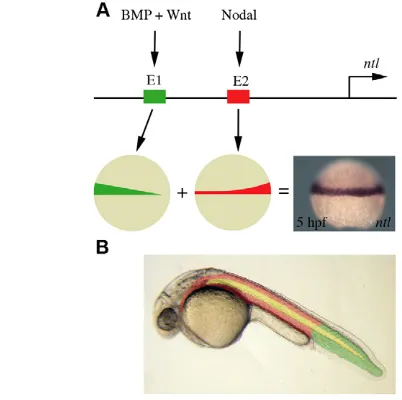
INTRODUCTION
During early zebrafish development a nodal morphogen gradient patterns the embryo into three germ layers by inducing the formation of endoderm and mesoderm (Schier and Talbot, 2005). Cells positioned at the margin of the developing embryo experience the highest levels of nodal signalling and adopt mesodermal and endodermal cell fates. Animal pole cells, which are positioned away from the margin, experience the lowest levels of nodal signalling and therefore become ectoderm. In the absence of nodal signalling all endoderm and almost all mesoderm fails to form, with just a few somites developing in the tail region (Feldman et al., 1998; Gritsman et al., 1999).
A key event in the patterning of the early embryo is the induction of goosecoid(gsc) at high levels of nodal signalling and the T-box transcription factor no tail(ntl) at lower levels (Chen and Schier, 2001). The expression of ntlat these stages is a reliable indicator of mesoderm formation, because the gene is expressed in mesodermal progenitors throughout the margin of the early embryo. At later stages, ntl expression is maintained in the tailbud and the developing notochord. In the absence of ntlfunction, notochord and posterior mesoderm fail to form, resulting in embryos without tails (Halpern et al., 1993; Odenthal et al., 1996). Anterior mesoderm continues to form
in such embryos, owing to the presence of the ntlhomologue, bra (Martin and Kimelman, 2008). However, loss of both braand ntlleads to a loss of almost all mesoderm (Martin and Kimelman, 2008).
Although graded nodal signalling is key to patterning the early embryo, an important observation suggests that mesoderm formation is not under the sole control of nodal ligands. In embryos in which nodal signalling has been completely inhibited, ntl expression is absent in the dorsal margin of the embryo, but persists ventrally and laterally (Feldman et al., 1998; Gritsman et al., 1999). This demonstrates that ntl, a key regulator of mesoderm formation, is under both nodal-dependent and nodal-independent regulation. Consistent with this observation, some somites form in the tail region of embryos lacking nodal signalling (Feldman et al., 1998; Gritsman et al., 1999). Further evidence pointing to the complexity of ntltranscriptional regulation comes from the observation that nodal signalling is higher, and extends further towards the animal pole, in the dorsal region of the embryo compared with lateral and ventral positions (Harvey and Smith, 2009). This would predict that ntlshould be expressed in more cell tiers on the dorsal side of the embryo than elsewhere, but in fact, the gene is expressed in approximately the same number of tiers throughout the margin.
In an effort to understand the regulation of ntl, and thereby mesoderm induction, we have mapped the regulatory elements required for ntlexpression in the developing embryo. As well as mapping the elements responsible for ntlexpression in the margin, we have also identified an ntlnotochord enhancer, the first such enhancer isolated for any ntlorthologue in any vertebrate (Lerchner et al., 2000; Yamaguchi et al., 1999). Our work shows that expression of ntlin the margin of the zebrafish embryo represents the effects of two independent enhancers – one that is responsive to nodal signalling and another that is regulated in a nodal-independent manner. We demonstrate that BMP and Wnt signals are responsible for nodal-independent regulation of ntl, and we have highlighted how this regulation is essential for tail formation.
Development 137, 1127-1135 (2010) doi:10.1242/dev.046318 © 2010. Published by The Company of Biologists Ltd
1Wellcome Trust and Cancer Research UK, Gurdon Institute and Department of Zoology, University of Cambridge, Tennis Court Road, Cambridge CB2 1QN, UK. 2The Wellcome Trust Sanger Institute, Hinxton, Cambridge CB10 1SA, UK. 3Department of Molecular and Cellular Biology, Centre for Brain Science, Broad Institute, Harvard Stem Cell Institute, Harvard University, Cambridge, MA 02138, USA. 4MRC National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA.
*Authors for correspondence (sh19@sanger.ac.uk;jim.smith@nimr.mrc.ac.uk)
Accepted 1 February 2010 SUMMARY
During early zebrafish development the nodal signalling pathway patterns the embryo into three germ layers, in part by inducing the expression of no tail(ntl), which is essential for correct mesoderm formation. When nodal signalling is inhibited ntl fails to be expressed in the dorsal margin, but ventral ntlexpression is unaffected. These observations indicate that ntl
transcription is under both nodal-dependent and nodal-independent regulation. Consistent with these observations and with a role for ntlin mesoderm formation, some somites form within the tail region of embryos lacking nodal signalling. In an effort to understand how ntlis regulated and thus how mesoderm forms, we have mapped the elements responsible for
nodal-dependent and nodal-innodal-dependent expression of ntlin the margin of the embryo. Our work demonstrates that expression of ntl in the margin is the consequence of two separate enhancers, which act to mediate different mechanisms of mesoderm
formation. One of these enhancers responds to nodal signalling, and the other to Wnt and BMP signalling. We demonstrate that the nodal-independent regulation of ntlis essential for tail formation. Misexpression of Wnt and BMP ligands can induce the formation of an ectopic tail, which contains somites, in embryos devoid of nodal signalling, and this tail formation is dependent on ntlfunction. Similarly, nodal-independent tail somite formation requires ntl. At later stages in development ntlis required for notochord formation, and our analysis has also led to the identification of the enhancer required for ntlexpression in the developing notochord.
KEY WORDS: Zebrafish, no tail, Mesoderm induction, Nodal signalling, Transcriptional regulation, Notochord
no tail
integrates two modes of mesoderm induction
Steven A. Harvey1,2,*, Stefan Tümpel1, Julien Dubrulle3, Alexander F. Schier3and James C. Smith1,4,*
D
E
V
E
LO
P
M
E
N
MATERIALS AND METHODS Luciferase assays
The –2.1 kb ntlpromoter fragment was cloned by PCR using the primers 5⬘
-TAACGCGTATACAATTCCTTTGTGCTGTTGCAACAC-3⬘ and 5⬘
-ATCTCGAGATTTCCGATCAAATAAAGCTTGAGAT-3⬘into the
pGL3-promoter luciferase plasmid (Promega). MluI and XhoI restriction enzyme sites (underlined) were incorporated into the primers to allow cloning. Embryos were injected at the 1-cell stage with 20 pg of the ntl promoter:luciferase plasmid and 1 pg of Renilla plasmid (pRL-TK, Promega). Assays were performed using the Dual-Luciferase reporter assay (Promega) according to the manufacturer’s instructions. All luciferase assays represent three different experiments each measured from 50 embryos at 6 hours postfertilisation (hpf). Data within each experiment were normalised using Renilla levels and data sets were normalised by assuming that luciferase levels in wild-type embryos injected with the –2.1 kb ntl promoter:luciferase plasmid were 100%. Error bars represent one standard deviation of the data set.
Constructs
PCR mutagenesis was performed using template plasmid DNA grown in TOP10 cells (dam+, Invitrogen). PCR products were treated with DpnI to digest dam+ template plasmid. Positive colonies were sequence verified.
Primers used for deletion of E1 were 5⬘GCTTAA AAAA
-TTGAATGCAACCAGAGAAATGAAATCGAACATTTTAATTG-3⬘and
5⬘CAATTAAAATGTTCGATTTCATTTCTCTGGTTGCATTCAATTTT
-TTAAGC-3⬘; those for deletion of E2 were 5⬘GCTTAAA
-AAATTGAATGCAACCAGAGAAATGAAATCGAACATTTTAATTG-3⬘
and 5⬘-AGACGGTGTCTGCTGCCTCGTCCCAGATACA CATGTG AGA-3⬘; and those for the removal of the Fast-1 binding site were 5⬘ - GGGACAACAAAAGATTAGCATTATTCGCACTGAGTTCAAAGGCA-ACGAGG-3⬘ and 5⬘CCTCGTTGCCTTTGAACTCAGTG CGAAT -AATGCTAATCTTTTGTTGTCCC-3⬘.
The Alk3* receptor (Piek et al., 1999) was subcloned into pCS2+. A 3⫻Flag-sequence was added to the 3⬘end of ntlin a bacterial artificial chromosome (BAC) (CHORI73_28E5, BACPAC Resources) via homologous recombination. We used a targeting construct that contained a kanamycin resistance gene cassette (Kan-r), flanked with FRT sites. Sequence 50 bp upstream and downstream of the ntlstop codon was amplified by PCR and used as homology arms (the sequence of the 5⬘arm
is 5⬘AGTTCGAGAGCTCCATCGCCCGGCTCACAGCATCATGGGC
-GCCTGTGGCT-3⬘, sequence of the 3⬘ arm is 5⬘GAT CGCTTC
-ACATTTAAGGACTGATGCTGCAGTTATGGACTTGATCTTGG-3⬘).
Sequencing confirmed the correct insertion of the Flag sequence and excision of the Kan-r cassette.
Manipulation of embryos
PCR-based mapping of the ntlregulatory elements was performed as previously described (Woolfe et al., 2005). Primers used to generate the –2.1
kb promoter fragment were (1) 5⬘ATACA ATTCC TTTGTGC
-TGTTGCAACAC-3⬘ and (2) 5⬘ATTTCCGATCAAAT AAAG CTTG
-AGAT–3⬘. For the two overlapping –1.4 kb fragments, primer (1) was used with (3) 5⬘-GTGTCTGCTGCCTCGTTGCCT-3⬘and primer (2) was used with (4) 5⬘-TTCAGTTCAGAATTATTTTAG-3⬘. All RNA was synthesised using mMessage mMachine (Ambion) according to the manufacturer’s protocol. Animal pole injections were performed at the 128-cell stage with 50 pg wnt8(Agathon et al., 2003), 50 pg bmp4(Thisse and Thisse, 1999) and 25 pg Venus RNA (to check injection). All other injections were performed at the one-cell stage by injecting 500 pg lefty (antivin), 10 pg Wnt8 (Sokol et al., 1991), 10 pg BMP4 (Jones et al., 1992), 600 pg dnBMPr (Suzuki et al., 1994), 1 pg of Taram-d* (Renucci et al., 1996) and 10 pg Alk3* encoding RNAs. The ntlmorpholino has been described previously (Feldman and Stemple, 2001).
Transgenic lines
For the generation of the ntlpromoter transgenic line, –1 kb of the ntl promoter was cloned upstream of CFP within the miniTol plasmid using the
primers 5⬘-ATCGAACATTTTAATTGTAGC-3⬘ and 5⬘ATTTCC
-GATCAAATAAAGCTTGAGAT-3⬘. One-cell stage embryos were injected with 25 pg of the –1 kb ntl:CFP miniTol plasmid and 25 pg of transposase
RNA. Injected fish were raised to adulthood and outcrossed. Embryos from outcrosses were used to perform PCR screening for the transgene. The ntl BAC transgenic line was generated by injecting embryos with 3 nl of BAC DNA at ~70 ng/ml. Before injection BAC DNA was dialyzed with Centriprep-YM30 column (Millipore) in microinjection buffer (10 mM Tris, pH 7.4, 0.15 mM EDTA, pH 8.0). The embryos were raised to adulthood and intercrossed. Embryos from intercrosses were used to perform PCR screening for the transgene with the following primers 5⬘
-GGATGACGATGACAAGTGATGAAG-3⬘ and 5⬘GCAATGGTTAC
-CAGTTTTGAACATT-3⬘.
Expression analysis
In situ hybridization was performed essentially as described (Thisse et al., 1993). Probes used were gata2(Dooley et al., 2005) and ntl(Schulte-Merker et al., 1994). Quantitative RT-PCR was performed using a Roche LightCycler 480. Data shown represent three different experiments each performed on 50 embryos at 6 hpf. Data within each set were normalised using the expression levels of ornithine decarboxylase (ODC) and data sets were normalised assuming the expression level in wild-type embryos was 100%. Primers used were: ODC 5⬘-GATGGCCTGGCTGTATGTTT-3⬘and
5⬘-GTTTGCAGGCTGAGTGTGAA-3⬘; ntl: 5⬘CGTCCGGTTTCA
-TTGTATCA-3⬘ and 5⬘-CGGTCACTTTTCAAAGCGTAT-3⬘; bra: 5⬘
-TCACCGGTGGAAGTATGTGA-3⬘ and 5⬘CCTCCGTTGAGTT TGT
-TGGT-3⬘. Immunostaining of ntlBAC transgenic embryos was performed with an HRP tagged anti-Flag antibody (DDDDK-HRP, ab1238, Abcam).
RESULTS
Identification of the regulatory elements responsible for ntlexpression
As a starting point for the identification of ntlregulatory elements, we used a BAC that spanned 133 kb of DNA surrounding the ntlopen reading frame, from –27.4 kb to +105.7 kb. Bacterial homologous recombination was used to introduce a FLAG tag into the ntlBAC, such that the FLAG amino acid sequence was positioned at the C-terminus of Ntl. We established a stable transgenic line with this BAC, and immunostaining of transgenic embryos demonstrated that the ntl BAC contained regulatory elements capable of driving expression in the developing margin and later in the notochord (Fig. 1A,B). However, in contrast to the endogenous gene, transgene expression was not observed in the tailbud. We used a PCR-based approach to identify these regulatory elements (Woolfe et al., 2005) and found that a –2.1 kb PCR fragment was capable of driving GFP expression in the developing margin (Fig. 1C) and later in the notochord (data not shown). Within this region we found that two overlapping 1.4 kb fragments (Fig. 1D) displayed similar enhancer abilities to those of the –2.1 kb fragment, suggesting that a 700 bp region (from –0.7 kb to –1.4 kb) can recapitulate the expression of ntlin the developing margin and notochord. We continued our analysis by focusing on two areas within the 700 bp region, which we called enhancers one (40 bp) and two (50 bp) (E1 and E2) (Fig. 1D). E1 was chosen because it contains a putative E-box binding site, which, although not directly required for Xbraand mouse brachyury expression, is present within genomic regions that are required for their expression (Lerchner et al., 2000; Yamaguchi et al., 1999). E2 contains a putative Fast-1 binding site, which is a co-factor of Smad2 and mediates the transcriptional response of nodal signalling (Schier, 2003). Alignments of E1 and E2 with stickleback and medaka ntlpromoters suggest that these regions are not highly conserved.
Nodal induction of ntlexpression
To understand the functions of E1 and E2 we cloned a –1 kb ntl promoter fragment, which excludes E1, upstream of cyan fluorescent protein (CFP) and used this construct to create a stable transgenic line (Fig. 2A,B). The –1 kb ntlpromoter fragment drove
D
E
V
E
LO
P
M
E
N
reporter gene expression within the developing margin and later in the notochord (Fig. 2A,B). At 5 hpf transgene expression was stronger in the dorsal margin, compared with ventral positions. Previous work has demonstrated that nodal signalling levels are higher on the dorsal side of the embryo (Harvey and Smith, 2009), suggesting that enhancer E2, within the –1 kb ntlpromoter fragment, is responsive to nodal signalling.
To test this idea we first performed luciferase assays on the –2.1 kb ntlpromoter fragment at 6 hpf, the time at which endogenous ntl is expressed. This fragment contains both the E1 and the E2 enhancers. We found that injection of the nodal antagonist lefty, at a concentration that fully inhibits nodal signalling, reduced luciferase levels but did not abolish them (Fig. 2C). When the nodal signalling pathway was activated we observed an increase in luciferase levels
(Fig. 2D, + Taram-d*). Removal of E2 (DE2) abolished the responsiveness to nodal signalling (Fig. 2D), and deletion of the putative Fast-1 binding site also eliminated nodal responsiveness (Fig. 2D, DF). When embryos were injected with a construct in which the –2.1 kb DE2 promoter was upstream of CFP, we observed expression in the margin at 6 hpf but not in the developing notochord (100% negative at 24 hpf, n=141) (Fig. 2E).
[image:3.612.52.361.58.311.2]Together, these results demonstrate that a 50 bp region (E2) within the –2.1 kb ntlpromoter responds to nodal signalling in the margin of the embryo and is required for maintenance of ntlexpression in the notochord at later stages of development. However, if the E2 element is removed, the –2.1 kb promoter is still capable of driving reporter gene expression within the margin (Fig. 2E) and it still produces some luciferase activity in the absence of nodal signalling
[image:3.612.51.363.518.738.2]Fig. 1. Mapping the ntlregulatory elements. (A,B) Anti-Flag immunostaining of ntl:Flag BAC transgenic embryos shows expression in the margin at 6 hpf (A) and in the notochord at 11 hpf (B). (C) An embryo injected with a –2.1 kb ntl promoter PCR fragment activates GFP expression in the margin at 6 hpf. (D) An illustration of the PCR fragments generated to map the ntl regulatory elements. Two overlapping 1.4 kb fragments displayed the same enhancer properties as the 2.1 kb fragment. Within the overlapping 700 bp region (–0.7 kb to –1.4 kb) we focused on enhancer 1 (E1, 40 bp) and enhancer 2 (E2, 50 bp). The sequences of E1 and E2 are shown.
Fig. 2. Nodal-dependent activation of ntl expression.(A,B) A transgenic line with –1 kb of the ntlpromoter upstream of CFP. (A) An animal pole view shows transgene expression in the developing margin. (B) The transgene is also expressed in the developing notochord as shown at 24 hpf. (C,D) Luciferase assays performed at 6 hpf with the –2.1 kb ntlpromoter fragment. (D) Wild type, –2.1 kb ntlpromoter. DE2, –2.1 kb ntl promoter, where E2 (50 bp) was removed. DF, –2.1 kb ntlpromoter with the putative fast-binding site (AAATATACA) removed from E2. (E) An embryo injected with a plasmid containing the –2.1 kb promoter fragment where E2 (50 bp) has been deleted (–2.1 kb DE2) upstream of CFP. Expression of CFP was observed in the developing margin at 6 hpf, but not in the notochord at 24 hpf.
D
E
V
E
LO
P
M
E
N
(Fig. 2C). Thus, in addition to the E2 nodal responsive element, the –2.1 kb ntl promoter fragment contains nodal-independent regulatory region(s) that are distinct from E2 and may be represented by the E1 region.
Nodal-independent regulation of ntl
If nodal signalling is abolished, some somites still form within the tail region of zebrafish embryos (Fig. 3A,B) (Feldman et al., 1998; Gritsman et al., 1999), and, consistent with this observation, ntl expression is retained in the ventral and lateral margin (see Fig. 4A,B) (Feldman et al., 1998; Gritsman et al., 1999). These observations suggest that factor(s) present in the ventral margin activate ntlexpression in a nodal-independent manner and that this regulation is required for formation of somites within the tail. Two candidates for the nodal-independent signal, both of which are expressed in the ventral margin, are wnt8and bmp4. In particular, we note that misexpression of these factors in the zebrafish animal pole region leads to the formation of ectopic tails that contain somites but no axial structures (Agathon et al., 2003) (Fig. 3C), also loss of BMP or Wnt function disrupts tail formation (Lekven et al., 2001; Pyati et al., 2005). To ask whether these factors can induce mesoderm and tail formation in a nodal-independent manner, we injected wnt8and bmp4into the animal pole regions of maternal and zygotic one-eyed pinhead (MZoep) embryos, which cannot transduce nodal signalling (Gritsman et al., 1999). Ectopic tails were indeed induced in MZoepembryos following the injection of bmp4 and wnt8(Fig. 3D), with somites visible within those ectopic tails (Fig. 3D, inset). Therefore, bmp4and wnt8can induce tail formation and mesoderm in a nodal-independent manner.
To ask whether these factors are the endogenous nodal-independent regulator of ntlexpression, we next injected embryos with RNA encoding the nodal antagonist leftyfollowed by RNA encoding Wnt8 and/or a constitutively active BMP receptor (Alk3*). In the absence of nodal signalling, Wnt8 had little effect on ntl
expression (Fig. 4A-C). However, activation of the BMP signalling pathway led to an increase in ntlexpression (Fig. 4B,D). Activation of both Wnt and BMP signalling led to a further mild increase in ntl expression (Fig. 4D,E). These results indicate that nodal independent induction of ntlexpression is mediated predominantly by BMP signalling. To test this we injected embryos with RNA encoding lefty and then with RNA encoding a dominant-negative BMP receptor (dnBMPr). Although ventral ntl expression was retained in embryos injected with only lefty(Fig. 4B), ntlexpression was completely abolished in embryos injected with lefty and dnBMPr (Fig. 4F).
The ntlpromoter contains a nodal-independent
regulatory element
[image:4.612.321.545.59.319.2]We went on to use luciferase assays to ask if the regulatory elements responsible for nodal-independent regulation of ntllie within the –2.1 kb promoter region. Activation of the BMP signalling pathway, in embryos also injected with lefty, caused an increase in luciferase levels, and further injection with Wnt8 produced an additional 35% increase (Fig. 4G). Deletion of E1 (DE1 2.1 kb) greatly reduced the ability of Wnt and BMP signalling to activate the promoter (Fig. 4G). Together these results demonstrate that E1 responds to BMP and Wnt signalling, whereas E2 responds to nodal signalling and is also required to maintain expression in the notochord. When we deleted both E1 and E2 (Fig. 4G, DE1, DE2) luciferase levels were reduced to 9.5% of wild-type levels.
Fig. 3. Tail formation in the absence of nodal signalling. (A) A wild-type embryo. (B) An MZoepembryo, with an arrowhead highlighting somites within the tail region. (C,D) Embryos previously injected at the 128-cell stage with zebrafish bmp4and wnt8(50 pg of each). (C) Injection of bmp4and wnt8into wild-type embryos produced an ectopic tail (arrow) containing somites. Ectopic tails formed in 22% of injected embryos (n=117). Injection of Xenopus laevisBMP4 and Wnt8 induced ectopic tails in 18% of injected embryos (data not shown, n=114). (D) Injection of bmp4and wnt8into MZoepembryos led to the formation of an ecoptic tail (arrow) in 29% of injected embryos (n=29). A magnified image of the ectopic tail (inset), which was identified by co-injection of a marker (not shown), highlights
somites within the tail (arrowhead). Fig. 4. Nodal-independent regulation of views of ntlRNA in situ hybridizations at 6 hpf. (A) A wild-type embryo.ntl.(A-F) Animal pole
Embryos injected with lefty(B) (100% with displayed expression, n=61), lefty+ wnt8(C) (100% with displayed expression, n=42), lefty+ Alk3* (D) (85% increased expression compared with B, n=40), lefty+ wnt8+ Alk3* (E) (87% increased expression compared with B, n=79), lefty+ dnBMPr (F) (80% no expression, n=40). (G) Luciferase assays performed at 6 hpf with the –2.1 kb ntlpromoter fragment in wild-type embryos and embryos injected with RNA encoding Wnt8 and/or Alk3*. DE1 = –2.1 kb ntlpromoter where E1 (40 bp) was present. DE1DE2 =–2.1 kb ntlpromoter where E1 and E2 were removed.
D
E
V
E
LO
P
M
E
N
[image:4.612.54.298.60.198.2]Spatial and temporal regulation of the ntl promoter
As nodal and BMP signalling are the main activators of ntl expression in the margin, we compared the spatial and temporal expression of the –1 kb ntlpromoter transgene, which contains only the nodal responsive enhancer, with ntlin wild-type embryos and in embryos in which BMP signalling was inhibited (Fig. 5). In wild-type embryos, at 5 hpf, ntlis expressed in approximately the same number of cell tiers throughout the margin (Fig. 5A,D,D⬘). At the same time point the –1 kb ntlpromoter transgene is expressed in more cell tiers on the dorsal side of the embryo (Fig. 5B,E,E⬘). A comparable expression pattern was observed in embryos in which BMP signalling had been inhibited (Fig. 5C,F,F⬘). Such dorsal enrichment of expression that was observed at 5 hpf was not detectable at 6 hpf. As gastrulation commences ntlbegins to be expressed in the developing notochord (Fig. 5G, arrow) and is enriched in the ventral margin (Fig. 5G, bracket). At this time point the –1 kb ntlpromoter transgene is also expressed in the developing notochord (Fig. 5H, arrow), but in contrast to ntlexpression in wild-type embryos, transgene expression is observed in many more cells (Fig. 5H). Closer inspection reveals that the transgene is expressed
in underlying cells of the endoderm at 7 hpf (Fig. 5J) and also at 8 hpf (not shown). Such expression suggests that ntl is actively repressed in endodermal cells. However, another plausible explanation is that the transgene transcripts are more stable than endogenous ntl mRNA and that transgene expression in the endoderm is not the consequence of de novo transcription. In embryos where BMP signalling is inhibited, ntlis expressed in the developing notochord but fails to be enriched in the ventral margin (Fig. 5I, bracket).
[image:5.612.55.296.61.337.2]As our previous experiments suggested that the –1 kb ntl promoter transgene contains only the nodal responsive enhancer, we injected –1 kb ntlpromoter transgenic embryos with a concentration range of the nodal antagonist lefty (Fig. 6). Injection of high concentrations of lefty(300 pg) resulted in the complete abolition of transgene expression within the margin (Fig. 6A,B). Injection of lower concentrations of lefty(2 pg) resulted in the transgene being expressed in fewer cell tiers on the dorsal side of the embryo (Fig. 6C,E), whereas expression in the ventral margin was completely lost (Fig. 6E,F). In these experiments we identified the ventral side of the embryo by co-staining embryos for gata2 expression, which marks the ventral ectoderm (Rentzsch et al., 2004). These results demonstrate that the –1 kb ntlpromoter responds to endogenous nodal signals in the developing embryo and that ventral expression driven by this promoter is the first to be abolished following a reduction in nodal signalling.
[image:5.612.362.513.65.260.2]Fig. 5. Spatial and temporal regulation of ntlexpression. (A,D,D⬘,G) ntlexpression in wild-type embryos. (B,E,E⬘,H,J) Expression of the –1 kb ntlpromoter transgene. (C,F,F⬘,I) The expression of ntlin embryos injected with a dnBMPr. (A-C) Animal pole views of expression at 5 hpf. (D-F⬘) Views of expression on opposing sides of individual embryos at 5 hpf (transgene expression 84%, n=51 and dnBMPRr injected 69%, n=46). (G-I) Lateral views of expression at 7 hpf. Arrows indicate expression in the developing notochord. Brackets in G and I highlight the enriched expression in the ventral margin of wild-type embryos, which is not observed in embryos injected with the dnBMPr (92%, n=37). (J) A magnified lateral view of a 7 hpf embryo shows the transgene is expressed in the underlying endodermal cells (arrow) and not the outer mesodermal cells (arrowhead) (100%, n=52).
Fig. 6. Attenuation of nodal signalling in Tg(–1 kb ntl:CFP) embryos.(A,B) Animal pole views showing transgene expression in Tg(–1 kb ntl:CFP) embryos. Injection of high concentrations of lefty (300 pg) completely abolished transgene expression (B) (100%, n=68). (C,D) A transgenic embryo processed to show transgene and gata2 expression, which marks the ventral ectoderm. (D) The white bracket highlights gata2 expression in the ventral ectoderm and the green bracket shows transgene expression in the margin. The red bracket shows a region that separates the expression domains of gata2and the transgene. (E,F) A transgenic embryo injected with a low concentration of lefty(2 pg). (E) On the dorsal side of the embryo the transgene is expressed in fewer cell tiers compared with uninjected embryos (C). However, within the same embryo, transgene expression is abolished in the ventral margin (arrow) (expression pattern observed in 43%; in 50% transgene expression was completely abolished, n=60).
D
E
V
E
LO
P
M
E
N
Tail formation is dependent on ntland not bra As the zebrafish genome contains two Brachyury homologues (ntl and bra) (Martin and Kimelman, 2008), a complete understanding of mesoderm formation during zebrafish development requires an understanding of the regulation of both ntland bra. To this end we carried out quantitative RT-PCR to ask whether braexpression is regulated in a similar manner to ntl(Fig. 7A,B). Both braand ntl expression increased in embryos injected with the constitutively active nodal receptor Taram-d* and, consistent with our previous experiments, ntlexpression was also activated by BMP and Wnt signalling (Fig. 7A). However, braexpression slightly decreased in embryos injected with Alk3* and wnt8, perhaps because these embryos become ventralised as a result of increased BMP signalling. Similarly, BMP and Wnt signalling activated ntlexpression in a nodal-independent manner, but braexpression decreased somewhat (Fig. 7B). These experiments show that the regulation of bradiffers from that of ntl, consistent with the observation that they play different roles in the early zebrafish embryo.
As previously shown, misexpression of wnt8and bmp4in the animal pole leads to the formation of an ectopic tail that contains somites but no axial structures (Fig. 3C) (Agathon et al., 2003). Because ntl, and not bra, is regulated by BMP and Wnt signalling, the formation of such ectopic tails is likely to require ntl. To test this idea we injected embryos with an ntlmorpholino and then further injected them, in the animal pole of the 128-cell embryo, with bmp4 and wnt8. Whereas injection of bmp4and wnt8 into wild-type embryos led to the formation of ectopic tails (see Fig. 3C), injection into ntlmorphants did not produce ectopic tails, but instead resulted in the formation of a small ectopic mass (Fig. 7C, 29%, n=77). Our observations suggest that the formation of somites within the tail
region of MZoepembryos must be dependent on ntland not bra. Injection of the ntlmorpholino into MZoepembryos abolished the formation of somites (Fig. 7D), thus confirming this idea.
DISCUSSION
Mesoderm induction during zebrafish embryonic development
An essential step in the patterning of the early vertebrate embryo is the commitment of cells to one of the three germ layers. At the core of this patterning is the transcription factor Brachyury, the zebrafish homologues of which are called ntland bra. The correct spatial and temporal regulation of these Brachyury genes is essential for the proper formation of mesoderm, because in their absence almost all mesoderm fails to develop (Martin and Kimelman, 2008). We also note that misexpression of XenopusBrachyury (Xbra) in cells of the animal pole, where endogenous Xbrais not expressed, leads to the formation of mesoderm in tissue that would normally become ectoderm (Cunliffe and Smith, 1992).
During zebrafish development a nodal morphogen gradient induces the formation of mesoderm by activating the expression of bra and ntl (Fig. 7A), and in the absence of nodal signalling mesoderm formation is greatly inhibited (Fig. 3B) (Feldman et al., 1998; Gritsman et al., 1999). However, our work, and that of others (Feldman et al., 1998; Gritsman et al., 1999), indicates that mesoderm can also form in the zebrafish embryo in a nodal-independent manner. In this paper we show that Wnt and BMP signalling activates the expression of ntlin the ventral margin and that such regulation is required for the formation of somites within the tail region of embryos.
Marginal expression of ntlrepresents the
combined effects of two enhancer regions
During normal zebrafish development, at 5 hpf, ntlis expressed in approximately the same numbers of cell tiers throughout the margin (Fig. 8A). We show here that this uniform spatial expression derives from the combined actions of two separate enhancers. One of these, E1, responds to signalling by BMPs and Wnts, whereas the other, E2, responds to nodal signalling (Fig. 8A). In the absence of nodal signalling there is a ‘wedge’ of ntlexpression, highest on the ventral side and low on the dorsal (Fig. 8A, green) (Thisse and Thisse, 1999). The reciprocal expression pattern is obtained by the activation of the promoter in response to nodal signalling (Fig. 8A, red). The combination of these enhancers drives ntlexpression throughout the margin.
[image:6.612.53.297.60.214.2]Injection of the nodal antagonist Cerberus-short (cer-s) into Xenopusembryos demonstrated that it is the ventral expression of Xbrathat is most sensitive to the loss of nodal signalling (Agius et al., 2000). By contrast, in previous studies, injection of lefty into zebrafish embryos had suggested that ntlexpression in the dorsal margin is the more sensitive to the loss of nodal signalling (Thisse and Thisse, 1999). Indeed, in the complete absence of nodal signalling ntlcontinues to be expressed in the ventral margin (Feldman et al., 1998; Gritsman et al., 1999). Our observation that ntlexpression is driven by the complementary actions of two enhancers resolves this apparent discrepancy: in zebrafish embryos in which nodal signalling is abolished, expression persists in ventral regions thanks to enhancer E1, which responds to BMPs and Wnts. In transgenic zebrafish embryos in which a reporter gene is driven only by the nodal responsive element E2, it is the ventral domain that is first abolished following loss of nodal signalling (Fig. 6C-F), as is the case for Xbrain the Xenopusembryo.
Fig. 7. Nodal-independent regulation of ntlis essential for tail formation. (A,B) Quantitative RT-PCR of ntl(red) and bra(blue) expression at 6 hpf. Absolute levels of wild-type ntlexpression were 1.44 times greater than that of bra. (A) Embryos injected with Alk3* and wnt8displayed an increase in ntl, but not braexpression. Injection of taram-d* produced an increase in both ntland braexpression. (B) Injection of embryos with leftyand then Alk3* and wnt8demonstrated that in the absence of nodal signalling BMP and Wnt signalling can activate the expression of ntl but not bra. (C) An embryo injected with an ntlmorpholino and then further injected in the animal pole with bmp4and wnt8leads to a small ectopic mass (arrow) in 29% of embryos (n=77) (compare with Fig. 3C). (D) An MZoepembryo injected at the one-cell stage with an ntlMO. Loss of ntlfunction abolished the formation of somites within the tail region of MZoepembryos (arrowhead, compare with Fig. 3B).
D
E
V
E
LO
P
M
E
N
Interestingly, the expression pattern of bra in the zebrafish resembles that of our –1 kb ntlpromoter transgenic line, which contains only the nodal responsive enhancer (Fig. 5B,E,E⬘) (Martin and Kimelman, 2008). Expression from the –1 kb ntltransgenic line and of brais enriched in the dorsal margin and is later maintained in the notochord (Martin and Kimelman, 2008). The dorsal enrichment of expression coincides with high levels of nodal signalling in the dorsal margin of the zebrafish embryo (Harvey and Smith, 2009). These observations are consistent with the conclusion that bra, like ntl, can be induced by nodal signalling, but that only ntlcan be activated by BMPs and Wnts (Fig. 7A,B). We note that Xenopus Brachyury, Xbra, can be induced by BMP signalling (Finley et al., 1999; Re’em-Kalma et al., 1995; Zhang et al., 2008), suggesting that brahas lost responsiveness to BMP and Wnt signalling following the duplication of their common ancestor. However, in the absence of data from a basal species such as amphioxus we cannot exclude the possibility that ntlacquired the ability to respond to BMPs and Wnts.
In the absence of ntl, posterior mesoderm and notochord formation is disrupted, but anterior mesoderm continues to develop (Halpern et al., 1993; Martin and Kimelman, 2008). Our work, together with that of others (Martin and Kimelman, 2008), suggests that differences in anterior and posterior (tail) mesoderm formation derive from the differential regulation of ntland bra. During anterior mesoderm formation, ntland bracompensate for the loss of one another, and our experiments suggest that this is because both genes are regulated by nodal signalling. In the absence of ntlfunction, bra continues to be activated by nodal signalling, and its expression ensures the correct formation of anterior mesoderm. However, the
differential requirement of ntland braduring posterior mesoderm formation derives from the fact that only ntlis regulated by BMP and Wnt signalling. In the absence of ntl, BMP and Wnt signalling cannot induce mesoderm, and subsequently tail formation is disrupted. By contrast, posterior mesoderm formation continues normally in the absence of bra, because ntlexpression is activated in the ventral margin in response to BMP and Wnt signals (Fig. 8). Consistent with this idea, cell transplantation experiments have shown that when cells are exposed to nodal signalling they form anterior somites, whereas exposure to BMP signalling leads to the formation of tail somites (Szeto and Kimelman, 2006). Similarly, it has been shown that oepgenetically interacts with ntl(Schier et al., 1997). Loss of both maternal and zygotic oepabolishes all nodal signalling and severely affects mesoderm formation (Gritsman et al., 1999), but loss of only zygotic oepdoes not affect the expression of the mesodermal markers myoD and a-tropomyosin (Schier et al., 1997). As expected, posterior expression of these markers was disrupted in ntl mutant embryos but anterior expression is unaffected. However, anterior mesoderm formation is severely affected in oep ntl double-mutant embryos. This suggests that maternal levels of oep are sufficient to activate bra and ntl expression to a level that induces anterior mesoderm formation. However, maternal oepis insufficient to induce anterior mesoderm with only bra (i.e. in the absence of ntl). Such a model would therefore predict a similar genetic interaction between zygotic oep and braloss of function.
With this in mind, why does notochord formation require ntl, when brais also expressed in the developing notochord in response to nodal signals? One possibility is that although the amino acid sequences of braand ntlare 65% identical (ClustalW alignment), bracannot substitute for ntlfunction in the notochord. Alternatively, formation of the notochord may require higher levels of Brachyury activity than are needed for anterior mesoderm formation, and perhaps brais not expressed at high enough levels to compensate for loss of ntl.
In the absence of nodal signalling braand ntlexpression levels are both reduced to 18% of wild-type levels (Fig. 7A,B). Our results indicate that the residual expression of ntlderives from BMP and Wnt signalling, but these pathways do not regulate bra, suggesting that it is regulated to some extent by pathways that are independent of nodals, BMPs and Wnts.
Vertebrate mesoderm formation
The regulation of Brachyury has been extensively studied during mouse and Xenopus development. In contrast to the zebrafish, inhibition of nodal signalling in Xenopusleads to a complete loss of Xbraexpression and of mesoderm formation (Agius et al., 2000; Dorey and Hill, 2006; Piccolo et al., 1999), suggesting that there is no nodal-independent induction of Xbra. However, as in the zebrafish, BMP signalling can nevertheless induce Xbrain Xenopus, and it can activate brachyury in mouse and human embryonic stem (ES) cells (Finley et al., 1999; Re’em-Kalma et al., 1995; Zhang et al., 2008). These species have therefore evolved different roles for BMP signalling in mesoderm formation. Interestingly, inhibition of Nodal signalling blocks the ability of BMPs to induce mesoderm in human ES cells (Zhang et al., 2008), suggesting that BMP signalling induces mesoderm in these cells by upregulating expression of a Nodal ligand.
[image:7.612.51.252.62.262.2]It has also been shown in Xenopusthat in the absence of FGF signalling Xbra expression is abolished dorsally but retained ventrally (Northrop et al., 1995), whereas simultaneous FGF and BMP inhibition leads to a complete loss of Xbraexpression. Thus, Fig. 8. A model of ntlregulation.(A) The ntlpromoter contains two
different regulatory elements that activate ntlexpression in the developing margin. BMP and Wnt signalling acts through one element (E1, shown in green) to activate ntlexpression in the ventral margin. Nodal signalling induces ntlexpression via a separate regulatory element (E2, shown in red), resulting in an enrichment of expression in the dorsal margin. (B) BMP and Wnt induction of ntlis required for tail mesoderm formation (shown in green). Anterior mesoderm (shown in red) results from the induction of ntland ntlexpression by nodal signalling. Notochord formation (shown in yellow) is dependent on regulatory elements that also lie within E2 of the ntlpromoter.
D
E
V
E
LO
P
M
E
N
while nodal signalling is required to induce Xbra, FGF and BMP pathways may be required to maintain expression. Interaction between the FGF and BMP pathways can occur through FGF-dependent phosphorylation of BMP-specific Smads (Pera et al., 2003), suggesting that in the normal embryo FGF signalling prevents BMPs from activating Xbra.
Previous work has demonstrated that Wnt signalling, although not required for the initiation of brachyury expression during mouse development, is required for its maintenance (Galceran et al., 2001; Yamaguchi et al., 1999). Our work shows that Wnt signalling does not play a major role in the induction of ntl expression, but it remains possible that Wnts are required to maintain ntlexpression later in development. Indeed, expression of wnt8in the early embryo depends on ntl, and there is a genetic interaction between ntland Wnt signalling (Marlow et al., 2004; Martin and Kimelman, 2008).
Finally, studies of –2.1 kb of the XenopusBrachyury (Xbra) promoter have shown that this fragment responds to Nodal/Activin signalling and is expressed throughout the margin of Xenopus embryos, but excluded from the organiser and notochord (Latinkic et al., 1997; Lerchner et al., 2000). A comparable region of the mouse brachyury promoter is similarly expressed in pre-somitic mesoderm but excluded from the notochord and node (Yamaguchi et al., 1999). Our work demonstrates that a 50 bp region within the –2.1 kb ntlpromoter is similarly responsive to nodal signalling, but also drives expression in the notochord (E2, Fig. 2B). Thus, although there has been conservation of the ability of comparable promoter regions to respond to Nodal/Activin signalling, there has been divergence with respect to notochord expression, with the Xenopus and mouse notochord enhancers located outside the studied regions.
Mesoderm and tail formation
Several lines of evidence suggest that zebrafish and Xenopushave evolved different mechanisms to induce mesoderm and tail formation. Transplantation of ventral tissue from 6 hpf zebrafish embryos into the animal pole of a host embryo induces the formation of an ectopic tail in a manner that does not require the dorsal organizer (shield) (Agathon et al., 2003). Comparable ventral tissue regions of the Xenopus embryo, however, do not posses tail-inducing activity (De Robertis and Kuroda, 2004). Rather, it is thought the tail is induced at later stages of development, near the end of gastrulation, by the progeny of the dorsal organizer (Gont et al., 1993).
During zebrafish development, BMP and Wnt signalling can recapitulate the tail organising activity of the ventral margin (Fig. 3C), and our work shows that this occurs through the activation of ntl(Fig. 7C,D). The timing of ntlexpression in response to BMP signalling at approximately 5 hpf coincides with the stage at which the tail is induced (Pyati et al., 2005; Szeto and Kimelman, 2006; Tucker et al., 2008). Specifically our results show that BMP and Wnt signalling induces tail formation in the absence of nodal signalling (Fig. 3), whereas it has previously been suggested that tail formation is nodal dependent (Agathon et al., 2003).
Despite the differences in tail formation during zebrafish and Xenopusdevelopment, one common feature is that BMP signalling is essential for tail formation in both species (Pyati et al., 2005; Reversade et al., 2005; Tucker et al., 2008). Although BMP signalling can induce the expression of both Xbraand ntl, only ntl is regulated by a nodal-independent mechanism (Fig. 4A-F) (Agius et al., 2000; Dorey and Hill, 2006; Re’em-Kalma et al., 1995). As nodal-independent regulation of ntlis essential for tail formation during zebrafish development, the differential regulation of
mesoderm induction by BMP signalling during Xenopus and zebrafish development may be central to differences in tail formation.
Acknowledgements
We thank past and present members of the Smith lab for their help and advice, and Caroline Hill, Fiona Wardle and Greg Elgar for reagents. This work is supported by the VolkswagenStiftung and by a Wellcome Trust Programme Grant awarded to J.C.S. A.F.S. is supported by the NIH. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
References
Agathon, A., Thisse, C. and Thisse, B.(2003). The molecular nature of the zebrafish tail organizer. Nature424, 448-452.
Agius, E., Oelgeschlager, M., Wessely, O., Kemp, C. and De Robertis, E. M.
(2000). Endodermal Nodal-related signals and mesoderm induction in Xenopus.
Development127, 1173-1183.
Chen, Y. and Schier, A. F.(2001). The zebrafish Nodal signal Squint functions as a morphogen. Nature411, 607-610.
Cunliffe, V. and Smith, J. C.(1992). Ectopic mesoderm formation in Xenopus embryos caused by widespread expression of a Brachyury homologue. Nature
358, 427-430.
De Robertis, E. M. and Kuroda, H.(2004). Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol.20, 285-308.
Dooley, K. A., Davidson, A. J. and Zon, L. I.(2005). Zebrafish scl functions independently in hematopoietic and endothelial development. Dev. Biol.277, 522-536.
Dorey, K. and Hill, C. S.(2006). A novel Cripto-related protein reveals an essential role for EGF-CFCs in Nodal signalling in Xenopus embryos. Dev. Biol.292, 303-316.
Feldman, B. and Stemple, D. L.(2001). Morpholino phenocopies of sqt, oep, and ntl mutations. Genesis30, 175-177.
Feldman, B., Gates, M. A., Egan, E. S., Dougan, S. T., Rennebeck, G., Sirotkin, H. I., Schier, A. F. and Talbot, W. S.(1998). Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature
395, 181-185.
Finley, M. F., Devata, S. and Huettner, J. E.(1999). BMP-4 inhibits neural differentiation of murine embryonic stem cells. J. Neurobiol.40, 271-287.
Galceran, J., Hsu, S. C. and Grosschedl, R.(2001). Rescue of a Wnt mutation by an activated form of LEF-1: regulation of maintenance but not initiation of Brachyury expression. Proc. Natl. Acad. Sci. USA98, 8668-8673.
Gont, L. K., Steinbeisser, H., Blumberg, B. and de Robertis, E. M.(1993). Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development119, 991-1004.
Gritsman, K., Zhang, J., Cheng, S., Heckscher, E., Talbot, W. S. and Schier, A. F.(1999). The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell97, 121-132.
Halpern, M. E., Ho, R. K., Walker, C. and Kimmel, C. B.(1993). Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell75, 99-111.
Harvey, S. A. and Smith, J. C.(2009). Visualisation and quantification of morphogen gradient formation in the zebrafish. PLoS Biol.7, e101.
Jones, C. M., Lyons, K. M., Lapan, P. M., Wright, C. V. and Hogan, B. L.
(1992). DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development115, 639-647.
Latinkic, B. V., Umbhauer, M., Neal, K. A., Lerchner, W., Smith, J. C. and Cunliffe, V.(1997). The Xenopus Brachyury promoter is activated by FGF and low concentrations of activin and suppressed by high concentrations of activin and by paired-type homeodomain proteins. Genes Dev.11, 3265-3276.
Lekven, A. C., Thorpe, C. J., Waxman, J. S. and Moon, R. T.(2001). Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell1, 103-114.
Lerchner, W., Latinkic, B. V., Remacle, J. E., Huylebroeck, D. and Smith, J. C.
(2000). Region-specific activation of the Xenopus brachyury promoter involves active repression in ectoderm and endoderm: a study using transgenic frog embryos. Development127, 2729-2739.
Marlow, F., Gonzalez, E. M., Yin, C., Rojo, C. and Solnica-Krezel, L.(2004). No tail co-operates with non-canonical Wnt signaling to regulate posterior body morphogenesis in zebrafish. Development131, 203-216.
Martin, B. L. and Kimelman, D.(2008). Regulation of canonical Wnt signaling by Brachyury is essential for posterior mesoderm formation. Dev. Cell15, 121-133.
Northrop, J., Woods, A., Seger, R., Suzuki, A., Ueno, N., Krebs, E. and
Kimelman, D.(1995). BMP-4 regulates the dorsal-ventral differences in
D
E
V
E
LO
P
M
E
N
FGF/MAPKK-mediated mesoderm induction in Xenopus. Dev. Biol.172, 242-252.
Odenthal, J., Haffter, P., Vogelsang, E., Brand, M., van Eeden, F. J., Furutani-Seiki, M., Granato, M., Hammerschmidt, M., Heisenberg, C. P., Jiang, Y. J. et al.(1996). Mutations affecting the formation of the notochord in the zebrafish, Danio rerio. Development123, 103-115.
Pera, E. M., Ikeda, A., Eivers, E. and De Robertis, E. M.(2003). Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction.
Genes Dev.17, 3023-3028.
Piccolo, S., Agius, E., Leyns, L., Bhattacharyya, S., Grunz, H., Bouwmeester, T. and De Robertis, E. M.(1999). The head inducer Cerberus is a
multifunctional antagonist of Nodal, BMP and Wnt signals. Nature397, 707-710.
Piek, E., Moustakas, A., Kurisaki, A., Heldin, C. H. and ten Dijke, P.(1999). TGF-(beta) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J. Cell Sci.
112, 4557-4568.
Pyati, U. J., Webb, A. E. and Kimelman, D.(2005). Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development132, 2333-2343.
Re’em-Kalma, Y., Lamb, T. and Frank, D.(1995). Competition between noggin and bone morphogenetic protein 4 activities may regulate dorsalization during Xenopus development. Proc. Natl. Acad. Sci. USA92, 12141-12145.
Rentzsch, F., Bakkers, J., Kramer, C. and Hammerschmidt, M.(2004). Fgf signaling induces posterior neuroectoderm independently of Bmp signaling inhibition. Dev. Dyn.231, 750-757.
Renucci, A., Lemarchandel, V. and Rosa, F.(1996). An activated form of type I serine/threonine kinase receptor TARAM-A reveals a specific signalling pathway involved in fish head organiser formation. Development122, 3735-3743.
Reversade, B., Kuroda, H., Lee, H., Mays, A. and De Robertis, E. M.(2005). Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development132, 3381-3392.
Schier, A. F.(2003). Nodal signaling in vertebrate development. Annu. Rev. Cell Dev. Biol.19, 589-621.
Schier, A. F. and Talbot, W. S.(2005). Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet.39, 561-613.
Schier, A. F., Neuhauss, S. C., Helde, K. A., Talbot, W. S. and Driever, W.
(1997). The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development124, 327-342.
Schulte-Merker, S., van Eeden, F. J., Halpern, M. E., Kimmel, C. B. and Nusslein-Volhard, C.(1994). no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development120, 1009-1015.
Sokol, S., Christian, J. L., Moon, R. T. and Melton, D. A.(1991). Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell67, 741-752.
Suzuki, A., Thies, R. S., Yamaji, N., Song, J. J., Wozney, J. M., Murakami, K. and Ueno, N.(1994). A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc. Natl. Acad. Sci. USA91, 10255-10259.
Szeto, D. P. and Kimelman, D.(2006). The regulation of mesodermal progenitor cell commitment to somitogenesis subdivides the zebrafish body musculature into distinct domains. Genes Dev.20, 1923-1932.
Thisse, C. and Thisse, B.(1999). Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development
126, 229-240.
Thisse, C., Thisse, B., Schilling, T. F. and Postlethwait, J. H.(1993). Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development119, 1203-1215.
Tucker, J. A., Mintzer, K. A. and Mullins, M. C.(2008). The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev. Cell14, 108-119.
Woolfe, A., Goodson, M., Goode, D. K., Snell, P., McEwen, G. K., Vavouri, T., Smith, S. F., North, P., Callaway, H., Kelly, K. et al.(2005). Highly conserved non-coding sequences are associated with vertebrate development. PLoS Biol.3, e7.
Yamaguchi, T. P., Takada, S., Yoshikawa, Y., Wu, N. and McMahon, A. P.
(1999). T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev.13, 3185-3190.
Zhang, P., Li, J., Tan, Z., Wang, C., Liu, T., Chen, L., Yong, J., Jiang, W., Sun, X., Du, L. et al.(2008). Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood111, 1933-1941.