Bloodstream Infection in Long-Term Catheters: a Prospective Study
M. Guembe,aP. Martín-Rabadán,aA. Echenagusia,bF. Camúñez,bG. Rodríguez-Rosales,bG. Simó,bM. Echenagusia,bE. Bouza,a,b on behalf of the GEIDI Study Group
Department of Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria del Hospital Gregorio Marañón, Universidad Complutense, Madrid, Spaina
; Department of Vascular Interventional Radiology, Hospital General Universitario Gregorio Marañón, Madrid, Spainb
Cultures taken from the skin and from the hubs of short-term central venous catheters can help us to predict catheter-related
bloodstream infections (C-RBSIs). The value of these cultures for such predictions has not been assessed in long-term catheters.
Our objective was to assess the value of superficial cultures for the prediction of C-RBSI among patients with long-term
cathe-ters. Over a 2-year period, we prospectively obtained cultures from the skin overlying reservoir ports (group A) and from the
skin insertion site and hubs of all tunneled catheters (group B). This routine was performed by vascular and interventional
radi-ologists immediately before catheter removal (irrespective of the reason for withdrawal). Swabs were processed
semiquantita-tively. Catheter tips from both groups were cultured using Maki’s semiquantitative technique and sonication. We also
per-formed cultures of the reservoir ports at different sites. C-RBSI was defined as the isolation of the same species of
microorganism(s) both in the colonized catheter and in at least 1 peripheral blood culture. We included 372 catheters (group A,
223; group B, 149) during the study period. The catheter colonization rate was 23.4% (87/372), and 28 patients had C-RBSI.
Va-lidity index values for the capacity of surface cultures to predict C-RBSI in groups A and B were, respectively, as follows:
sensitiv-ity, 23.5% and 45.5%; specificsensitiv-ity, 59.7% and 63.0%; positive predictive value, 4.6% and 8.9%; and negative predictive value,
90.4% and 93.5%. Superficial cultures of patients with long-term catheters could help us to rule out the catheter as the portal of
entry of bloodstream infections. Superficial cultures (from skin and hubs) proved to be a useful conservative diagnostic tool for
ruling out C-RBSI among patients with long-term tunneled catheters and totally implantable venous access ports.
C
atheter-related bloodstream infection (C-RBSI) is an
impor-tant nosocomial entity with high rates of morbidity and
mor-tality (
1–5
). Patients undergoing chemotherapy or hemodialysis
are at risk of developing C-RBSI, as they need a permanent
intra-vascular device. Although C-RBSI rates are low in these patients
(
6–12
), the difficulties and complications attributable to catheter
replacement require the use of alternative diagnostic tools which
allow us to predict infection without having to withdraw the
cath-eter.
Microorganisms colonizing skin, hubs, or both are considered
the first step to catheter tip colonization and, consequently, to
C-RBSI (
13–15
). Therefore, superficial cultures (skin and hubs)
are good diagnostic tools for predicting catheter colonization and
C-RBSI. However, they have been tested only in patients with
short-term central venous catheters, who are mainly admitted to
intensive care units (
13
,
16
). Data on the usefulness of superficial
cultures in a subpopulation using long-term catheters are scarce.
The purpose of our study was to assess the validity values of
superficial cultures for the prediction of C-RBSI in patients with
long-term tunneled catheters and totally implantable venous
ac-cess ports.
MATERIALS AND METHODS
Setting.We performed a prospective study between July 2009 and April 2011 at a large tertiary institution in Madrid, Spain.
We included all long-term central venous catheters that were routinely removed in the Vascular and Interventional Radiology Department, irre-spective of the reason for withdrawal. No antimicrobial-coated catheters were used during the study period.
Catheters were classified into two groups: group A, totally implantable venous access ports; and group B, tunneled central venous catheters.
Laboratory procedures.Catheter tips from groups A and B were an-alyzed using Maki’s semiquantitative roll-plate technique and the sonica-tion method in a random order (1:1). The roll-plate technique was applied by transferring each catheter tip to a plate with Columbia agar supple-mented with 5% sheep blood and rolling the tip back and forth across the surface at least 3 to 4 times (17). Sonication was performed by placing the catheter tip in 10 ml of brain heart infusion broth, sonicating for 1 min (at 55,000 Hz and 125 W), and vortexing for 15 s. Then, 0.1 ml of the soni-cated broth and 0.1 ml of a 1:100 dilution of the broth were streaked onto sheep blood agar plates. The plates were incubated aerobically for 48 h at 37°C. The colonies recovered were counted (18).
We performed venous access port cultures (Columbia blood agar) using the following samples and sites: port content aspirate before and after sonication, port sonication fluid, and port internal surface biofilm. The laboratory management of venous access port sites was as described in a previous study by our group (19).
The microorganisms recovered from catheter cultures were fully iden-tified by standard microbiologic methods using an automated MicroScan system with POS Combo Panel Type 2S and NEG Combo Panel Type 1S (Dade Behring, Sacramento, CA).
Superficial cultures.Cultures from the skin insertion site (groups A and B) and all hubs (group B) were collected immediately before catheter withdrawal. For skin samples, a dry cotton swab was rubbed over a 2-cm2
area around the insertion site. For hub samples, an alginate swab was
Received23 May 2013Returned for modification1 July 2013
Accepted10 July 2013
Published ahead of print12 July 2013
Address correspondence to M. Guembe, mariaguembe@hotmail.com.
Copyright © 2013, American Society for Microbiology. All Rights Reserved.
doi:10.1128/JCM.01351-13
on May 16, 2020 by guest
http://jcm.asm.org/
introduced in each hub and rubbed 3 times against the inner surface. When swabs arrived at the laboratory, they were rubbed onto Columbia blood agar and incubated aerobically for 48 h at 37°C. The colonies recov-ered were counted. Cultures withⱖ15 CFU/plate were considered posi-tive (13). The microorganisms recovered from cultures were identified by their phenotypic characteristics.
Definitions. (i) Tunneled catheter colonization.Tunneled catheter colonization was defined as detection of the presence of a positive semi-quantitative tip culture by either the roll-plate technique (ⱖ15 CFU/ plate) or the sonication method (ⱖ100 CFU/plate) (20).
(ii) Venous access port colonization.Venous access port colonization was defined as detection of the presence of a positive semiquantitative tip culture by either the roll-plate technique (ⱖ15 CFU/plate) or the sonica-tion method (ⱖ100 CFU/plate) and/or a positive quantitative culture (ⱖ100 CFU/ml) of port content aspirate before or after sonication or of
port sonication fluid or a positive qualitative culture of port internal sur-face biofilm.
(iii) Gold standard for C-RBSI.The gold standard for C-RBSI detec-tion was defined as isoladetec-tion of the same microorganism(s) in both the colonized catheter and at least 1 peripheral blood culture obtained 7 days before or after catheter withdrawal (21).
Statistical analysis.Qualitative variables are expressed as a frequency distribution and quantitative variables as means and standard deviations or median and interquartile ranges (nonnormal distribution).
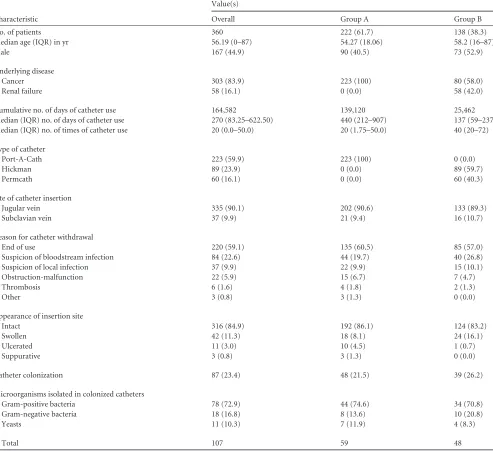
[image:2.585.47.540.81.532.2]Validity values were defined as follows: sensitivity, proportion of col-onized catheters causing C-RBSI detected using the tested culture with respect to the total number of colonized catheters causing C-RBSI de-tected by the gold standard; specificity, proportion of noncolonized cath-eters not causing C-RBSI detected using the tested culture with respect to the total noncolonized catheters not causing C-RBSI detected by the gold TABLE 1Patient and catheter characteristicsa
Characteristic
Value(s)
Overall Group A Group B
No. of patients 360 222 (61.7) 138 (38.3)
Median age (IQR) in yr 56.19 (0–87) 54.27 (18.06) 58.2 (16–87)
Male 167 (44.9) 90 (40.5) 73 (52.9)
Underlying disease
Cancer 303 (83.9) 223 (100) 80 (58.0)
Renal failure 58 (16.1) 0 (0.0) 58 (42.0)
Cumulative no. of days of catheter use 164,582 139,120 25,462
Median (IQR) no. of days of catheter use 270 (83.25–622.50) 440 (212–907) 137 (59–237) Median (IQR) no. of times of catheter use 20 (0.0–50.0) 20 (1.75–50.0) 40 (20–72)
Type of catheter
Port-A-Cath 223 (59.9) 223 (100) 0 (0.0)
Hickman 89 (23.9) 0 (0.0) 89 (59.7)
Permcath 60 (16.1) 0 (0.0) 60 (40.3)
Site of catheter insertion
Jugular vein 335 (90.1) 202 (90.6) 133 (89.3)
Subclavian vein 37 (9.9) 21 (9.4) 16 (10.7)
Reason for catheter withdrawal
End of use 220 (59.1) 135 (60.5) 85 (57.0)
Suspicion of bloodstream infection 84 (22.6) 44 (19.7) 40 (26.8)
Suspicion of local infection 37 (9.9) 22 (9.9) 15 (10.1)
Obstruction-malfunction 22 (5.9) 15 (6.7) 7 (4.7)
Thrombosis 6 (1.6) 4 (1.8) 2 (1.3)
Other 3 (0.8) 3 (1.3) 0 (0.0)
Appearance of insertion site
Intact 316 (84.9) 192 (86.1) 124 (83.2)
Swollen 42 (11.3) 18 (8.1) 24 (16.1)
Ulcerated 11 (3.0) 10 (4.5) 1 (0.7)
Suppurative 3 (0.8) 3 (1.3) 0 (0.0)
Catheter colonization 87 (23.4) 48 (21.5) 39 (26.2)
Microorganisms isolated in colonized catheters
Gram-positive bacteria 78 (72.9) 44 (74.6) 34 (70.8)
Gram-negative bacteria 18 (16.8) 8 (13.6) 10 (20.8)
Yeasts 11 (10.3) 7 (11.9) 4 (8.3)
Total 107 59 48
a
Values represent numbers (%) of patients except where otherwise indicated. Group A, totally implantable venous access ports; group B, long-term tunneled catheters; IQR, interquartile range.
on May 16, 2020 by guest
http://jcm.asm.org/
standard; positive predictive value, proportion of colonized catheters causing C-RBSI detected using the tested culture matching colonized catheters causing C-RBSI detected by the gold standard with respect to the total colonized catheters causing C-RBSI detected by the tested culture; negative predictive value, proportion of noncolonized catheters not caus-ing C-RBSI detected uscaus-ing the tested culture matchcaus-ing noncolonized
catheters not causing C-RBSI detected by the gold standard with respect to the total noncolonized catheters not causing C-RBSI detected by the tested culture.
Statistical significance was set atPⱕ0.05.
[image:3.585.44.545.81.622.2]The statistical analysis was performed using SPSS 16.0 and EPIDAT. Ethics.The study was approved by the local ethics committee. TABLE 2Description of the 28 C-RBSI episodesa
Characteristic
Value(s)
Overall Group A Group B
No. of C-RBSI episodes 28 17 (60.7) 11 (39.3)
Median (IQR) age in yr 59.28 (47.03–67.86) 55.05 (18.75) 59.8 (42.5–68.6)
Male 15 (53.6) 11 (64.7) 4 (36.4)
Underlying disease
Cancer 22 (78.6) 17 (100) 5 (45.5)
Renal failure 6 (21.4) 0 (0.0) 6 (54.5)
Median (IQR) Charlson comorbidity index 4 (2–5.75) 5 (2.0–7.5) 4 (2–5)
Mean (SD) McCabe-Jackson index 2.68 (0.670) 2.59 (0.712) 2.82 (0.603)
Cumulative no. of days of catheter use 13,838 12,303 1,535
Median (IQR) no. of days of catheter use 248 (55.75–726.75) 613 (163.50–1,082.50) 119.5 (37.8–246.3)
Site of catheter insertion
Jugular vein 24 (85.7) 13 (76.5) 11 (100)
Subclavian vein 4 (14.3) 4 (23.5) 0 (0.0)
Time that blood cultures were drawn
Before catheter withdrawal 25 (89.3) 15 (88.2) 10 (90.9)
At catheter withdrawal 1 (3.6) 0 (0.0) 1 (9.1)
After catheter withdrawal 2 (7.1) 2 (11.8) 0 (0.0)
Reason for catheter withdrawal
Suspicion of bloodstream infection 23 (82.1) 13 (76.5) 10 (90.9)
Suspicion of local infection 5 (17.9) 4 (23.5) 1 (9.1)
Appearance of insertion site
Intact 12 (42.9) 5 (29.4) 7 (63.6)
Ulcerated 6 (21.4) 6 (35.3) 0 (0.0)
Swollen 8 (28.6) 4 (23.5) 4 (36.4)
Suppurative 2 (7.1) 2 (11.8) 0 (0.0)
Median (IQR) DDDs 23.85 (10.63–55.50) 29.4 (18.05–63.20) 11 (4.80–50.00)
Median (IQR) total days of therapy 16 (10.25–27.75) 19 (14–28) 16 (6–27)
Microorganisms causing C-RBSI
Gram-positive bacteria 18 (62.1) 11 (61.1) 7 (63.6)
Staphylococcus aureus 9 (31.3) 6 (33.3) 3 (27.3)
Staphylococcus epidermidis 7 (24.1) 3 (16.7) 4 (36.4)
Enterococcus faecalis 2 (6.9) 2 (11.1) 0 (0.0)
Gram-negative bacteria 7 (24.1) 4 (22.2) 3 (27.3)
Stenotrophomonas maltophilia 2 (6.9) 0 (0.0) 2 (18.2)
Proteus mirabilis 2 (6.9) 1 (5.6) 1 (9.1)
Escherichia coli 1 (3.4) 1 (5.6) 0 (0.0)
Enterobacter cloacae 1 (3.4) 1 (5.6) 0 (0.0)
Serratia marcescens 1 (3.4) 1 (5.6) 0 (0.0)
Yeasts 4 (13.8) 3 (16.7) 1 (9.1)
Candida parapsilosis 2 (6.9) 2 (11.1) 0 (0.0)
Candida glabrata 1 (3.4) 1 (5.6) 0 (0.0)
Candida tropicalis 1 (3.4) 0 (0.0) 1 (9.1)
Total 29 18 11
a
Values represent numbers (%) of patients except where otherwise indicated. C-RBSI, catheter-related bloodstream infection, IQR, interquartile range; SD, standard deviation; DDDs, defined daily doses; Group A, totally implantable venous access ports; group B, long-term tunneled catheters.
on May 16, 2020 by guest
http://jcm.asm.org/
RESULTS
We included 372 long-term central venous catheters from 360
patients during the study period. Of these, 223 (59.9%) were
ve-nous access ports and 149 (40.1%) were tunneled catheters. The
median catheter indwelling time was 270 days (interquartile range
[IQR], 83.25 to 622.50). The main underlying disease was cancer
(83.9%), followed by renal failure (16.1%). Most catheters were
removed because of end of use (59.1%), followed by suspicion of
bloodstream infection (22.6%). Other patient and catheter
char-acteristics are detailed in
Table 1
.
The overall catheter colonization rate was 23.4% (87/372), and
the distribution of the isolated microorganisms was as follows:
Gram-positive bacteria, 72.9%; Gram-negative bacteria, 16.8%;
and yeast species, 10.3% (
Table 1
).
During the total of 164,582 cumulative catheter days, we found
28 episodes of C-RBSI (incidence density, 0.17 episodes/1,000
catheter days). The main underlying disease with C-RBSI was
can-cer (78.6%), and many patients showed no external signs of
infec-tion (42.9%). The microorganisms isolated from the C-RBSI
ep-isodes were distributed as follows: Gram-positive bacteria, 62.1%;
Gram-negative bacteria, 24.1%; and yeast species, 13.8%. The
most frequently isolated microorganism causing C-RBSI was
Staphylococcus aureus
(31.0%) (
Table 2
).
The colonization rates of superficial cultures were 39.0% (86/
223) and 37.6% (56/149) in groups A and B, respectively. The
most predominant microorganisms were coagulase-negative
staphylococci (66.7% in group A and 65.4% in group B) (
Table 3
).
The validity values of superficial cultures for prediction of C-RBSI
in groups A and B were, respectively, as follows: sensitivity, 23.5%
and 45.5%; specificity, 59.7% and 63.0%; positive predictive
value, 4.6% and 8.9%; and negative predictive value, 90.4% and
93.5% (
Table 4
).
In group B (patients with tunneled catheters), catheter hub
cultures proved to have good (98.6%) specificity for the
predic-tion of C-RBSI.
DISCUSSION
Our study showed that negative superficial cultures allow us to
rule out C-RBSI in patients with long-term catheters. Long-term
central venous catheters are widely used in patients with cancer or
renal failure, as these populations need a permanent vascular
ac-cess for chemotherapy and hemodialysis. Therefore, they are at
risk of developing C-RBSI. Rates of C-RBSI range from 0.10 to
0.37 episodes/catheter days among patients undergoing
chemo-therapy and 0.5 to 7.6 episodes/1,000 catheter days among
pa-tients undergoing hemodialysis (
6–8
,
11
,
22–27
).
The most appropriate procedure for confirming an episode of
C-RBSI is microbiological culture, which requires the catheter to
be withdrawn (
20
). However, in this subpopulation of patients,
catheter replacement is not always possible, since it involves severe
complications during the surgical insertion procedure (
28–30
).
Therefore, conservative methods may be required for the
diagno-sis of C-RBSI. Evaluation of differential times to positivity
(DTTP) has proven effective for the diagnosis of C-RBSI in the
general population (
16
,
31–33
). However, it requires drawn blood
from all catheter hubs and from a peripheral vein, which in most
cases represents a great amount of blood. This, particularly,
rep-resents a big problem in the neonatal population and in patients
whose catheters have persistent occlusion. Moreover, the
applica-tion of DTTP evaluaapplica-tion for catheter-related candidemia has not
been already established (
34
,
35
). Therefore, the use of a rapid and
easy-to-perform technique such as superficial culture analysis
may allow us to solve these problems.
Superficial culture analyses have proven to be useful diagnostic
methods that do not require the catheter to be withdrawn, as they
are performed by taking cultures from the skin around the
cathe-ter insertion site and from the incathe-ternal surface of all hubs
(super-ficial cultures). However, studies evaluating this method have
been tested mainly in critical ill patients with short-term central
venous catheters (
13
,
16
). We provide novel data regarding the
validity values of superficial cultures tested in patients with
long-TABLE 3Etiology of positive superficial culturesaMicroorganism
Value(s)
Group A (skin)
Group B
Global Skin Hubs
Gram-positive bacteria 133 (92.4) 68 (84.0) 67 (87.0) 1 (25.0) CoNS 96 (66.7) 53 (65.4) 52 (67.5) 1 (25.0)
Corynebacterium
spp.
15 (10.4) 5 (6.2) 5 (6.5) 0 (0.0)
Staphylococcus aureus
13 (9.0) 9 (11.1) 9 (11.7) 0 (0.0)
Micrococcusspp. 5 (3.5) 1 (1.2) 1 (1.3) 0 (0.0)
Enterococcusspp. 3 (2.1) 0 (0.0) 0 (0.0) 0 (0.0)
Streptococcus viridans
1 (0.7) 0 (0.0) 0 (0.0) 0 (0.0)
Gram-negative bacteria
10 (6.9) 7 (8.6) 6 (7.8) 1 (25.0)
Enterobacteriaceae 4 (2.8) 5 (6.2) 4 (5.2) 1 (25.0)
NFGNB 6 (4.2) 2 (2.5) 2 (2.6) 0 (0.0)
Yeasts 1 (0.7) 6 (7.4) 4 (5.2) 2 (50.0)
Candida albicans 0 (0.0) 2 (2.5) 1 (1.3) 1 (25.0)
Candida parapsilosis 1 (0.7) 2 (2.5) 2 (2.6) 0 (0.0)
Candida glabrata 0 (0.0) 2 (2.5) 1 (1.3) 1 (25.0)
Total 144 81 77 4
a
[image:4.585.40.288.77.344.2]Values represent numbers (%) of patients except where otherwise indicated. CoNS, coagulase-negative staphylococci; NFGNB, nonfermenting Gram-negative bacilli; Group A, totally implantable venous access ports; group B, long-term tunneled catheters.
TABLE 4Validity values of superficial cultures for the prediction of catheter-related bloodstream infectiona
Group Parameter
Value (%)
Overall Skin Hubs
A S 23.5
SP 59.7
PPV 4.6
NPV 90.4
B S 45.5 36.4 18.2
SP 63.0 63.8 98.6
PPV 8.9 7.4 50.0
NPV 93.5 92.6 93.8
a
S, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; group A, totally implantable venous access ports; group B, long-term tunneled catheters. Values of⬎90% are shown in boldface type.
on May 16, 2020 by guest
http://jcm.asm.org/
[image:4.585.300.544.86.208.2]term catheters. We demonstrate that superficial cultures had a
good (93.5%) negative predictive value for C-RBSI in long-term
catheters and could help us to rule out the catheter as the origin of
the bacteremia without needing to remove it. Moreover,
long-term catheters are colonized mainly by the intraluminal route
(
36
), and our data showed that hub cultures from tunneled
cath-eters had high (98.6%) specificity for predicting C-RBSI. This may
enable C-RBSIs to be managed by combining systemic and lock
antimicrobial therapy, thus obviating the need for catheter
with-drawal.
The main limitations of our study were the low number of
C-RBSI episodes and that we performed only superficial cultures
before catheter withdrawal. Besides, we have no data available
regarding antibiotic use before catheter withdrawal, which could
partially explain the low sensitivity and the low positive predictive
value. Moreover, our results may not be entirely applicable to all
patients with long-term tunneled catheters or port reservoirs, as
we did not include patients whose device was removed because of
failure of conservative treatment. Future studies must evaluate the
validity of superficial cultures while the catheter is being used as a
surveillance measure.
In conclusion, superficial cultures performed on long-term
central venous catheters may be useful for ruling out an episode of
C-RBSI.
ACKNOWLEDGMENTS
This study was supported by Ministerio de Sanidad y Consumo (Instituto de Salud Carlos III) and Fundación para la Investigación Biomédica del Hospital General Gregorio Marañón (FIBHGM) (CM09/00028). This study was partially financed by grants from Fundación Mutua Madrileña de Madrid.
We thank Thomas O’Boyle for his help in the preparation of the man-uscript.
We thank the members of the GEIDI Study Group for their contribu-tion to the work as follows: José Eugenio Guerrero, Milagros Sancho, Braulio de la Calle, Carlos Sotillo, Guiomar Sánchez, Esther Bermejo López, Lorenzo Fernández Quero, Ana Lajara, Isabel Frías, Carmen Heras, María Jesús Pérez, José Maria Barrio, Alejandro Garrido Sanchez, Patricia Muñoz, Marta Rodríguez-Créixems, Mar Santos, Eduardo Verde, Fernando González García, Emilia Bastida, Maite López Gil, Teresa Blanco, Cristina Cuerda, Laura Frías, José María Tellado, Antonio Ech-enagusia, Fernando Camúñez, Gracia Rodríguez Rosales, Gonzalo Simó, Mikel Echenagusia, Sonia Zamorano Caballero, Ana Barrientos Guerrero, Abilio Calderón Martín, Carmen Flores Sánchez, M. Jesús Ruano Sta. Engracia, Esperanza Arranz García, M. Ana Luna Caballero, Mar San Segundo Sánchez, Amelia V. Fernández Alonso, and M. Nieves Moro Tejedor.
We declare that we have no conflicts of interest.
REFERENCES
1.Blot SI, Depuydt P, Annemans L, Benoit D, Hoste E, De Waele JJ, Decruyenaere J, Vogelaers D, Colardyn F, Vandewoude KH. 2005. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin. Infect. Dis.41:1591–1598. 2.Dimick JB, Pelz RK, Consunji R, Swoboda SM, Hendrix CW, Lipsett PA.2001. Increased resource use associated with catheter-related blood-stream infection in the surgical intensive care unit. Arch. Surg.136:229 – 234.
3.Maki DG, Kluger DM, Crnich CJ.2006. The risk of bloodstream infec-tion in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin. Proc.81:1159 –1171. 4.Renaud B, Brun-Buisson C; ICU-Bacteremia Study Group.2001.
Out-comes of primary and catheter-related bacteremia. A cohort and case-control study in critically ill patients. Am. J. Respir. Crit. Care Med.163: 1584 –1590.
5.Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, Fraser VJ.2006. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit. Care Med. 34:2084 –2089.
6.Biffi R, de Braud F, Orsi F, Pozzi S, Mauri S, Goldhirsch A, Nole F, Andreoni B.1998. Totally implantable central venous access ports for long-term chemotherapy. A prospective study analyzing complications and costs of 333 devices with a minimum follow-up of 180 days. Ann. Oncol.9:767–773.
7.Crisinel M, Mahy S, Ortega-Debalon P, Buisson M, Favre JP, Chavanet P, Piroth L.2009. Incidence, prevalence and risk factors for a first infec-tious complication on a totally implantable venous-access port. Med. Mal. Infect.39:252–258. (In French.)
8.Groeger JS, Lucas AB, Thaler HT, Friedlander-Klar H, Brown AE, Kiehn TE, Armstrong D. 1993. Infectious morbidity associated with long-term use of venous access devices in patients with cancer. Ann. In-tern. Med.119:1168 –1174.
9.Hoen B, Paul-Dauphin A, Hestin D, Kessler M.1998. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J. Am. Soc. Nephrol.9:869 – 876.
10. Kessler M, Hoen B, Mayeux D, Hestin D, Fontenaille C.1993. Bacte-remia in patients on chronic hemodialysis. A multicenter prospective sur-vey. Nephron64:95–100.
11. Kuizon D, Gordon SM, Dolmatch BL.2001. Single-lumen subcutaneous ports inserted by interventional radiologists in patients undergoing che-motherapy: incidence of infection and outcome of attempted catheter salvage. Arch. Intern. Med.161:406 – 410.
12. Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB.2003. Current trends in the epidemiology of nosocomial bloodstream infections in pa-tients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin. Infect. Dis.36:1103–1110.
13. Cercenado E, Ena J, Rodriguez-Creixems M, Romero I, Bouza E.1990. A conservative procedure for the diagnosis of catheter-related infections. Arch. Intern. Med.150:1417–1420.
14. Liñares J, Sitges-Serra A, Garau J, Pérez JL, Martín R.1985. Pathogen-esis of catheter sepsis: a prospective study with quantitative and semiquan-titative cultures of catheter hub and segments. J. Clin. Microbiol.21:357– 360.
15. Templeton A, Schlegel M, Fleisch F, Rettenmund G, Schobi B, Henz S, Eich G.2008. Multilumen central venous catheters increase risk for cath-eter-related bloodstream infection: prospective surveillance study. Infec-tion36:322–327.
16. Bouza E, Alvarado N, Alcala L, Perez MJ, Rincon C, Munoz P.2007. A randomized and prospective study of 3 procedures for the diagnosis of catheter-related bloodstream infection without catheter withdrawal. Clin. Infect. Dis.44:820 – 826.
17. Maki DG, Weise CE, Sarafin HW. 1977. A semiquantitative culture method for identifying intravenous-catheter-related infection. N. Engl. J. Med.296:1305–1309.
18. Sherertz RJ, Heard SO, Raad II.1997. Diagnosis of triple-lumen catheter infection: comparison of roll plate, sonication, and flushing methodolo-gies. J. Clin. Microbiol.35:641– 646.
19. Guembe M, Marin M, Martin-Rabadan P, Echenagusia A, Camunez F, Rodriguez-Rosales G, Simo G, Echenagusia M, Bouza E.2013. Use of universal 16S rRNA gene PCR as a diagnostic tool for venous access port-related bloodstream infections. J. Clin. Microbiol.51:799 – 804. 20. Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad
II, Rijnders BJ, Sherertz RJ, Warren DK.2009. Clinical practice guide-lines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis.49:1– 45.
21. O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S; Healthcare Infection Control Practices Advisory Committee (HICPAC).2011. Guidelines for the prevention of intravas-cular catheter-related infections. Clin. Infect. Dis.52:e162– e193. 22. Chang L, Tsai JS, Huang SJ, Shih CC.2003. Evaluation of infectious
complications of the implantable venous access system in a general onco-logic population. Am. J. Infect. Control31:34 –39.
23. Fariñas MC, García-Palomo JD, Gutiérrez-Cuadra M.2008. Infection associated with hemodialysis and peritoneal dialysis catheters. Enferm. Infecc. Microbiol. Clin.26:518 –526. (In Spanish.)
24. Grothe C, da Silva Belasco AG, de Cassia Bittencourt AR, Vianna LA,
on May 16, 2020 by guest
http://jcm.asm.org/
de Castro Cintra Sesso R, Barbosa DA.2010. Incidence of bloodstream infection among patients on hemodialysis by central venous catheter. Rev. Lat. Am. Enfermagem.18:73– 80.
25. Klevens RM, Edwards JR, Andrus ML, Peterson KD, Dudeck MA, Horan TC.2008. Dialysis surveillance report: National Healthcare Safety Network (NHSN)— data summary for 2006. Semin. Dial.21:24 –28. 26. Powe NR, Jaar B, Furth SL, Hermann J, Briggs W.1999. Septicemia in
dialysis patients: incidence, risk factors, and prognosis. Kidney Int.55: 1081–1090.
27. Saeed Abdulrahman I, Mueilo SH, Bokhary HA, Ladipo GO, Al-Rubaish A.2002. A prospective study of hemodialysis access-related bac-terial infections. J. Infect. Chemother.8:242–246.
28. Eastridge BJ, Lefor AT.1995. Complications of indwelling venous access devices in cancer patients. J. Clin. Oncol.13:233–238.
29. Silas AM, Perrich KD, Hoffer EK, McNulty NJ.2010. Complication rates and outcomes of 536 implanted subcutaneous chest ports: do rates differ based on the primary operator’s level of training? Acad. Radiol.17:464 – 467.
30. Walser EM.2012. Venous access ports: indications, implantation tech-nique, follow-up, and complications. Cardiovasc. Intervent. Radiol.35: 751–764.
31. Blot F, Nitenberg G, Chachaty E, Raynard B, Germann N, Antoun S, Laplanche A, Brun-Buisson C, Tancrede C.1999. Diagnosis of
catheter-related bacteraemia: a prospective comparison of the time to positivity of hub-blood versus peripheral-blood cultures. Lancet354:1071–1077. 32. Catton JA, Dobbins BM, Kite P, Wood JM, Eastwood K, Sugden S,
Sandoe JA, Burke D, McMahon MJ, Wilcox MH.2005. In situ diagnosis of intravascular catheter-related bloodstream infection: a comparison of quantitative culture, differential time to positivity, and endoluminal brushing. Crit. Care Med.33:787–791.
33. Raad I, Hanna HA, Alakech B, Chatzinikolaou I, Johnson MM, Tarrand J.2004. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann. Intern. Med.140:18 –25. 34. Ben-Ami R, Weinberger M, Orni-Wasserlauff R, Schwartz D, Itzhaki A,
Lazarovitch T, Bash E, Aharoni Y, Moroz I, Giladi M.2008. Time to blood culture positivity as a marker for catheter-related candidemia. J. Clin. Microbiol.46:2222–2226.
35. Bouza E, Alcala L, Munoz P, Martin-Rabadan P, Guembe M, Rodri-guez-Creixems M.2013. Can. microbiologists help to assess catheter in-volvement in candidaemic patients before removal? Clin. Microbiol. In-fect.19:E129 –E135.
36. Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP. 1993. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis.168:400 – 407.