan, B. L.(1997a). Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development124, 53-63.
Bellusci, S., Grindley, J., Emoto, H., Itoh, N. and Hogan, B. L.(1997b). Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development124, 4867-4878.
Bitgood, M. J. and McMahon, A. P.(1995). Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 172, 126-138.
Brunet, L. J., McMahon, J. A., McMahon, A. P. and Harland, R. M.(1998). Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science280, 1455-1457.
Cardoso, W. V., Itoh, A., Nogawa, H., Mason, I. and Brody, J. S.(1997). FGF-1 and FGF-7 induce distinct patterns of growth and differentiation in embryonic lung epithelium. Dev. Dyn. 208, 398-405.
Celli, G., LaRochelle, W. J., Mackem, S., Sharp, R. and Merlino, G.(1998). Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J.17, 1642-1655.
Chuang, P. T., Kawcak, T. and McMahon, A. P.(2003). Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev.17, 342-347.
Colvin, J. S., Feldman, B., Nadeau, J. H., Goldfarb, M. and Ornitz, D. M.
(1999). Genomic organization and embryonic expression of the mouse fibroblast growth factor 9 gene. Dev. Dyn. 216, 72-88.
Colvin, J. S., White, A., Pratt, S. J. and Ornitz, D. M.(2001). Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development128, 2095-2106.
De Moerlooze, L., Spencer-Dene, B., Revest, J., Hajihosseini, M., Rosewell, I. and Dickson, C.(2000). An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development127, 483-492.
Fadel, B. M., Boutet, S. C. and Quertermous, T.(1999). Octamer-dependent in vivo expression of the endothelial cell-specific TIE2 gene. J. Biol. Chem.274, 20376-20383.
Grindley, J. C., Bellusci, S., Perkins, D. and Hogan, B. L.(1997). Evidence for the involvement of the Gli gene family in embryonic mouse lung development. Dev. Biol.188, 337-348.
Guo, L., Degenstein, L. and Fuchs, E.(1996). Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev.10, 165-175.
Hanafusa, H., Torii, S., Yasunaga, T. and Nishida, E.(2002). Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol. 4, 850-858.
Ingham, P. W. and McMahon, A. P.(2001). Hedgehog signaling in animal development: paradigms and principles. Genes Dev.15, 3059-3087.
Lebeche, D., Malpel, S. and Cardoso, W. V.(1999). Fibroblast growth factor interactions in the developing lung. Mech. Dev.86, 125-136.
Litingtung, Y., Lei, L., Westphal, H. and Chiang, C.(1998). Sonic hedgehog is essential to foregut development. Nat. Genet. 20, 58-61.
Mailleux, A. A., Tefft, D., Ndiaye, D., Itoh, N., Thiery, J. P., Warburton, D. and Bellusci, S.(2001). Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech. Dev.102, 81-94.
Mailleux, A. A., Kelly, R., Veltmaat, J. M., De Langhe, S. P., Zaffran, S., Thiery, J. P. and Bellusci, S.(2005). Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development132, 2157-2166.
Malpel, S., Mendelsohn, C. and Cardoso, W. V.(2000). Regulation of retinoic acid signaling during lung morphogenesis. Development127, 3057-3067.
Miller, L. A., Wert, S. E. and Whitsett, J. A.(2001). Immunolocalization of sonic hedgehog (Shh) in developing mouse lung. J. Histochem. Cytochem.49, 1593-1604.
Min, H., Danilenko, D. M., Scully, S. A., Bolon, B., Ring, B. D., Tarpley, J. E., DeRose, M. and Simonet, W. S.(1998). Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 12, 3156-3161.
Ornitz, D. M., Xu, J., Colvin, J. S., McEwen, D. G., MacArthur, C. A., Coulier, F., Gao, G. and Goldfarb, M.(1996). Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292-15297.
Parera, M. C., van Dooren, M., van Kempen, M., de Krijger, R., Grosveld, F., Tibboel, D. and Rottier, R.(2005). Distal angiogenesis: a new concept for lung vascular morphogenesis. Am. J. Physiol. Lung Cell Mol. Physiol.288, L141-L149.
Park, W. Y., Miranda, B., Lebeche, D., Hashimoto, G. and Cardoso, W. V.
(1998). FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Dev. Biol.201, 125-134.
Pepicelli, C. V., Lewis, P. M. and McMahon, A. P.(1998). Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol.8, 1083-1086.
Perl, A. K., Tichelaar, J. W. and Whitsett, J. A.(2002). Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res.11, 21-29.
Peters, K. G., Werner, S., Chen, G. and Williams, L. T.(1992). Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development
114, 233-243.
Peters, K., Werner, S., Liao, X., Wert, S., Whitsett, J. and Williams, L.(1995). Targeted expression of a dominant negative FGF receptor blocks branching morphogenesis and epithelial differentiation of the mouse lung. EMBO J. 13, 3296-3301.
Post, M., Souza, P., Liu, J., Tseu, I., Wang, J. X., Kuliszewski, M. and Tanswell, A. K.(1996). Keratinocyte growth factor and its receptor are involved in regulating early lung branching. Development122, 3107-3115.
Santos-Ocampo, S., Colvin, J. S., Chellaiah, A. T. and Ornitz, D. M.(1996). Expression and biological activity of mouse fibroblast growth factor-9. J. Biol. Chem. 271, 1726-1731.
Sekine, K., Ohuchi, H., Fujiwara, M., Yamasaki, M., Yoshizawa, T., Sato, T., Yagishita, N., Matsui, D., Koga, Y., Itoh, N. et al.(1999). Fgf10 is essential for limb and lung formation. Nat. Genet.21, 138-141.
Shannon, J. M. and Hyatt, B. A.(2004). Epithelial-mesenchymal interactions in the developing lung. Annu. Rev. Physiol.66, 625-645.
D
E
V
E
LO
P
M
E
N
T
Pulmonary malformation in transgenic mice expressing human keratinocyte growth factor in the lung. Proc. Natl. Acad. Sci. USA 92, 12461-12465.
Soriano, P.(1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71.
Tefft, D., Lee, M., Smith, S., Crowe, D. L., Bellusci, S. and Warburton, D.(2002). mSprouty2 inhibits FGF10-activated MAP kinase by differentially binding to upstream target proteins. Am. J. Physiol. Lung Cell Mol. Physiol.283, L700-L706.
Tefft, J. D., Lee, M., Smith, S., Leinwand, M., Zhao, J., Bringas, P., Jr, Crowe, D. L. and Warburton, D.(1999). Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr. Biol.9, 219-222.
Tichelaar, J. W., Lu, W. and Whitsett, J. K.(2000). Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem.
275, 11858-11864.
Trokovic, R., Trokovic, N., Hernesniemi, S., Pirvola, U., Vogt Weisenhorn, D. M., Rossant, J., McMahon, A. P., Wurst, W. and Partanen, J.(2003). FGFR1
is independently required in both developing mid- and hindbrain for sustained response to isthmic signals. EMBO J.22, 1811-1823.
van Tuyl, M. and Post, M.(2000). From fruitflies to mammals: mechanisms of signalling via the Sonic hedgehog pathway in lung development. Respir. Res.1, 30-35.
Weaver, M., Dunn, N. R. and Hogan, B. L.(2000). Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development127, 2695-2704.
Weaver, M., Batts, L. and Hogan, B. L.(2003). Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev. Biol.258, 169-184.
Yu, K., Xu, J., Liu, Z., Sosic, D., Shao, J., Olson, E. N., Towler, D. A. and Ornitz, D. M.(2003). Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development130, 3063-3074.