International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 9, Issue 8, August 2019)
27
Effects of Some Amine Treatments on Corrosion of Mild Steel
in Acidic Chloride Environment
T.N. Guma
1, K.O. Daniel
2Department of Mechanical Engineering, Nigerian Defence Academy, Kaduna, Nigeria
Abstract-- Inhibitory aftermaths of 40-520ppm treatment concentrations of cyclohexylamine, dicyclohexylamine and
dimethylamine on mild steel corrosion in 0.5M H2SO4
containing 3.5%-wt NaCl were separately investigated at ambient laboratory temperature for various exposure durations of 6 to 30 days with the steel coupons. Obtained information on gravimetrically determined corrosion rates of the coupons indicated different considerable inhibition protection efficiencies of the steel corrosion by the amine concentrations in the medium. The protection efficiencies by
cyclohexylamine, dicyclohexylamine and dimethylamine
increased respectivelyfrom 45.1%, 54.1% and 19.8% with the
40ppm treatment concentration on the 6th day of exposure and
increase in exposure duration and concentration to maximum values of 84.8%, 91.3%, and 72.8% with the 520-ppm
treatment on the 30th day of exposure. To gain insight into the
inhibition mechanisms of the amines, surface morphological characteristics of coupons that exhibited the least and highest corrosion rates were studied by scanning electron microscopy technique. Microphotographs of the scanning indicated protective layer formations of decreasing coarseness with exposure time on the coupons’ surfaces to more compact and uniform formations that attained maximum coalescences on
the 30th day of exposure resulting in drastic decrease in
corrosion rates and better inhibition protection efficiencies of the steel coupons.
Keywords-- Metals, corrosive environments, multifarious prospective inhibitors, test needs for all, inhibition efficiencies, optimal performance concentrations, correct application information.
I. INTRODUCTION
Corrosion is a natural material-environment interaction process by which the material becomes degraded with attenuation in its prized physical and mechanical properties which need to be protected. If material corrosion remains unrestrained it will eventually cause failure of structural engineering material in its service applications with bad consequences. An environment is any other physical thing or various combined levels of things whether visible or not visible that the material is in contact with. There are macro and countless micro-environments where materials are applied in service. These environments are corrosive to various levels to a wide range of engineering materials.
The serious consequences of the corrosion process have been a problem of worldwide significance in our technological era. In addition to our everyday encounters with this form of degradation, corrosion causes plant shutdowns, waste of valuable resources, loss or contamination of product, reduction in efficiency, costly maintenance, and expensive over-design. Its combined effects manifest to jeopardize safety, and inhibit economic and technological progress [1]. Carbon steel is the most important structural material so its corrosion problem has been of tremendous concern in all spheres of engineering technology and economy world over. A lot of money equivalent to several millions of American dollars are spent worldwide annually on researches on the science and methods of preventing corrosion of steel, yet the up-to-date efforts and technological sophistication on the subject are far from utopian achievement [2, 3]. The practicable technological achievements have been by various levels of cost-incurring control of its corrosion. This apparently indicates that control of corrosion is primarily an economic problem. Whether or not to apply a control method in any corrosion problem is usually determined by the cost justification [2, 3].
International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 9, Issue 8, August 2019)
28
Inhibitor performance however depends on effectiveness of the inhibitor, type and nature of the metal surface, composition and corrosivity level of the liquid, the liquid temperature, concentration of the inhibitor in the liquid, mechanical effects, degree of aeration and movement of the liquid, presence of crevices and dead ends, effects of microorganisms, scale formation, toxicity disposal and effluent problems [4, 5, 6].
By design standard, optimal methodical corrosion protection of any steel type can be based on information from mild steel as the commonly used and least corrosion-resistant type [3]. Inhibitive prevention or mitigation of corrosion of mild to the barest rate has been widely studied. Most of what is known has grown from trial and error experiments, both in the laboratories and in the field. Rules, equations, and theories to guide inhibitor development or use are very limited. Most good and cheap inhibitors used in aqueous systems are organic compounds that mainly contain nitrogen, oxygen, sulfur atoms, and heterocyclic compounds containing functional groups and conjugated double bonds, and multiple bonds in their molecules through which they are adsorbed on metal surface by the formation of an adherent film. Amines and their salts are a group of abundant promising organic chemicals in the regards.
Amines are being studied and exploited singly or in various combinations with other chemicals for protecting metal works in their different service environments [7, 8, 9]. Good understanding of optimal levels and mechanisms of corrosion inhibition by all amines and their derivatives or combinations with regard to mild steel in the wide range of environments is noted to be highly desirable in their design selections and uses as corrosion inhibitors. When there is no inhibitor information on corrosion behavior of a metal or alloy or a fabrication under specific environmental conditions such as a newly formulated alloy and/or a new environment, it is essential to carry out all relevant corrosion tests to provide data that can be used by the designer, or user for best service performance of the inhibitor in the environment [9, 10, 11].
Chlorides are aggressive ions that abound in some natural environments such as surf beaches and other maritime locations and also exist to various levels in industrial environments. They are great agents of steel corrosion with capability to even attack and break down natural protective coating barriers that can form on its surface. They can also exist inadvertently by contamination in association with industrial solutions containing various levels of acid or as a result of some reactions of acids with alkalis and synergistically aggravate corrosion [12-15].
In engineering point of view, corrosion in acid media is one of the important aspects for corrosion industry. Acid solutions are often used in drilling operations in oil and gas exploration, as well as for cleaning, descaling and pickling of steel structures. These processes are normally accompanied by considerable dissolution of the metal and it is very important to add corrosion inhibitors to decrease the corrosion rate in such situations [16, 17, 18]. There are so many acid media but hydrochloric and sulphuric acids’ are more commonly used and notably very harmful to mild steel. Sulphuric acid is a strong and basically non-oxidizing acid. It is used directly or indirectly in nearly all industries and is a vital commodity in Nigerian and other national economies. Its maximum corrosion occurs at the concentration of 60 to 70%. Increasing the temperature, concentration and velocity, all tend to accelerate the attack. Materials that show good corrosion rates in other environments are often not economically feasible in others. Good judgment is required to obtain balance between service life costs of equipment [16, 17, 18].
Aqueous solutions containing chlorides have been reported to attain high corrosivity at about 3.5% chloride compositions [8, 19]. However, there is scarcity of meaningful applicable information from the literatures on the use of cyclohexylamine, dicyclohexylamine and dimethylamine to inhibit corrosion of mild steel in acidic chloride environments.
The purpose of this paper is to present a laboratory study on aqueous-phase capability levels of various separate small concentrations of cyclohexylamine, dicyclohexylamine and dimethylamine to inhibit corrosion of mild steel in 0.5M H2S04 containing 3.5%Wt NaCl at
ambient temperature under stagnant conditions. The objective of the research work was:
i. To contribute in providing information for practical application consideration and positive research interests in using the amines for corrosion protection of steel works in acidic chloride environments.
II.LITERATURE REVIEW
International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 9, Issue 8, August 2019)
29
Amines and their salts are for example used as corrosion inhibitors in boilers and in lubricating oils (morpholine), antioxidants for rubber and roofing asphalt, stabilizers for cellulose nitrate explosives, protective against damage from gamma radiation, developers in photograph, flotation agents in mining, anticling and waterproofing agents for textiles, fabric softeners, in paper coating, and for solubilizing herbicides. .Although amines and their salts have various levels of toxicities and recent interests for corrosion inhibition focus on non-toxic and environmental-friendly inhibitors due to more stringent environment quality requirements, these chemical groups have a number of promising advantages if properly used as inhibitors. These include abundance from various sources, availability at cheap cost, ease to obtain, and ability of being used in vapour or liquid phases [7, 9, 20- 25].
Cyclohexylamine is an aliphatic amine. Although it has no color, it can be colored by contamination. It mixes with water and can be recognized by its fishy odor. Like other amines, it is a weak base, compared to strong bases such as NaOH. It is a useful product for producing many other organic compounds. The main way of producing it is by hydrogenation of aniline using cobalt or nickel catalysts: C6H5NH2 + 3H2 → C6H11NH2. It is also prepared by
ammonia alkylation with cyclohexanol. Cyclohexylamine is an important product in synthesis of many other organic compounds. It is the forerunner to sulfenamide-based reagents for aiding vulcanization. It is a basic material for pharmaceuticals such as analgesics, bronchodilators, and mucolytics, It has been used as a flushing aid in the printing ink industry. The amine itself has been found to be an effective corrosion inhibitor for some metals in some environments. It is toxic by both ingestion and inhalation and can cause irritation by absorption through skin. Its inhalation per se can be fatal. It is flammable and has a flash point at 28.6 °C. In regards to occupational exposures, the National Institute for Occupational Safety and Health has suggested workers not be exposed to a recommended exposure limit of over 10ppm (40 mg/m3) over an
eight-hour work shift [22-30] Dicyclohexylamine is a secondary amine. Its chemical
formula is HN(C6H11)2. In its natural form
dicyclohexylamine has no color but it can be commercially available in more or less yellow color. It is associated with fishy odor, typical for all amines. It is sparingly soluble in water. It is an organic base and forerunner to other chemicals. dicyclohexylamine is prepared as mixture with cyclohexylamine by catalytic hydrogenation of aniline (phenylamine), with ruthenium and/or palladium catalysts. However the quantity of dicyclohexylamine produced by this method is much less compared to cyclohexylamine.
Greater yields of dicyclohexylamine are known to occur by applying the catalyst in support of niobic acid and/or tantalic acid. It is also obtained by reductive amination of cyclohexanone with ammonia or cyclohexylamine. It can also be prepared by pressure hydrogenation of diphenylamine using a ruthenium catalyst, or by the reaction of cyclohexanone with cyclohexylamine in the presence of a palladium/carbon catalyst under a hydrogen pressure of about 4 mm Hg. Applications of dicyclohexylamine are more or less the same with those of cyclohexylamine. Its applications include; vulcanization accelerators for rubber, corrosion inhibitors in steam pipes and boilers, textile chemicals and catalysts for flexible polyurethane foams, production of antioxidants in rubber and plastics, agrochemicals [25, 30].
Dimethylamine is secondary amine. Its chemical formula is (CH3)2NH. It is frequently available for sale in
aqueous solution. The current worldwide industrial production of dimethylamine is reckoned at over 300,000 tons apart from its being obtained naturally. In structural terms, dimethylamine molecule is made up of a nitrogen atom with two methyl substituent and one proton. It is a colorless flammable gas with a fishy ammoniacal odor and density of 649.6kg/m3. It reacts with acids to form salts, such as dimethylamine hydrochloride. It is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure. It is found quite widely distributed in animals and plants, and is present in many foods at the level of a few mg/kg. It is a forerunner to many important compounds produced in some industries. By reaction with carbon disulfide it yields dimethyl dithiocarbonate, a forerunner to a family of chemicals that is extensively employed in vulcanizing rubber. It is a forerunner for producing many agrochemicals and pharmaceauticals, some chemical weapons, soaps and cleaning compounds, and rocket fuel. It is toxic to various degrees to both humans and some animals [25, 31].
III. MATERIALS AND METHODOLOGY
A.Materials
Mild steel, amines, sulphuric acid, and sodium chloride were procured for the study.
1. Mild steel
International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 9, Issue 8, August 2019)
30
2. AminesCyclohexalamine, dicyclohexalamine and dimethylamine were obtained in Kaduna metropolis in Nigeria from a company that supplied laboratory facilities and chemicals.
3. Sulphuric acid and sodium chloride
Analytical grade sulphuric acid and sodium chloride were obtained from the Chemistry Laboratory of Department of Chemistry, Nigerian Defence Academy Kaduna, Nigeria and used as corrosive constituents in the study aqueous media in which the mild steel coupons were exposed.
IV. METHODOLOGY
1. Analysis of chemical composition of the procured steel rods
The chemical compositions of the procured mild steel rods were determined using the Japanese-made Shimadzu-model-PDA-7000 optical emission spectrometer metal analyzer at the Research and Development Unit of the Defence Industries Corporation of Nigeria (DICON). Three specimens each of about 20mm long, suitable for accommodation on the specimen stand of the analyzer were sawn out of each rod; one at each end, and middle of the rod.
Any rust on the specimens was then brushed off with a polishing paper so as not to include the chemical composition of the rust in the chemical analysis of the specimen. Each specimen was loaded on the specimen stand of the analyzer and the equipment was switched on. This created an arc or spark discharge that resulted in vaporization of material from the surface of the steel specimen. The atoms and ions contained in the atomic vapour were excited into emission radiation. The emitted radiation passed to the spectrometer optics via an optical fibre, where it was dispersed into its distinct spectral components that were each the characteristic of a chemical element of the steel specimen. These spectral components were automatically and appropriately analyzed by the integral computer accessory of the unit into percentage elemental weight quantities and the results were printed out by the printer unit. This was repeated with each specimen in the rod set and for all rods. Rods whose elemental chemical compositions were found to have very minimal differences by composition from one another and also from the three locations on the rod with percentage carbon contents of less than 0.3% were earmarked for the study. Any rods whose elemental weight compositions were found to deviate by more than one percent from the rest, even from one result from its specimens was discarded and results for each analysed element of the correct rods for the test were averaged. Plate I shows a side view of the PDA 7000 optical emission metal analyzer used to determine the .
Plate I: Side View of the Shimadzu PDA 7000 spectrometer metal analyzer with its integral computer accessory
2. Preparation of coupons
The ascertained mild steel rods were cut into similar cylindrical solid pieces to produce 80 coupons each of diameter 10mm and length 12mm. Grease and natural corrosion products formed on the coupons and machining burrs on them were first removed by wire brushing. The coupons were polished manually using abrasive papers of grits 80, 320, 500, and 800.
International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 9, Issue 8, August 2019)
31
Plate II: Some coupons before cleaning them
Plate III: Some coupons after cleaning them
3. Preparation of the test medium
The test acidic chloride medium was prepared by admixing 96.5% Wt 0.5M H2SO4 with 3.5% Wt NaCl in a
glass container by weight (Wt) determinations using an accurate balance. The 0.5M H2SO4 was prepared in the
laboratory using concentrated sulphuric acid of 98% purity and distilled water by adding slowly with stirring 30ml of the acid to 800ml of the distilled water in a glass container and making the glass content up to 1000ml with the distilled water and allowing the mixture to cool to room temperature. The solution was divided in equal measures into separate 1.5-litre plastic container in which different small separate quantities of 0ppm (control), 40ppm, 120ppm, 190ppm, 240ppm, and 520ppm concentrations of each of the amines were separately admixed. The extraction value in part per million (ppm) were calculated as follows using equation (1);
………… (1)
4.Exposure and determination of corrosion rates of specimens
Each coupon was weighed to the nearest 0.001mg, tong-held and immersed in the test acidic chloride medium in the separate plastic containers with labels indicating the treatment concentrations of the amine therein. The containers were left open-ended with their contents in the laboratory at ambient temperature undisturbed for various durations of up 6, 12, 18, 24, and 30 days. Thereto, the coupons were removed and rinsed to remove residual test solution and any loose corrosion products, cleaned with detergent using bristle brush under running tap water, rinsed in distilled water, dried with a lint-free towel, and reweighted in line with the ASTM G-1 method [3]. The change in weight of each coupon during its immersion period was used to determine its corrosion penetration rate (CPR. Plate IV shows some coupons after their immersions.
International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 9, Issue 8, August 2019)
32
The CPR of each coupon for each specified exposure duration and amine concentration in parts per million (ppm) was evaluated according to [2], [3];
CPR =
……….. (2a)
Where: W = weight loss in milligram of the coupon, D = density of the steel coupons (7.75 g/cm3 ); A = evaluated exposed surface area of each coupon in square centimeters before exposing them to the medium, and T = exposure time in hours [2].
The surface area of each the solid cylindrical coupon was evaluated before exposure to the medium as;
…… ……… (2b)
Where‘d’ = 9.98mm was the average diameter of each coupon after cleaning them and h = 11.99mm was the average length of each cleaned coupon as determined with a functional hand-held micrometer that measured to accuracy of 0.01mm. All handlings of the coupons were with clean hand gloves.
The obtained CPRs from equation 2a were used to calculate the percentage corrosion inhibition efficiency (IE) of the coupons by the various amine treatment concentrations according to equation (3) given as;
IE = (
) …….. ………….. (3)
Where, CPRO and CPR are the corrosion rates in
absence and in presence of the amines respectively [2, 17].
V. RESULTS AND DISCUSSIONS
A.Results
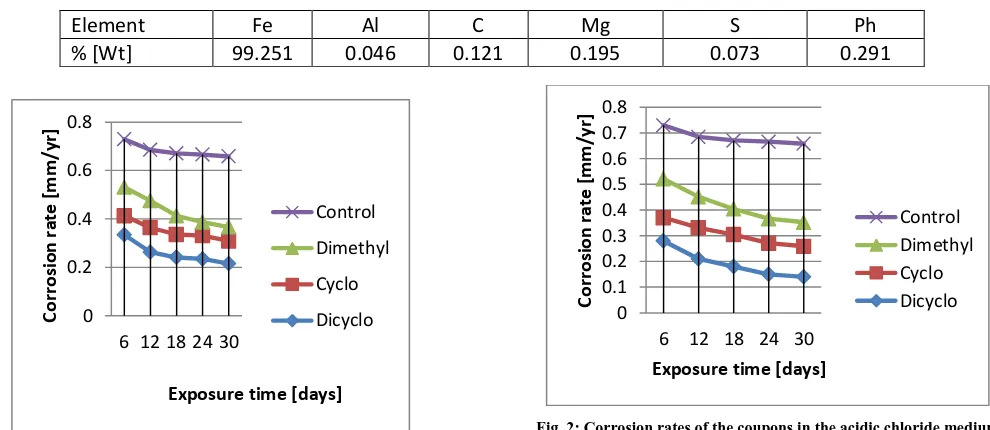
Results of the analytically obtained percentage elemental weight of the mild steel used to produce coupons for the study is shown in Table 1. Figs 1-5 show results of effects of treating 0.5M H2SO4 containing 3.5% sodium chloride
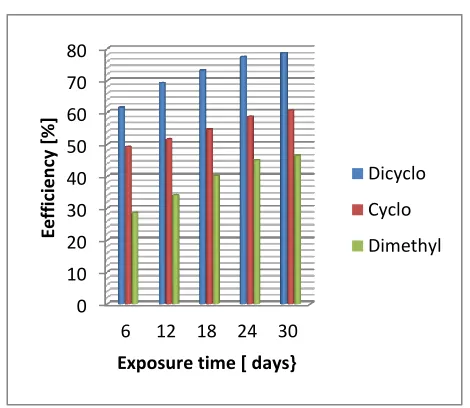
with various separate concentrations of cyclohexalamine, dicyclohexalamine and dimethylamine up to 520ppm on corrosion rate of mild steel at the various exposure durations up to 30 days at ambient temperatures in the laboratory. Fig. 6-10 on the other hand show the corresponding corrosion inhibition efficiencies attained by the separate amine treatment concentrations for the steel coupons
Table 1:
Average elemental weight chemical composition of mild steel material used for the study
Element
Fe
Al
C
Mg
S
Ph
% [Wt]
99.251
0.046
0.121
0.195
0.073
0.291
[image:6.612.60.555.444.659.2]Fig. 1: Corrosion rates of the coupons in the acidic chloride medium treated to 40ppm with the amines
Fig. 2: Corrosion rates of the coupons in the acidic chloride medium treated to 120ppm with the amines
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8
6 12 18 24 30
Co
rr
o
si
o
n
r
ate
[m
m
/y
r]
Exposure time [days]
Control Dimethyl Cyclo Dicyclo 0
0.2 0.4 0.6 0.8
6 12 18 24 30
Co
rr
o
si
o
n
r
ate
[m
m
/y
r]
Exposure time [days]
Control
Dimethyl
Cyclo
International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 9, Issue 8, August 2019)
[image:7.612.48.296.110.298.2]33
[image:7.612.47.290.327.491.2]Fig. 3: Corrosion rates of the coupons in the acidic chloride medium treated to 190ppm with the amines
Fig. 4: Corrosion rates of the coupons in the acidic chloride medium treated to 240ppm with the amines
[image:7.612.327.562.340.544.2]Fig. 5: Corrosion rates of the coupons in the acidic chloride medium treated to 520ppm with the amines
Fig. 6: Inhibition protection efficiency of the coupons from corrosion in the acidic chloride medium when treated with 40ppm of the
amines
Fig. 7: Inhibition protection efficiency of the coupons from corrosion in the acidic chloride medium when treated with 120ppm of the
amines
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8
6 12 18 24 30
Cor
rosi
on
r
ate
[m
m
/yr
]
Exposure time [days]
Control
Dimethyl
Cyclo
Dicyclo
0 0.2 0.4 0.6 0.8
6 12 18 24 30
Co
rr
o
si
o
n
r
ate
[d
ay
s]
Exposure time [days]
Control
Dimethyl
Cyclo
Dicyclo
0 10 20 30 40 50 60 70
6 12 18 24 30
Ee
ff
ic
ie
n
cy
[%
]
Exposure time [ days]
Dicyclo
Cyclo
Dimethyl
0 10 20 30 40 50 60 70 80
6 12 18 24 30
Ee
ff
ic
ie
n
cy
[%
]
Exposure time [ days}
Dicyclo
Cyclo
[image:7.612.48.291.519.683.2]International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 9, Issue 8, August 2019)
[image:8.612.323.560.105.326.2]34
Fig. 8: Inhibition protection efficiency of the coupons from corrosion in the acidic chloride medium when treated with 190ppm of the
[image:8.612.53.287.107.302.2]amines
Fig. 9: Inhibition protection efficiency of the coupons from corrosion in the acidic chloride medium when treated with 240ppm of the
[image:8.612.51.284.111.507.2]amines
Fig. 10: Inhibition protection efficiency of the coupons from corrosion in the acidic chloride medium when treated with 520ppm of the
amines
B.Discussion of Results
Table 1 shows that the material procured for the tested coupons was indeed mild steel with a carbon content of 0.121% [2].
From corrosion inhibition performances by the amines on the coupons shown in Figs 1-5, it is apparent that each treatment concentration of the amines differently decreased corrosion rate of the steel coupons in the acidic chloride medium as the exposure duration approaches the 30th day. From Figs 6-10 it is also clear that each of the amines had appreciable inhibitory effect on corrosion of the steel coupons in the medium. The inhibition efficiencies of corrosion in the acidic chloride medium by the amines increased from 45.1%, 54.1% and 19.8% on the 6th day of exposure with increase in exposure duration to maximum efficiencies of 84.8%, 91.3%, and 72.8% on the 30th day of expposure with the cyclohexylamine, dicyclohexalamine, dimethylamine treatments respectively. It is also clear from
0 20 40 60 80 100
6 12 18 24 30
Eff
ic
ie
n
cy
[%
]
Exposure time [ days]
Dicyclo
Cyclo
Dimethyl
0 20 40 60 80 100
6 12 18 24 30
Eff
ic
ie
n
cy
[%
]
Exposure time [ days]
Dicyclo
Cyclo
Dimethyl
0 20 40 60 80 100
6 12 18 24 30
Eff
ic
ie
n
cy
[
%
]
Exposure time [days]
Dicyclo
Cyclo
[image:8.612.55.281.332.506.2]International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 9, Issue 8, August 2019)
35
Figs 6-10 that among the three amines, dicyclohexalamine exhibited relatively better inhibition performance than cyclohexylamine and cyclohexylamine better than dimethylamine. Inhibitors can interact with metallic surface or the environment to which the surface is exposed to give the surface a certain level of protection. Mechanism of corrosion inhibition include formation of a passivation layer-a thin film on the surface of the material to be protected that stops access of the corrosive substance to it, inhibiting either the oxidation or reduction part of the redox corrosion system, or scavenging the dissolved oxygen [22, 23]. The differences in the corrosion inhibition performances of the three amines can therefore be attributed to variations in their inhibitory mechanisms such as their levels of solubility in the test medium and ability to
create barriers between the corrosive species and ability to scavenge corrosion-active ions on the surface of the steel, ability to neutralize the test medium to less corrosivity levels, and ability to cathodically or anodically depolarize the steel surface in the medium. To have an insight into the mechanisms of corrosion inhibition by the amines, scanning microscopy of the surface morphologies of another similarly prepared and exposed set of the steel in the similarly amines-treated acidic chloride medium were investigated after the 6th, 18th, and 30th day of the coupons’ exposure with the 520ppm-dicyclohexalamine treated medium and the 520ppm-dimethylamine treated medium for each exposure duration. The SEM microphotographs of surface morphologies of the coupons were as shown in Plates V, VI, and VII.
6th Day 18th Day 30th Day
Plate V: Microphotographs of surface morphologies of exposed steel coupons after the 6th, 18th, and 30th day exposure to the acidic chloride medium
treated with 520ppm Dicyclohexalamine
6th Day 18th Day 30th Day
Plate VI: Microphotographs of surface morphologies of the exposed steel coupons after the 6th, 18th, and 30th day exposure to the acidic chloride
medium treated with 520ppm Dimethylamine.
500µm
500µmm
500µm
500µm 500µmm
m
International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 9, Issue 8, August 2019)
36
Plate VII: Microphotograph of surface morphology of the control coupon in the non-amine treated acidic chloride medium after its 30th day of exposure in the medium
Comparing the SEM microphotographs shown in Plates V and VI with that shown in plate VII, it is apparently indicative that there were protective layer formations on the’ surfaces of the coupons which were coarse after the 6th day exposure of the coupons in the medium. These layer formations however became more compact and finer in structure as the exposure time extended to 30th day. One reason attributed to this variation of layer formations was due to increasing concentrations of the amines on the surfaces of the coupons with exposure time which possibly attained maximum thickness on the 30th day of exposure. This trend of layer formation was reasoned to have resulted in drastic decrease in corrosion rates and better inhibition protection efficiencies of the steel coupons with the exposure time and the amines’ concentration increments in the acidic chloride medium.
IV. CONCLUSIONS AND RECOMMENDATIONS
A.Concluding Remarks
The paper has reaffirmed that the use of corrosion inhibitors is the most suitable and economical way of protecting metalwork especially steelwork in extraction and processing industries, heavy industrial manufacturing, water treatment facility, etc to minimize localized corrosions and unexpected sudden failures. Multifarious materials in existence are known to have different ability levels to inhibit corrosion of other materials in their countless number of service environments, yet researches have been conducted only a relatively small number of them for proper engineering applications. Amines especially cyclohexylamine, dicyclohexylamine and dimethylamine were seen among the organic materials to form the most promising groups in corrosion inhibition of metals because of their favorable chemical structures, abundance and availability at relatively cheaper costs.
The inhibitory actions of various small treatment concentrations of 40-520 ppm of cyclohexylamine, dicyclohexylamine and dimethylamine on corrosion of mild steel in 0.5M sulphuric acid containing 3.5% sodium chloride were individually studied for various exposure durations of 6 to 30 days. Obtained results indicated that all the amines can have strong inhibitory effects on mild steel corrosion in the acidic chloride medium at ambient temperature with inhibition protection efficiencies that increase with increase in concentrations of the amines. Maximum efficiencies of 84.8%, 91.3%, and 72.8% were obtained at 520ppm-concentration of cyclohexylamine, methyclohexylamine dimethylamine respectively after 30 day-exposure of the steel coupons to the test medium. Further analysis indicated that the basic corrosion inhibition mechanism of the amines was by greater and more uniform protective layer formations with time on the surfaces of the steel coupons.
B.Recommendations
The following recommendations are hereby made from the research:
1.Field studies of corrosion inhibition of the mild steel by the amines need to be undertaken to supplement results obtained in this study.
2.A study of inhibitory mechanisms of the amines such as their solubility levels and ability to create barriers between the corrosive species and the steel surfaces, ability to scavenge corrosion-active ions, ability to neutralize the test medium to less corrosive levels and ability to cathodically or anodically depolarize the steel surface in the medium also needs be undertaken in greater details.
3.A study of effective service duration of inhibitory effects of the amines should also be undertaken.
International Journal of Emerging Technology and Advanced Engineering
Website: www.ijetae.com (ISSN 2250-2459, ISO 9001:2008 Certified Journal, Volume 9, Issue 8, August 2019)
37
4. The work and results from it are recommended for application and positive research interests in considering the amines as inhibitors.
REFERENCES
[1] Mars G. Fontana (1987). Corrosion engineering-international edn.
McGraw-Hill Book Co, pp. 15-120
[2] T.N. Guma, S.Y. Aku, D.S. Yawas, and M. Dauda. Bitumen in
Coating Corrosion Protection of Steel-The Position and Prognosis of Nigerian Bitumen. American Journal of Engineering Research (AJER), 4(12), 2015. www.ajer.org.
[3] T.N. Guma and James Abu. A Field Survey of Outdoor
Atmospheric Corrosion Rates of Mild Steel around Kaduna Metropolis. SSRG International Journal of Mechanical Engineering, Volume 5, Issue 11, 2018.
[4] Shreir, L (1979). Corrosion Volume 2, Principles of Corrosion
Control. Butterworth Publishers, London, England, pp. 10.1-20.3.
[5] Harrop, D (1990) Chemical Inhibitors for Corrosion Control In:
Chubley BG (Ed), Chemical Inhibitor for Corrosion Control Royal Society of Chemistry, Cambridge, England, pp. 1-20.
[6] Guma, T.N; Madakson, P.B; Yawas, D.S; and Aku, S.Y. Sodium
Benzoate and Bitumen Coatings as Inhibitors of Corrosion Deterioration of Mechanical Properties of Low Carbon Steel. Journal of Chemical Mechanical and Engineering Practice, Vol. 2, No. 3, 2012.
[7] M. Ramananda Singh, Kalpana Bhrara and Gurmeet Singh. The
Inhibitory Effect of Diethanolamine on Corrosion of Mild Steel in 0.5M Sulphuric Acidic Medium. Portugaliae Electrochimica Acta 26/6 (2008) 479-492.
[8] Arockia Selvi, P. Kamaraj, M. Arthanareswari and V. Saranya.
Trisodium Citrate as an Effective Corrosion Inhibitor for Carbon Steel in Chloride Environment. International Journal of Advance Chemical Science and Applications, 2 (1), 2014, pp. 54-58.
[9] K.S Rajagopalan, S. K. Gupta, V.P. Khanna and B. Sanyal. Amine
Salts and Amine Absorbed Cation Exchangers. Defence Research Laboratory (Stores) Kanpur.
[10] Oguzie, Mater. Chem. Phys.99 (2006) 441-446.
[11] Ramananda S. Mayanglambam, Vivek Sharma, Gurmeet Singh.
Musa Paradisiaca Extract as a Green Inhibitor for Corrosion of Mild
Steel in 0.5M Sulphuric Acid Solution. Portugaliae
ElectrochimicaActa 2011, 29(6),405-417, DOI:
10.4152/pea.201106405
[12] Ambler, H.R. and Bain, A.A.J. (2012). In: Atmospheric Corrosion
by G. O. Llyod.
http://www.npl.co.uk/upload/pdf/atmosphericcorrosion.pdf. Accessed on 20/10/2012.
[13] T.N. Guma S.Y. Aku, D.S. Yawas and M. Dauda. An Overview
Assessmentof Various Surveyed Corrosion Protection Approaches for Steel. IOSR Journal of Engineering IOSRJEN, 4(11), 2014, pp.48-56.
[14] Legrand, L. and Leroy, P. (1990). Prevention of Corrosion and
Scaling in Water Supply Systems. Ellis Horwood Ltd. Chichester, England, pp. 15-143
[15] Laque, F.L. (1975). Marine Corrosion. A Wiley-Intersceince
Publication, New York, pp. 12-317.
[16] Bikash Kar. Study of Mitigation of Corrosion Rate of Mild Steel Using Green Inhibitors; Thesis submitted in Partial Fulfillment of the Requirements for the Degree of Master of Technology in Materials Engineering. Department of Metallurgical and Materials Engineering, Faculty of Engineering and Technology, Jadavpur University Kolkata-700032, 2010.
[17] S.B. Ulaeto, U.J. Ekpe, M.A. Chidiebere, E.E. Oguzie. Corrosion
Inhibition of Mild Steel in Hydrochloric Acid by Acid Extracts of Eichhornia Crassipes. International Journal of Materials and Chemistry, 2(4), 2012: 158-164 DOI: 10.5923/j.ijmc.20120204.08, pp. 158-164.
[18] Matjaz Finsgar and Jennifer Jackson (2014). Application of
Corrosion Inhibitors of Steels in Acidic Media for Oil and Gas Industry: A Review, Corrosion Science Vol. 86, pp: 17-41. DOI 10:10:1016/j.corsci.2014.04.044.
[19] Kahraman, R (2002). Effect of Sodium Benzoate in Atmospheric
Corrosion of Mild Steel. The 6th Saudi Engineering Conference
KFUPM Dhahran, December.
[20] J McMurry, John E (1992), Organic Chemistry (3rd ed.), Belmont: Wadsworth, ISBN 0-534-16218-5.
[21] Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut
(2000). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a02_001. ISBN 3527306730.
[22] Amine chemical compounds Brittanica.
www.brittanica.com/science/amine
[23] What is amine inhibitor….Definition from
www.coorosionpedia.com/definition/1436.
[24] Merck Index, 11th Edition, 2735.
[25] K.O. Daniel. Effects of Some Amine Treatments on Corrosion of
Mild Steel in Acidic Chloride Medium. A Research Project Report Submitted to the Department of Mechanical Engineering, Nigerian Defence Academy, Kaduna, Nigeria, for Award of the Degree of Bachelor of Engineering ( B. Eng) in Mechanical Engineering, June, 2016.
[26] "NIOSH Pocket Guide to Chemical Hazards #0168". National
Institute for Occupational Safety and Health (NIOSH.
[27] H. K. Hall, J. Am. Chem. Soc. (1957) 79 5441.
[28] Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke
"Amines, Aliphatic" in Ullmann's Encyclopedia of Industrial
Chemistry, Wiley-VCH Weinheim, 2005.
doi:10.1002/14356007.a02_001.
[29] The Merck Index, 10th Ed. (1983) p.392, Rahway: Merck and Co.
[30] U.S. Patent 5322965, "Process for the preparation of a mixture of cyclohexylamine and dicyclohexylamine using a supported noble metal catalyst", Bayer AG, 21 June 1994
[31] Dimethylamine-wikipedia, the free encyclopedia.