Original Article
Decreased expression of miR-218 is associated with
poor prognosis in patients with colorectal cancer
Hong Yu1*, Guangzhong Gao2*, Lin Jiang3*, Lingchuan Guo4, Mei Lin5, Xiao Jiao1, Weiguang Jia6, Junxing Huang5
Departments of 1Pathology, 2Neurosurgery, 3Anesthesiology, 5Oncology, 6Cardiothoracic Surgery, Taizhou People’s Hospital, Jiangsu, China; 4Department of Pathology, The First Affiliated Hospital of Soochow University, Jiangsu, China. *Equal contributors.
Received October 15, 2013; Accepted November 10, 2013; Epub November 15, 2013; Published December 1, 2013
Abstract:The identification of biomarkers in colorectal cancer (CRC) diagnosis and therapy is important in achieving early cancer diagnosis and improving patient outcomes. The aim of this study was to examine clinical significance of miR-218 expression in sera and tissues from CRC patients. A total of 189 cases and 30 healthy subjects were in
-cluded. The expression levels of miR-218, SLIT2 and SLIT3 were measured by quantitative reverse transcription PCR (qRT-PCR). The relationship between miR-218 expression and clinicopathological characteristics was investigated. The expression levels of miR-218, SLIT2 and SLIT3 in CRC tissues were decreased than those in adjacent normal tis -sues (all P < 0.05). miR-218 expression was significantly associated with TNM stage, lymph node metastasis (LNM) and differentiation (all P < 0.05). Patients with low miR-218 expression had shorter survival time than those with high miR-218 expression (P = 0.036). Furthermore, the expression levels of serum miR-218 in CRC patients were
lower than those in controls (P = 0.005). An increased level of serum miR-218 was found 1 month after surgery (P = 0.026). In conclusion, the miR-218 may has important roles in the development and progression of CRC and be a potential diagnostic and prognostic biomarker of CRC.
Keywords: Colorectal cancer, miR-218, microRNA, survival, expression
Introduction
Colorectal cancer (CRC) is the most common gastrointestinal cancer, with over 1.2 million new cancer cases and 608,700 cancer deaths estimated to have occurred in 2008 [1]. In china, CRC is the third most common cancer by annual incidence and the fifth leading cause of cancer-related death [2], with an upward trend in incidence rate in recent decades. Given that there are typically no specific symptoms in the early stage of CRC, most patients are diag -nosed in an advanced stage. Current treat-ments for CRC include surgery, radiotherapy, chemotherapy and targeted therapy, but the five-year survival rate is still not high, especially in patients with advanced CRC [3]. Early diag -nosis and prognostic evaluation of CRC are cru -cial for timely and appropriate treatment. Thus, an urgent need exists to develop new screening tools and identify biomarkers for CRC.
MicroRNAs (miRNAs) are a large class of evolu -tionarily conserved endogenous non-coding RNAs of about 20 to 25 nucleotides with intrin -sic regulatory functions. miRNAs negatively regulate expression of target genes at the post-transcriptional level through mRNA degrada-tion, translational repression, or miRNA-mediat-ed mRNA decay [4]. It is well known that miRNAs play crucial roles in diverse biological process -es, including cell proliferation, differentiation, apoptosis and tumorigenesis. miRNAs can function as oncogenes and/or tumor suppres -sors [5], depending on their specific gene tar -gets. Different types of cancer show different and specific miRNA expression profiles [5, 6]. miRNAs could be promising biomarkers for can -cer diagnosis [7], prognosis [8-10] and predic-tion of treatment response [11-13].
pros-tate and bladder cancer [14-22]. In colon cancer, miR-218 inhibits cancer cell prolifera -tion, suppresses cycle progression, and pro -motes apoptosis by down-regulating BMI-1 expression [19]. In the present study, we inves -tigated whether miR-218 expression was asso-ciated with outcome of CRC patients.
Materials and methods Patients and samples
This study was conducted with the approval of the Ethical and Scientific Committees of Taizhou People’s Hospital. Primary tumor speci -mens were obtained from 189 patients (79 females and 110 males) diagnosed with CRC who underwent complete resection in Taizhou People’s Hospital between 2006 and 2008. Patient characteristics, including age, gender, TNM stage, lymph node status, tumor size and histological grade, were retrospectively collect -ed (Table 1). Twenty-six fresh specimens includ -ing both CRC tissue and correspond-ing normal tissue were also collected and stored at -80 °C
immediately after resection. A total of 60 sera were collected from 30 CRC patients. Serum samples were collected prior to the surgery and 1 month after surgery. No patient was treated with radiotherapy or chemotherapy prior to sur -gical resection. Furthermore, 30 age and gen-der matched healthy individuals were included in our study. Written informed consent was obtained from each participant prior to sample collection.
RNA isolation
RNA was extracted from fresh-frozen and for -malin-fixed tissues, and serum samples using TRIzol reagent, PureLink™ FFPE RNA Isolation Kit and mirVana PARIS kit (Life Technology, California, USA), respectively, following the manufacturer’s instructions. RNA was recov -ered in 50 µl of RNase-free water and stored at -80 °C before use. The RNA concentration was measured by NanoDrop 2000 Spectrophoto-meter (Thermo Fisher Scientific, DE, USA).
Protocol for the detection of miR-218
The reverse transcription reaction was carried out using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, CA, USA) in a total volume of 10 μL containing 3.33 μL RNA, 1 μl 10 × reverse transcription buffer, 0.67 μL Mutiscribe Reverse Transcriptase, 0.13 μL RNase Inhibitor. The reaction mixture was incubated for 30 min at 16 °C, 30 min at 42 °C, 5 min at 85 °C for 5 min, and then held at 4 °C. Next, quantitative reverse transcription PCR (qRT-PCR) was run on a 7500 Real-Time PCR System (Applied Biosystems, CA, USA). qRT-PCR was carried out in a total of 20 μl vol -ume containing 1.33 μl cDNA, 1 × Universal PCR Master Mix and 1 μl gene-specific primers and probe. PCR parameters were as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. All reactions were performed in triplicate. The expression of miR-218 from tissue sample was normalized to the endogenous reference gene RNU6, whereas the cel-miR-39 was used as the reference con -trol for serum samples [23].
Protocol for the detection of SLIT2 and SLIT3
[image:2.612.91.288.94.411.2]The reverse transcription reaction was per-formed using PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Dalian, China) following
Table 1. Clinicopathological characteristics of CRC patients
Characteristics No. of Patients %
Age (years)
Mean 65.3
range 30-83
Gender
female 79 41.8
male 110 58.2
TNM stage
I 24 12.7
II 73 38.6
III 81 42.9
IV 10 5.3
unknown 1 0.5
lymph node metastasis
no 104 55.0
yes 85 45.0
Tumor size
> 4 129 68.3
≤ 4 58 30.7
unknown 2 1.1
Differentiation
well 36 19.0
moderate 102 54.0
the manufacturer’s instructions. qRT-PCR was carried out using SYBR® Premix Ex Taq™ (Takara, Dalian, China) in a total volume of 20 μl on a 7500 Real-Time PCR System (Applied Biosystems, CA, USA) as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 3 s, and 60 °C for 30 s. The sequences of the primer pairs were as follows: SLIT2 forward (5’-AGCCGAGG-TTCAAAAACGAGA-3’) and SLIT2 reverse (5’-GG- CAGTGCAAAACACTACAAGA-3’); SLIT3 forward (5’-GAATATGTCACCGACCTGCGACT-3’) and SL- IT3 reverse (5’-GCAGGTTGGGCAACTTCTTGA-3’); RPL13A forward (5’-CCTGGAGGAGAAGAGGAA-AGAGA-3’) and RPL13A reverse (5’-TTGAG- GACCTCTGTGTATTTGTCAA-3’). RPL13A was used as the reference gene.
Statistical analysis
The relative changes in gene expression was calculated using the 2−ΔΔCT method. Cases were divided into 2 groups, high or low, using the median miR-218 expression as a cutoff. The chi square or Fisher’s exact probability test was
used to examine possible correlations between miR-218 expression and clinicopathologic fea -tures. The Kaplan–Meier method was used to estimate the survival rate, and differences in survival of subgroups of the study were com -pared by log-rank test. A P value < 0.05 was considered significant. The statistical analyses were performed using the SPSS version 19.0 (SPSS Inc, IL, USA). All analyses were performed in all cases by using Mann–Whitney U-test or Wilcoxon nonparametric test.
Results
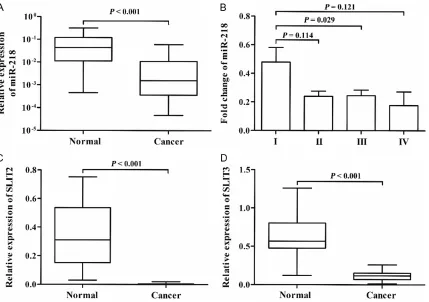
The expression levels of miR-218 and SLIT2 in CRC
[image:3.612.95.524.70.372.2]miR-218 in patients with TNM stage I were higher than those in patients with TNM stage II~IV (P = 0.038). In addition, to confirm predict -ed host gene down-regulation, we also examine the expression levels of SLIT2 and SLIT3 in 26 pairs of CRC and adjacent normal tissues. As expected, the expression levels of SLT2 and SLT3 in CRC tissues were significantly lower than those in the corresponding noncancerous tissues (all P < 0.001). The expression of miR-218 was co-regulated with SLIT2 and SLIT3 expression (all P < 0.05).
Relationship between miR-218 expression and clinicopathological features in CRC patients
Patient characteristics with respect to miR-218 expression were shown in Table 2. The Chi-square test showed that miR-218 expression was associated with TNM stage, lymph node metastasis (LNM) and differentiation (P = 0.001, 0.004 and 0.025, respectively). No association was found between miR-218 expression and age, sex, as well as tumor size (all P > 0.05).
Association between miR-218 expression and survival in CRC patients
During the follow-up period of 73 months, 77 of 189 (40.7%) CRC patients died due to disease progression. Twenty seven of 95 (28.4%) patients with high miR-218 expression died from CRC. In the group of patients with low miR-218 expression, 50 of 94 (53.2%) died from CRC. Median survival time (MST) of patients with low miR-218 was 45.0 months (range 2 to 73 months), and MST of patients with high miR-218 expression was 58.6 months (range 4 to 73 months). Kaplan-Meier analysis revealed a statistically significant association between low miR-218 expression and worse survival (P = 3.9 × 10-4, Figure 2). Univariate Cox regression analysis showed that overall survival was strongly influenced by TNM, differentiation, LNM and miR-218 (Table 3). Similar results were obtained in multivariate COX regression analysis.
Evaluation of miR-218 expression in serum of CRC patients
Finally, we hypothesized that low miR-218 expression in CRC tissues might influence serum levels of miR-218 in CRC patients. To evaluate serum expression of miR-218 in can -cer surveillance, we performed qRT-PCR on 30 sera from CRC patients and 30 sera from healthy controls. The expression levels of serum miR-218 in CRC patients were signifi -cantly lower than those in controls (P = 0.005,
Figure 3A). Furthermore, the expression of serum miR-218 was increased after surgery (P
= 0.026, Figure 3B). Twenty five of 30 patients showed increased levels of miR-218 after sur -gery, with a 135.5% mean increased, whereas 5 patients showed a decreased. All these 30 CRC patients showed no evidence of progres -sion after initial surgery.
Discussion
A large number of genetic and epigenetic alter -ations are involved in carcinogenesis and tumor progression. Among these alterations, miRNA has been shown to regulate the expression of multiple target genes at the transcriptional level. Abnormal expression of miRNA is associ -ated with cancer initiation, progression and metastasis [5, 6]. Thus, miRNAs provide a use-ful starting point in understanding cancer etiol
-Table 2. Association between miR-218 ex-pression and the clinicopathological features
Characteristics miR-218
Low High P value
Age (years) 0.461
> 60 64 (68.8) 70 (73.7)
≤ 60 29 (31.2) 25 (26.3)
Sex 0.932
female 39 (41.5) 40 (42.1) male 55 (58.5) 55 (57.9)
TNM stage 0.001
I 5 (5.3) 19 (20.2) II 32 (34.0) 41 (43.6) III 49 (52.1) 32 (34.0) IV 8 (8.5) 2 (2.1)
LNM 0.004
no 42 (44.7) 62 (65.3)
yes 52 (55.3) 33 (34.7)
Tumor size (cm) 0.715
> 4 63 (67.7) 66 (70.2)
≤ 4 30 (32.3) 28 (29.8)
Histology grade 0.025
[image:4.612.91.288.96.400.2]ogy at the molecular level, specifically in look -ing for optimal biomarkers and explor-ing gene therapy. These may in turn improve cancer diagnosis and treatment.
miR-218 is dysregulated in different cancers [14-18]. miR-218 precursors, miR-218-1 and miR-218-2, are produced from two different genomic loci. Both miR-218-1 and miR-218-2 are located in the intronic regions of the SLIT2 gene on chromosome 4p15.2 and SLIT3 on chromosome 5q35–q34, respectively [24]. Allelic losses of chromosome 4p15.2 and 5q35–q34 are frequently found in a variety of cancers, including CRC [24, 25]. In addition, SLIT2 and SLIT3 are also frequently hypermeth -ylated in human cancer [21, 24, 26]. Therefore, SLIT2 and SLIT3 are commonly downregulated in human cancer due to allelic loss and promot-er hyppromot-ermethylation. In the present study, we also found that the expression levels of miR-218, SLIT2 and SLIT3 were lower in CRC tis-sues, and downregulation of SLIT2 and SLIT3 was concordant with decreased expression of
28]. Leite et al. [20] reported that the expres-sion level of miR-218 in high grade prostate cancer was high than those in metastatic pros-tate cancer. Wu et al. [29] found that low miR-218 expression was associated with worse sur-vival time. In this study, we found that miR-218 expression was closely related to TNM Stage, LNM, differentiation and worse survival time. Furthermore, the expression levels of miR-218 were significantly decreased in patients with TNM stages II~IV compared with those with TNM stage I. These results indicated that decreased miR-218 expression may be associ -ated with CRC progression.
[image:5.612.92.386.71.387.2]Although the source of the endogenous circu -lating miRNAs and the precise cellular release mechanisms of miRNAs remain unknown, many studies have revealed that circulating miRNAs are not only derived from circulating tumor cells and blood cells, but also originate from cancer tissues or other tissue cells affected by disease [30]. Aberrant miRNA expression in circulation has been observed in various cancers [6, Figure 2. Kaplan-Meier curves for overall survival and miR-218 expression in
group of 189 CRC patients.
miR-218. The expression levels of miR218, SLIT2 and SLIT3 were restored in cancer cells treated with 5-Aza-2’-deoxycytidine [24, 27]. Underexpression of SLIT2 and SLIT3 is the main cause for reduced miR-218 expression [14, 27]. Fur- thermore, a high frequency of promoter hypermethyl -ation of SLIT2 and loss of heterozygosity at 4p15.1– 4p15.31 occur at an very early stage in the pathogen -esis of CRC [26]. Down- regulation of miR-218 may play a vital role in CRC initiation.
secretion in normal cells affected by their inter -action with cancer cells. We propose that the curative resection lead to removing influence of cancer cells on normal cells, the level of miR-218 in turn elevated. Further studies with larg-er samples are required to validate these results.
In summary, we provide the evidence that miR-218 was downregulated in tissues and sera of CRC patients, and may act as independent prognostic factors for CRC patients. miR-218 is a candidate target gene for future cancer therapeutics.
Acknowledgements
This work was supported by Natural Science Fund programs of Nantong University (10Z088).
Disclosure of conflict of interest
The authors have no conflicts of interest. 30-32]. Furthermore, circulating miRNAs
[image:6.612.90.526.80.393.2]remain stable even after exposure to severe physicochemical conditions, such as extreme pH, long-time storage at room temperature [23, 31]. Many studies have reported on the use of circulating miRNAs as diagnostic or prognostic biomarker for human cancer, including lung cancer, CRC. In the present study, we found that serum miR-218 levels were dramatically reduced in CRC patients. This was in keeping with previous study which found reduced miR-218 expression in plasma of gastric cancer [22]. Some studies revealed that expression levels of circulating miRNAs changed after anti-cancer treatment [33]. Therefore, we further explore the potential impact of CRC surgery on serum miR-218. The expression level of serum miR-218 was weak but significantly elevated after surgery. Since cancer cells account for only a very tiny fraction of the body mass, circu -lating miR-218 may be mostly derived from nor -mal cells. Decreased serum miR-218 level in CRC patients may be the result of reduced
Table 3. Univariate and multivariate Cox regression analysis of overall survival
Features Univariate analysis Multivariate analysis HR (95% CI) P value HR (95% CI) P value
age (years), > 60 v ≤ 60 0.814 (0.488-1.356) 0.429
sex, male v female 0.994 (0.631-1.563) 0.978
tumor size (cm), > 4 v ≤ 4 0.743 (0.450-1.227) 0.246
tumor differentiation, moderate, poor v well 2.684 (1.853-3.886) 1.72 × 10-7 2.067 (1.412-3.026) 1.90 × 10-4
TNM stage, III+IV v I+II 1.790 (1.131-2.832) 0.013 1.662 (1.048-2.634) 0.031
LNM, yes v no 3.323 (2.066-5.344) 7.33 × 10-7 2.305 (1.403-3.788) 0.001
[image:6.612.93.521.87.200.2]miR-218, low v high 2.276 (1.423-3. 640) 0.001 1.681 (1.034-2.731) 0.036
profiles as a predictor of chemoresponsive -ness in Wilms’ tumor blastema. PLoS One 2013; 8: e53417.
[13] Svoboda M, Sana J, Fabian P, Kocakova I, Gombosova J, Nekvindova J, Radova L, Vyzula R and Slaby O. MicroRNA expression profile as -sociated with response to neoadjuvant
chemo-radiotherapy in locally advanced rectal cancer
patients. Radiat Oncol 2012; 7: 195.
[14] Davidson MR, Larsen JE, Yang IA, Hayward NK, Clarke BE, Duhig EE, Passmore LH, Bowman
RV and Fong KM. MicroRNA-218 is deleted
and downregulated in lung squamous cell car -cinoma. PLoS One 2010; 5: e12560.
[15] Chiyomaru T, Enokida H, Kawakami K, Tatara
-no S, Uchida Y, Kawahara K, Nishiyama K, Seki N and Nakagawa M. Functional role of LASP1 in cell viability and its regulation by microRNAs in bladder cancer. Urol Oncol 2012; 30:
434-443.
[16] Song L, Huang Q, Chen K, Liu L, Lin C, Dai T, Yu
C, Wu Z and Li J. miR-218 inhibits the invasive ability of glioma cells by direct downregulation of IKK-beta. Biochem Biophys Res Commun
2010; 402: 135-140.
[17] Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, Guo X, Wang B, Gang Y, Zhang Y, Li Q, Qiao T, Zhao Q,
Nie Y and Fan D. MiR-218 inhibits invasion and
metastasis of gastric cancer by targeting the
Robo1 receptor. PLoS Genet 2010; 6: e1000879.
[18] Li J, Ping Z and Ning H. MiR-218 Impairs Tumor Growth and Increases Chemo-Sensitivity to
Cisplatin in Cervical Cancer. Int J Mol Sci 2012; 13: 16053-16064.
[19] He X, Dong Y, Wu CW, Zhao Z, Ng SS, Chan FK,
Sung JJ and Yu J. MicroRNA-218 inhibits cell
cycle progression and promotes apoptosis in colon cancer by downregulating BMI1 poly
-comb ring finger oncogene. Mol Med 2012;
18: 1491-1498.
[20] Leite KR, Sousa-Canavez JM, Reis ST, Tomiya -ma AH, Ca-mara-Lopes LH, Sanudo A, Antunes
AA and Srougi M. Change in expression of miR-let7c, miR-100, and miR-218 from high grade localized prostate cancer to metastasis. Urol
Oncol 2011; 29: 265-269.
[21] Uesugi A, Kozaki K, Tsuruta T, Furuta M, Morita
K, Imoto I, Omura K and Inazawa J. The tumor suppressive microRNA miR-218 targets the mTOR component Rictor and inhibits AKT
phosphorylation in oral cancer. Cancer Res
2011; 71: 5765-5778.
[22] Li BS, Zhao YL, Guo G, Li W, Zhu ED, Luo X, Mao XH, Zou QM, Yu PW, Zuo QF, Li N, Tang B, Liu KY and Xiao B. Plasma microRNAs,
miR-223, miR-21 and miR-218, as novel potential
biomarkers for gastric cancer detection. PLoS
One 2012; 7: e41629.
Address correspondence to: Dr. Junxing Huang,
Department of Oncology, Taizhou People’s Hospintal,
210 Yingchun Road, Taizhou, Jiangsu 225300, China. Tel: +86 523 86333483; Fax: +86 523
86333483; E-mail: hjxtz@sina.cn; hjxtz@outlook.
com
References
[1] Jemal A, Bray F, Center MM, Ferlay J, Ward E
and Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90.
[2] He J and Chen WQ. 2012 Chinese Cancer
Reg-istry Annual Report. Military Medical Science
Press 2012; pp: 28-57.
[3] Hill DA, Furman WL, Billups CA, Riedley SE, Cain AM, Rao BN, Pratt CB and Spunt SL.
Colorectal carcinoma in childhood and adoles-cence: a clinicopathologic review. J Clin Oncol 2007; 25: 5808-5814.
[4] Cordes KR and Srivastava D. MicroRNA
regula-tion of cardiovascular development. Circ Res
2009; 104: 724-732.
[5] Calin GA and Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6: 857-866.
[6] Shen J, Stass SA and Jiang F. MicroRNAs as
potential biomarkers in human solid tumors.
Cancer Lett 2013; 329: 125-136.
[7] Ulivi P, Foschi G, Mengozzi M, Scarpi E, Silves
-trini R, Amadori D and Zoli W. Peripheral Blood miR-328 Expression as a Potential Biomarker for the Early Diagnosis of NSCLC. Int J Mol Sci
2013; 14: 10332-10342.
[8] Chen Q, Si Q, Xiao S, Xie Q, Lin J, Wang C, Chen
L, Chen Q and Wang L. Prognostic significance of serum miR-17-5p in lung cancer. Med Oncol
2013; 30: 353.
[9] Volinia S and Croce CM. Prognostic microRNA/
mRNA signature from the integrated analysis of patients with invasive breast cancer. Proc Natl Acad Sci U S A 2013; 110: 7413-7417.
[10] Lin Q, Chen T, Lin Q, Lin G, Lin J, Chen G and Guo L. Serum miR-19a expression correlates
with worse prognosis of patients with
non-small cell lung cancer. J Surg Oncol 2013; 107: 767-771.
[11] Gamez-Pozo A, Anton-Aparicio LM, Bayona C, Borrega P, Gallegos Sancho MI, Garcia-Domin
-guez R, de Portugal T, Ramos-Vazquez M, Per
-ez-Carrion R, Bolos MV, Madero R,
Sanchez-Navarro I, Fresno Vara JA and Espinosa Arranz
E. MicroRNA expression profiling of peripheral blood samples predicts resistance to first-line
sunitinib in advanced renal cell carcinoma pa-tients. Neoplasia 2012; 14: 1144-1152. [12] Watson JA, Bryan K, Williams R, Popov S, Vu
-janic G, Coulomb A, Boccon-Gibod L, Graf N,
[29] Wu DW, Cheng YW, Wang J, Chen CY and Lee H. Paxillin predicts survival and relapse in
non-small cell lung cancer by microRNA-218 target -ing. Cancer Res 2010; 70: 10392-10401. [30] Mo MH, Chen L, Fu Y, Wang W and Fu SW.
Cell-free Circulating miRNA Biomarkers in Cancer. J
Cancer 2012; 3: 432-448.
[31] Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J and Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of can -cer and other diseases. Cell Res 2008; 18: 997-1006.
[32] Chin LJ and Slack FJ. A truth serum for
cancer--microRNAs have major potential as cancer
biomarkers. Cell Res 2008; 18: 983-984.
[33] Sun Y, Wang M, Lin G, Sun S, Li X, Qi J and Li J.
Serum microRNA-155 as a potential biomarker to track disease in breast cancer. PLoS One
2012; 7: e47003. [23] Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peter
-son A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stire -walt DL, Gentleman R, Vessella RL, Nelson PS,
Martin DB and Tewari M. Circulating microR
-NAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105:
10513-10518.
[24] Dickinson RE, Dallol A, Bieche I, Krex D, Mor
-ton D, Maher ER and Latif F. Epigenetic inacti
-vation of SLIT3 and SLIT1 genes in human can
-cers. Br J Cancer 2004; 91: 2071-2078.
[25] Shivapurkar N, Maitra A, Milchgrub S and Gaz
-dar AF. Deletions of chromosome 4 occur early during the pathogenesis of colorectal carcino -ma. Hum Pathol 2001; 32: 169-177.
[26] Beggs AD, Jones A, Shepherd N, Arnaout A, Fin
-layson C, Abulafi AM, Morton DG, Matthews GM, Hodgson SV and Tomlinson IP. Loss of ex
-pression and promoter methylation of SLIT2
are associated with sessile serrated adenoma
formation. PLoS Genet 2013; 9: e1003488.
[27] Alajez NM, Lenarduzzi M, Ito E, Hui AB, Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J, O’Sullivan B and Liu FF. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-RO
-BO1 pathway. Cancer Res 2011; 71:
2381-2391.
[28] Yamamoto N, Kinoshita T, Nohata N, Itesako T, Yoshino H, Enokida H, Nakagawa M, Shozu M and Seki N. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion by targeting focal adhesion pathways in cervical squamous cell carcinoma. Int J Oncol 2013;