IJPSR (2019), Volume 10, Issue 7 (Research Article)
Received on 31 October 2018; received in revised form, 09 January 2019; accepted, 31 January 2019; published 01 July 2019
2D QSAR STUDY OF INDOLE DERIVATIVES AS SELECTIVE COX-2 INHIBITORS
R. S. Chavan * 1, H. N. More 2 and A. V. Bhosale 1
Department of Pharmaceutical Chemistry 1, Pune District Education Association’s Seth Govind Raghunath Sable College of Pharmacy, Saswad, Pune - 412301, Maharashtra, India.
Bharati Vidyapeeth’s College of Pharmacy 2
, Kolhapur-400614, Maharashtra, India.
ABSTRACT: The NSAIDs are popular in reducing acute and chronic
inflammation as they have no abuse liability. QSAR (Quantitative structure-activity relationship) approach is a very useful and widespread technique for drug design. 2D QSAR models are based on descriptors derived from a two-dimensional graph representation of a molecule. The 2D QSAR study was performed on selected twenty-four compounds from synthesized indole derivatives for elucidating the structural requirements for COX-2 inhibition using multiple linear regression method. Statistically, significant models were generated using VLife Molecular Design Suite 3.5 software. The physicochemical parameters contributed significantly to biological activity. Amongst all the models generated, model 3 was found to be best with high r2 (squared correlation coefficient) of 0.9382. Model is robust as q2 (cross-validated squared correlation coefficient) value is also high as 0.8557 with good predictive power as indicated by pred_r2 = 0.7443. The model showed two alignment independent (AI) descriptors T_2_O_0 and T_2_N_7 as well as two physicochemical descriptors –ve Potential Surface Area and SA Most Hydrophobic contributing for activity. The present study may prove to be helpful in the development and optimization of existing indole derivatives as anti-inflammatory agents with selective COX-2 inhibition.
INTRODUCTION: NSAIDs inhibit cyclo-oxygenase (COX), the enzyme responsible for the conversion of arachidonic acid to prostaglandins. COX exists in 2 isoforms. COX-1 is a ubiquitous constitutive isozyme producing prostaglandins responsible for homeostatic functions such as maintenance of GI mucosal integrity. COX-2 is largely a cytokine-induced isozyme producing prostaglandins that mediate pain and inflammation 1
. NSAIDs inhibit both COX-1 and COX-2 to varying degrees.
QUICK RESPONSE CODE
DOI:
10.13040/IJPSR.0975-8232.10(7).3378-85
The article can be accessed online on
www.ijpsr.com
DOI link: http://dx.doi.org/10.13040/IJPSR.0975-8232.10(7).3378-85
Thus, the therapeutic effects of conventional NSAIDs are derived from the inhibition of COX-2, while the adverse effects of these agents, particularly in the upper GI tract, arise from inhibition of COX-1 activity 2. Much recent effort thus has been made to produce selective inhibitors of cyclooxygenase-2 (COX-2) in the belief that these will lack the gastrointestinal damaging effects of traditional non-steroidal anti-inflammatory drugs (NSAIDs) 3, 4, 5.
Diarylheterocycle class of compounds has been investigated extensively as COX-2 inhibitors. Literature survey revealed that indole derivatives, pyrazoline derivatives, and pyrimidine derivatives independently possess good anti-inflammatory, analgesic activity, and selective COX-2 inhibitory effects 6, 7, 8, 9, 10, 11. Hence, we focused at achieving greater selectivity for COX-2 enzymes with the use
Keywords:
Anti-inflammatory, Indole, COX-2, 2D QSAR
Correspondence to Author: Dr. Mrs. Rajashree S. Chavan
Associate Professor,
Department of Pharmaceutical Chemistry, PDEA’s Seth Govind Raghunath Sable College of Pharmacy, Saswad, Pune - 412301, Maharashtra, India.
of indole nucleus and other structural features of different COX-2 inhibitors (pyrazoline derivatives, pyrimidine derivatives, and sulfinyl methyl group) in the designed molecules. Thus, the concept of chemical derivatization of the indole nucleus with pyrazoline and pyrimidine was attempted. Three series of the target molecules are synthesized. The compounds synthesized were subjected to preliminary pharmacological evaluation for anti-inflammatory and analgesic activity by using models like carrageenin-induced rat paw edema method and acetic acid-induced writhing in mice, respectively. The compounds were also screened for acute ulcerogenicity by using Wistar rats. The compounds viz. IA7, IA9, IA11, IA12, IB3, IB7, IB12, IIA2, IIA3, IIA4, IIA5, IIA10, IIB2, IIB3, IIB4, IIB5, IIB7, IIIA4, IIIA10, IIIA11, IIIA17, IIIB10, IIIB11, IIIB17 showing comparable anti-inflammatory, analgesic activities with less ulceration were subjected to in-vitro cyclo-oxygenase (COX) inhibition assays using Celecoxib as the reference 12.
The computer-aided prediction of biological activity about the chemical structure of a compound is now a commonly used technique in
drug discovery. Computational chemistry represents
molecular structures as a numerical model and simulates their behavior with the equations of quantum and classical physics. Available programs enable scientists to easily generate and present molecular data including geometries, energies, and associated properties (electronic, spectroscopic, and bulk). The usual paradigm for displaying and manipulating these data is a table in which compounds are defined by individual rows, and molecular properties (or descriptors) are defined by the associated columns 13, 14, 15.
QSAR (Quantitative structure-activity relationship) attempts to find consistent relationships between the variations in the values of molecular properties and the biological activity (% activity, IC50, ED50, MIC) for a series of compounds to generate a mathematical expression so that these rules can be used to evaluate new chemical entities. The mathematical expression can then be used to predict the biological response of other chemical structures. 2D QSAR models are based on descriptors derived from a two-dimensional graph representation of a molecule.
A QSAR generally takes the form of a linear equation:
Biological Activity = Const + (C1 × P1) + (C 2 × P2) + (C3 ×P3) +...
Where the P1 to Pn are physicochemical parameters value computed for each molecule in the series and C1 to Cn are the coefficients of parameters. Physicochemical descriptors are based on the physicochemical properties of the molecule.
In the present research work, a data set of twenty-four molecules showing comparable COX-2 inhibitory activity was subjected to 2D quantitative structure-activity relationship (QSAR) analyses, in search of newer and potent anti-inflammatory agents with selective COX-2 inhibition. Statistically, significant models were generated, and the most robust models for 2D QSAR were obtained using partial least square regression method coupled with a stepwise forward-backward method using V-Life Molecular Design Suite software version 3.5.
MATERIALS AND METHODS:
Optimization of Molecules Structure: A data set of twenty-four molecules showing comparable COX-2 inhibitory activity measured was chosen for the present 2D QSAR study. Table 1 the biological activity was expressed as IC50 values measured on COX-2 enzyme.
TABLE 1: THE CHEMICAL STRUCTURES OF COMPOUNDS USED FOR 2D QSAR STUDY
N
R2
R1
R3
S. no. Compd R1 R2 R3
1 IA7
5-(4-methoxyphenyl)-1-phenylpyrazoline
4-chlorophenyl H
2 IA9 1,5-diphenylpyrazoline 4-methoxyphenyl H
3 IA11
5-(4-methoxyphenyl)-1-phenylpyrazoline
4-methoxyphenyl H
4 IA12
5-(4-dimethyaminophenyl)-1-phenylpyrazoline
4-methoxyphenyl H
5 IB3 4(4-methoxyphenyl)
pyrimidin-2-amine
Phenyl Phenyl
6 IB7 4(4-methoxyphenyl)
pyrimidin-2-amine
4-chlorophenyl Phenyl
7 IB12 4(4-(dimethylamino)
phenyl)pyrimidin-2-amine
4-methoxyphenyl Phenyl
8 IIA2 methylsulfonyl
5-(4-chlorophenyl)-1-phenylpyrazoline
Phenyl
9 IIA3 methylsulfonyl
(4-bromophenyl)-1-phenylpyrazoline
Phenyl
10 IIA4 methylsulfonyl
5-(4-methoxyphenyl)-1-phenylpyrazoline
Phenyl
11 IIA5 methylsulfonyl
5-(4-dimethyaminophenyl)-1-phenylpyrazoline
Phenyl
12 IIA10 Tosyl
(5-(4-methoxyphenyl)-1-phenylpyrazoline
Phenyl
13 IIB2 methylsulfonyl 4-(4-chlorophenyl)
pyrimidin-2-amine
Phenyl
14 IIB3 methylsulfonyl 4-(4-bromophenyl)
pyrimidine-2-amine
Phenyl
15 IIB4 methylsulfonyl 4-(4-methoxyphenyl)
pyrimidin-2-amine
Phenyl
16 IIB5 methylsulfonyl 4-(4-(dimethylamino)
phenyl)pyrimidin-2-amine
Phenyl
17 IIB7 Tosyl 4-phenylpyrimidin-2-amine Phenyl
18 IIIA4 H Phenyl
3-(4-hydroxyphenyl)-1-phenylpyrazoline
19 IIIA10 H 4-chlorophenyl
3-(4-hydroxyphenyl)-1-phenylpyrazoline
20 IIIA11 H 4-chlorophenyl
3-(4-aminophenyl)-1-phenylpyrazoline
21 IIIA17 H 4-methoxyphenyl
3-(4-aminophenyl)-1-phenylpyrazoline
22 IIIB10 H 4-chlorophenyl 4-(2-amino)
pyrimidin-4-yl) phenol
23 IIIB11 H 4-chlorophenyl 4-(4-aminophenyl)
pyrimidin-2-amine
24 IIIB17 H 4-methoxyphenyl 4-(4-aminophenyl)
pyrimidin-2-amine
The optimization process was started. The energy and gradient of each iteration were reported in Output Window. After the successful termination of the optimization process, the final output in the
Calculation of Descriptors for QSAR: The 2D QSAR was launched by picking Modules in an appropriate sequence as per the manual. By selecting the QSAR Tool ‘Calculate Descriptors’ various physicochemical descriptors were selected. For performing QSAR analysis, descriptors that show variation for all the molecules are required. As the descriptors that were constant for all the molecules were not considered to contribute to QSAR and hence removed from the worksheet. Training and test set from the data were selected by manual selection method, ensuring the molecules have uniform spread (train and test) in terms of both activity and chemical space. In the generation of the QSAR model, we have selected eight molecules in test and sixteen in the training set. Table 2 all calculations were run on a Pentium IV personal computer with the window XP operating system. Total of 213 Descriptors was evaluated.
TABLE 2: LIST OF SETS OF TRAINING AND TEST COMPOUNDS FOR QSAR STUDIES
Training Set (16 Molecules)
IA7 IIA10
IA11 IIB4
IB3 IIB5
IB7 IIIA4
IB12 IIIA11
IIA3 IIIA17
IIA4 IIIB10
IIA5 IIIB11
Test Set (08 Molecules)
IA9 IIB3
IA12 IIB7
IIA2 IIIA10
IIB2 IIIB17
Then data selection was done considering the negative log of IC50 values under the dependent variable and the remaining variables considered as independent. Regression methods selected were Multiple and Forward-Backward as the Stepwise Variable Selection from the Select Variable Selection Method panel. Stepwise parameter setting was done as cross-correlation limit =0.5, number of variable in final equation = 4, term selection criteria as r, F test in as 4 and F test out as 3.99. Additional parameter settings as: Variance Cut-Off = 0, Scaling option = Auto Scaling. The best model was selected based on various statistical parameters such as squared correlation coefficient (r2), standard error of estimation (SE), sequential Fischer test (F). Quality and predictability of the model were estimated from the cross-validated
squared correlation coefficient. The various 2D-QSAR models were developed using the MLR method. 2D-QSAR equations were selected by optimizing the statistical results generated along with a variation of the descriptors in these models. The fitness/pattern plots were also generated for evaluating the dependence of the biological activity on various types of descriptors Fig. 1.
FIG. 1: FITNESS GRAPH OF OBSERVED AND PREDICTED IC50 DATA FOR ALL 24 COMPOUNDS
DERIVED FROM MODEL NO. 3
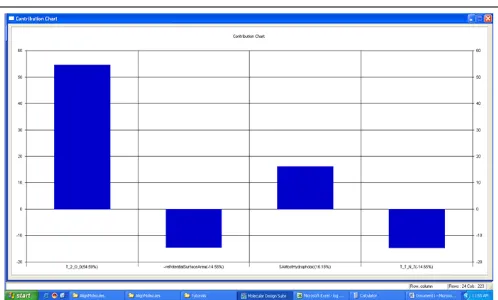
The frequency of use of a particular descriptor in the population of equations indicated the relevant contributions of the descriptors. Contribution chart Fig. 2 signifies that the descriptors below the zero line have negative contribution and above the zero lines has a positive contribution. The best regression equation obtained is represented as model 3.
FIG. 2: CONTRIBUTION PLOT DERIVED IN MODEL NO. 3
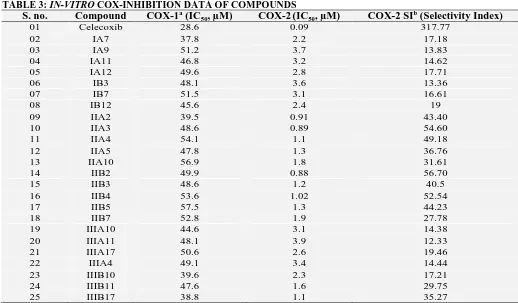
[image:4.612.48.298.329.507.2] [image:4.612.315.564.487.637.2]54.60, 56.70 and 52.54 for COX-2 vs. COX-1. The compounds IIIB11 and IIIB17 from series III also showed a selectivity index of 29.75 and 35.27, respectively. The remaining compounds in series IA, IB, IIIA and IIIB have shown less selectivity index as compared to compounds in IIA and IIB
[image:5.612.46.564.147.450.2]series. The results show that the presence of a sulfonyl group in series IIA and IIB is favorable for maximum drug-receptor interactions. In the compounds IIIB11 and IIIB17, presence of hydrogen bonding groups is important for optimum drug-receptor interactions Table 3.
TABLE 3: IN-VITRO COX-INHIBITION DATA OF COMPOUNDS
S. no. Compound COX-1a (IC50, µM) COX-2(IC50, µM) COX-2 SI
b
(Selectivity Index)
01 Celecoxib 28.6 0.09 317.77
02 IA7 37.8 2.2 17.18
03 IA9 51.2 3.7 13.83
04 IA11 46.8 3.2 14.62
05 IA12 49.6 2.8 17.71
06 IB3 48.1 3.6 13.36
07 IB7 51.5 3.1 16.61
08 IB12 45.6 2.4 19
09 IIA2 39.5 0.91 43.40
10 IIA3 48.6 0.89 54.60
11 IIA4 54.1 1.1 49.18
12 IIA5 47.8 1.3 36.76
13 IIA10 56.9 1.8 31.61
14 IIB2 49.9 0.88 56.70
15 IIB3 48.6 1.2 40.5
16 IIB4 53.6 1.02 52.54
17 IIB5 57.5 1.3 44.23
18 IIB7 52.8 1.9 27.78
19 IIIA10 44.6 3.1 14.38
20 IIIA11 48.1 3.9 12.33
21 IIIA17 50.6 2.6 19.46
22 IIIA4 49.1 3.4 14.44
23 IIIB10 39.6 2.3 17.21
24 IIIB11 47.6 1.6 29.75
25 IIIB17 38.8 1.1 35.27
a Values are means of two determinations acquired using an ovine COX-1/COX-2 assay kit and the deviation from the mean is
<10% of the mean value. bIn-vitro COX-2 selectivity index (COX-1 IC50/COX-2 IC50).
2D-QSAR Equation Interpretation: Among the generated QSAR models; model 3 was selected based on various statistical parameters such as squared correlation coefficient (r2), which is a relative measure of the quality of fit.
Fischer’s value (F test) which represents F-ratio between the variance of calculated and observed activity, standard error (r2_se) representing absolute measure of quality of fit, and cross-validated square correlation coefficient (q2), standard error of cross-validated square correlation coefficient (q2_se), predicted squared regression (pred_r2) and standard error of predicted squared regression (pred_r2se) to estimate the predictive potential of the models, respectively.
Model 1:
IC50 = 3.4687 (± 1.1887) SA Most Hydrophobic + 10.9861(± 4.2795) Average +ve Potential -3.5648.
Statistical Data: n = 16, Degree of freedom = 13, r2 = 0.8120, q2 = 0.6542 F test = 21.4359, r2 se = 0.1728, q2 se = 0.2937, pred_r2 = 0.6180, pred_r2se = 0.1883.
Model 2:
IC50 = -2.2496 (± 0.0314) XA Most Hydrophilic + 4.5372 (± 1.1283) SA Most Hydrophobic + 13.7287 (±5.1352) Average +ve Potential -2.3174.
Statistical Data: n = 16, Degree of freedom = 12, r2 = 0.8636, q2 = 0.7264, F test = 36.6845, r2 se = 0.1278, q2 se = 0.1145, pred_r2 = 0.7180, pred_r2se = 0.1183.
Model 3:
IC50 = 0.2167 (±0.0015) T_2_O_0 - 0.0014 (±0.0000) –ve Potential Surface Area + 4.0448
(±0.9698) SA Most Hydrophobic -0.0143
Statistical Data: n = 16, Degree of freedom = 11, r2 = 0.9382 , q2 = 0.8557, F test = 41.7199, r2 se = 0.0596, q2 se = 0.0911, pred_r2 = 0.7443, pred_r2se = 0.1274.
Interpretation of Model 3: Several models were generated for 2 D QSAR model development. Model 3 was found to be best with high r2 of 0.9382. Model is robust as q2 value is also high as 0.8557 with good predictive power as indicated by pred_r2=0.7443. The model showed two alignment independent (AI) descriptors T_2_O_0 and T_2_N_7 as well as two physicochemical descriptors -ve Potential Surface Area and SA Most Hydrophobic contributing for activity. From the derived QSAR model, it can be concluded that anti inflammatory activity of indole derivatives is
strongly influenced by physicochemical descriptors.
Alignment Independent (AI) Descriptors: T_2_O_0 is the count of a number of double bounded atoms (i.e., any double bonded atom, T_2) separated from oxygen atom by 0 bonds in a molecule and is positively contributing (54.59%). This indicates that double bonded oxygen atoms should be more for increasing activity. Thus, it can
be predicted that the sulfonyl group in series II is responsible for high COX-2 selectivity of the few of the compounds in the series.T_2_N_7 (-14.65% contribution) is the count of the number of double bounded atoms (i.e., any double bonded atom, T_2) separated from nitrogen atom by seven bonds in a molecule and is negatively contributing (-14.65%). This indicates that a reduction in double bond characteristics surrounding nitrogen of indole ring at a 7th bond distance will lead to an increase in activity.
Physicochemical Descriptor: -ve Potential Surface Area is the descriptor which signifies total van der Waals surface area with the negative electrostatic potential of the molecule. This is contributing negatively (-14.58%) indicate that negative electrostatic potential on the surface should be reduced by attaching electron donating groups.
SA Most Hydrophobic is the most hydrophobic value on the vdW surface, which is positively contributing (16.18%) indicates that more bulky groups will further increase the activity by increasing surface hydrophobicity.
TABLE 4: INTERCORRELATION MATRIX OF DESCRIPTORS USED IN MODEL NO. 3
Parameter T_2_O_0 –ve Potential Surface
Area
SA Most Hydrophobic
T_2_N_7 IC50
T_2_O_0 1
–ve Potential Surface Area 0.2081 1
SA Most Hydrophobic -0.1463 0.1695 1
T_2_N_7 0.1678 0.1986 0.3151 1
IC50 0.5192 -0.12674 -0.1687 -0.1378 1
TABLE 5: OBSERVED AND PREDICTED IC50 VALUE DATA FOR TRAINING SET COMPOUNDS (16
MOLECULES) OBTAINED FROM MODEL NO. 3
Compound Code Observed Value Predicted Value Residual Value Residual Variance
IA7 -0.342423 -0.376169 0.033746 0.0043
IA11 -0.505150 -0.460390 -0.04476 0.1590
IB3 -0.556303 -0.582001 0.025698 0.0044
IB7 -0.491362 -0.486880 -0.004482 0.0049
IB12 -0.380211 -0.422895 0.042684 0.1770
IIA3 0.050610 0.010688 0.039922 0.0019
IIA4 -0.041393 -0.074962 0.033569 0.0035
IIA5 -0.176091 -0.116537 -0.059554 0.0006
IIA10 -0.255273 -0.167558 -0.087715 0.0060
IIB4 -0.041393 -0.073966 0.032573 0.0573
IIB5 -0.113943 -0.155171 0.041228 0.0535
IIIA4 -0.531479 -0.599633 0.068154 0.0093
IIIA11 -0.591065 -0.541233 -0.049832 0.0157
IIIA17 -0.414973 -0.400449 -0.014524 0.0002
IIIB10 -0.361728 -0.395808 0.03408 0.0104
[image:6.612.53.565.534.743.2]TABLE 6: OBSERVED AND PREDICTED IC50 VALUE DATA FOR TEST SET COMPOUNDS (08 MOLECULES)
OBTAINED FROM MODEL NO. 3
Compound Code Observed Value Predicted Value Residual Value Residual Variance
IA9 -0.568202 -0.430638 -0.137564 0.1900
IA12 -0.447158 -0.522218 0.07506 0.0409
IIA2 0.040959 0.004411 0.036548 0.0713
IIB2 0.055517 -0.045410 0.100927 0.0803
IIB3 -0.079181 -0.043709 -0.035472 0.0122
IIB7 -0.278754 -0.084583 -0.194171 0.0049
IIIA10 -0.491362 -0.492239 0.000877 0.0924
IIIB17 -0.322219 -0.518414 0.196195 0.0557
The plot of observed versus predicted activity provides an idea about how well the model was trained and how well it predicts the activity of the external test set Table 5 and Table 6. From the plots it can be seen that the model is able to predict the activity of training set quite well (all points are close to regression line) as well as external test set up to 62% (only few points are relatively apart from the regression line) providing confidence in predictive ability of the model Fig. 1.
CONCLUSION: The present work describes the 2D QSAR study which generated an equation which signifies that the count of number of double bounded atoms separated from oxygen atom by 0 bonds in a molecule is positively contributing thus indicating double bonded oxygen atoms should be more for increasing activity and total van der Waals surface area with negative electrostatic potential of the molecule contributing negatively indicating that negative electrostatic potential on surface should be reduced by attaching electron donating groups.
It also signifies that most hydrophobic value on the vdW surface positively contributing and thus indicating that more bulky groups will further increase the activity by increasing surface hydrophobicity. It further signifies the count of the number of double bounded atoms (i.e. any double bonded atom, T_2) separated from nitrogen atom by seven bonds in a molecule which is negatively contributing (-14.65%). Thus, the results obtained can be used for further modification and optimization of the indole derivatives for better anti-inflammatory activity with selective COX-2 inhibition.
ACKNOWLEDGEMENT: The authors are grateful to Principal Dr. Ashok V. Bhosale for providing infrastructure and facilities to conduct the research activity. We are also thankful to VLife
Sciences for providing technical support from time to time.
CONFLICT OF INTEREST: All authors have none to declare.
REFERENCES:
1. Abdellatif KR, Abdelall EK, Fadaly WA and Kamel GM: Synthesis, cyclooxygenase inhibition, and anti-inflammatory evaluation of novel diarylheterocycles with a central pyrazole, pyrazoline, or pyridine ring. Medicinal Chemistry Research 2015; 24: 2632-44.
2. Husain A, Ahmad A, Alam MM, Ajmal M and Ahuja P: Fenbufen based 3-[5-(substituted aryl)-1,3,4-oxadiazol-2-yl]-1 -(biphenyl-4-yl) propan-1-ones as safer anti-inflammatory and analgesic agents. European Journal of Medicinal Chemistry 2009; 44: 3798-04.
3. Ross E: Goodman & Gilaman’s, the pharmacological basis of therapeutics. McGrahill Medical Publishing Division, New York, Edition 10th, 2000: 31-38.
4. Zarghi A and Arfaei S: Selective COX-2 inhibitors: A review of their structure-activity relationships. Iranian Journal of Pharmaceutical Research 2011; 10(4): 655-83. 5. Abdelazeem AH, Abdelatef SA, El-Saadi MT, Omar HA,
Khan SI, McCurdy CR and El-Moghazy SM: Novel pyrazolopyrimidine derivatives targeting COXs and iNOS enzymes; design, synthesis and biological evaluation as potential anti-inflammatory agents. European Journal of Pharmaceutical Sciences 2014; 62: 197-11.
6. Abdellatif KR, Abdelgawad MA, Labib MB and Zidan TH: Synthesis, cyclooxygenase inhibition, anti-inflammatory evaluation and ulcerogenic liability of novel triaryl pyrazoline derivatives as selective COX-2 inhibitors. Bioorganic Medicinal Chemistry Letters 2015; 25: 5787-91.
7. Barsoum FF and Girgis AS: Facile synthesis of bis (4, 5-dihydro-1Hpyrazole-1-carboxamides) and their thio-analogues of potential PGE2 inhibitory properties. European Journal of Medicinal Chemistry 2009; 44: 2172-77.
8. Tewari AK, Singh VP, Yadav P, Gupta G, Singh A, Goel RK, Shinde P and Mohan CG: Synthesis, biological evaluation and molecular modeling study of pyrazole derivatives as selective COX-2 inhibitors and anti-inflammatory agents. Bioorganic Chemistry 2014; 56: 8-15.
[image:7.612.52.574.78.184.2]10. Kalgutkar AS, Marnett AB, Crews BC, Remmel RP and Marnett LJ: Ester and amide derivatives of the nonsteroidal anti-inflammatory drug, indomethacin, as selective COX-2 inhibitors. Journal of Medicinal Chemistry 2000; 43: 2860-70.
11. Rani P, Srivastava VK and Kumar A: Synthesis and anti-inflammatory activity of heterocyclic indole derivatives. European Jour of Medicinal Chemistry 2004; 39: 449-52. 12. Chakraborti AK, Garg SK, Motiwala HF and Jadhavar PS:
Progress in COX-2 Inhibitors: A journey so far. Current Medicinal Chemistry 2010; 17: 1563-93.
13. Katritzky A: QSAR modeling, synthesis and bioassay of diverse leukemia RPMI-8226 cell line active agents. Bioorganic and Medicinal Chemistry Letters 2010; 45: 5183-99.
14. Rapatri V, Chitre T and Bothara K: Novel 4-(morpholin-4-yl)-N -(arylidene) benzohydrazides: Synthesis, anti-mycobacterial activity and QSAR investigations. European Journal of Medicinal Chemistry 2009; 44: 3954-60. 15. VLife Molecular Design Suite version 3.5; VLife Sciences
Technologies Pvt. Ltd., 2010, Pune, India.
All © 2013 are reserved by International Journal of Pharmaceutical Sciences and Research. This Journal licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.
This article can be downloaded to Android OS based mobile. Scan QR Code using Code/Bar Scanner from your mobile. (Scanners are available on Google Play store)
How to cite this article: