metal-organic papers
m478
Peter G. Joneset al. [Cr(C24H20P2S)(CO)5] DOI: 10.1107/S1600536802014125 Acta Cryst.(2002). E58, m478±m479 Acta Crystallographica Section EStructure Reports
Online ISSN 1600-5368
Pentacarbonyl(tetraphenyldiphosphine
monosulfide-
P
)chromium(0)
Peter G. Jones,* Axel K. Fischer, Michael Farkens and Reinhard Schmutzler
Institut fuÈr Anorganische und Analytische Chemie, Technische UniversitaÈt Braunschweig, Postfach 3329, 38023 Braunschweig, Germany
Correspondence e-mail: jones@xray36.anchem.nat.tu-bs.de
Key indicators Single-crystal X-ray study
T= 178 K
Mean(C±C) = 0.005 AÊ
Rfactor = 0.040
wRfactor = 0.108
Data-to-parameter ratio = 13.3
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2002 International Union of Crystallography Printed in Great Britain ± all rights reserved
In the title compound, [Cr(C24H20P2S)(CO)5], the key bond
lengths are P1ÐP2 2.2659 (15), P2ÐS 1.9531 (15), P1ÐC 1.830, 1.833 (3), P2ÐC 1.821, 1.822 (3) and P1ÐCr 2.3921 (13) AÊ. The conformation about the PÐP bond is de®ned by the torsion angle SÐP2ÐP1ÐCr ofÿ58.60 (7).
Comment
The title compound, (I), arose during studies (see Experi-mental) of thioureas substituted with phosphorus-containing groups (Farkens, 1991). It was characterized by this structure determination.
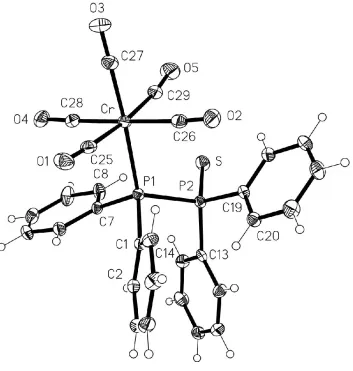
The molecular structure of (I) is shown in Fig. 1. A search of the Cambridge Structural Database (CSD, Version of April 2002; Allen & Kennard, 1993) revealed no other complexes of any tetra(organyl)diphosphine monosul®de (in the strict sense of a compound containing a PÐP bond). The key bond lengths are P1ÐP2 2.2659 (15), P2ÐS 1.9531 (15), P1ÐC 1.830, 1.833 (3) and P2ÐC 1.821 1.822 (3) AÊ. These may be compared, although the two systems clearly differ chemically in many respects, with values for uncomplexed tetramethyl-diphosphane monosul®de (Gruberet al., 1990): PÐP 2.201 (1), PÐS 1.970 (1), PIIIÐC 1.830 and 1.834 (1), and P(S)ÐC 1.803
and 1.803 (1) AÊ. The PÐP bond in (I) may be regarded as long, and it is reasonable to propose steric effects as the cause. A CSD search revealed 57 structures with (C)2PÐP(C)2single
bonds, involving 69 bonds in the range 2.104±2.310 AÊ (average 2.216 AÊ).
The PÐCr distance is 2.3921 (13) AÊ, and the CrÐC bond
transto CrÐP is by far the shortest, at 1.865 (4) AÊ,cf. average 1.903 AÊ for the other four CrÐC bonds. The conformation about the PÐP bond is de®ned by the torsion angle SÐP2Ð P1ÐCr ofÿ58.60 (7) (in the above-mentioned diphosphine monosul®de, the S atom istransto the lone pair at the other phosphorus). As expected, the largest angles at phosphorus are those involving the Cr atom at P1 and the S atom at P2.
Two short H O contacts could reasonably be interpreted as weak hydrogen bonds (Table 2).
Experimental
The reaction between (norbornadiene)Cr(CO)4 and N,N0 -bis(di-phenylphosphino)±N,N0-dimethylthiourea in dichloromethane led to
a complex mixture of products. The mixture was dissolved in diethyl ether, from which the title compound crystallized in 13% yield (Farkens, 1991). The decomposition of the thiourea to tetraphenyl-diphosphine monosul®de has been observed before in our laboratory (Gruber, 1989).
Crystal data
[Cr(C24H20P2S)(CO)5]
Mr= 594.45
Triclinic,P1
a= 9.840 (3) AÊ
b= 10.089 (5) AÊ
c= 14.967 (6) AÊ = 77.90 (3) = 84.43 (3) = 68.02 (3)
V= 1347.0 (10) AÊ3
Z= 2
Dx= 1.466 Mg mÿ3
MoKradiation Cell parameters from 50
re¯ections = 10±11.5 = 0.66 mmÿ1
T= 178 (2) K Tablet, yellow 0.50.50.2 mm
Data collection
NicoletR3 diffractometer !scans
Absorption correction: scan (XEMP; Nicolet, 1987)
Tmin= 0.801,Tmax= 0.991 5120 measured re¯ections 4578 independent re¯ections 3476 re¯ections withI> 2(I)
Rint= 0.038 max= 25.1
h=ÿ11!11
k=ÿ11!11
l=ÿ12!17 3 standard re¯ections
every 147 re¯ections intensity decay: none
Re®nement
Re®nement onF2
R[F2> 2(F2)] = 0.040
wR(F2) = 0.108
S= 1.03 4578 re¯ections 343 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0464P)2
+ 1.6663P]
whereP= (Fo2+ 2Fc2)/3
(/)max< 0.001
max= 0.48 e AÊÿ3
min=ÿ0.47 e AÊÿ3
?show
Table 1
Selected geometric parameters (AÊ,).
CrÐC27 1.865 (4) CrÐC25 1.893 (4) CrÐC28 1.895 (4) CrÐC26 1.909 (4) CrÐC29 1.916 (4) CrÐP1 2.3921 (13)
SÐP2 1.9531 (15) P1ÐC1 1.830 (3) P1ÐC7 1.833 (3) P1ÐP2 2.2659 (15) P2ÐC13 1.821 (3) P2ÐC19 1.822 (3)
C27ÐCrÐC25 88.48 (15) C27ÐCrÐC28 88.55 (15) C25ÐCrÐC28 87.44 (15) C27ÐCrÐC26 89.65 (15) C25ÐCrÐC26 91.50 (15) C28ÐCrÐC26 177.94 (14) C27ÐCrÐC29 85.89 (15) C25ÐCrÐC29 173.94 (14) C28ÐCrÐC29 90.19 (15) C26ÐCrÐC29 90.69 (16) C27ÐCrÐP1 176.55 (11) C25ÐCrÐP1 89.77 (10) C28ÐCrÐP1 88.40 (10) C26ÐCrÐP1 93.36 (10)
C29ÐCrÐP1 95.75 (11) C1ÐP1ÐC7 103.54 (14) C1ÐP1ÐP2 101.21 (10) C7ÐP1ÐP2 104.56 (10) C1ÐP1ÐCr 119.02 (11) C7ÐP1ÐCr 110.78 (10) P2ÐP1ÐCr 116.02 (5) C13ÐP2ÐC19 110.90 (14) C13ÐP2ÐS 111.01 (11) C19ÐP2ÐS 111.67 (11) C13ÐP2ÐP1 107.35 (10) C19ÐP2ÐP1 103.03 (11) SÐP2ÐP1 112.55 (6)
C1ÐP1ÐP2ÐC13 48.63 (15) C7ÐP1ÐP2ÐC13 ÿ58.72 (15) CrÐP1ÐP2ÐC13 178.96 (10) C1ÐP1ÐP2ÐC19 ÿ68.50 (15) C7ÐP1ÐP2ÐC19 ÿ175.85 (14)
CrÐP1ÐP2ÐC19 61.83 (11) C1ÐP1ÐP2ÐS 171.07 (11) C7ÐP1ÐP2ÐS 63.72 (11) CrÐP1ÐP2ÐS ÿ58.60 (7)
Table 2
Hydrogen-bonding geometry (AÊ,).
DÐH A DÐH H A D A DÐH A
C22ÐH22 O2i 0.95 2.65 3.581 (4) 166 C21ÐH21 O4ii 0.95 2.57 3.426 (5) 149 Symmetry codes: (i) 1ÿx;1ÿy;1ÿz; (ii) 1x;y;z.
H atoms were included using a riding model with ®xed CÐH bond lengths of 0.95 AÊ;Uiso(H) values were ®xed at 1.2 times theUeqof the parent atom.
Data collection: P3 (Nicolet, 1987); cell re®nement: P3; data reduction:XDISK(Nicolet, 1987); program(s) used to solve struc-ture:SHELXS97 (Sheldrick, 1990); program(s) used to re®ne struc-ture: SHELXL97 (Sheldrick, 1997); molecular graphics: XP
(Siemens, 1994); software used to prepare material for publication:
SHELXL97.
Financial support from the Fonds der Chemischen Industrie is gratefully acknowledged. The authors thank Mr A. Wein-kauf for technical assistance.
References
Allen, F. H. & Kennard, O. (1993).Chem. Des. Autom. News,8, 1, 31±37. Farkens, M. (1991). Diplomarbeit, Technical University of Braunschweig,
Germany.
Gruber, M. (1989). PhD Thesis, Technical University of Braunschweig, Germany.
Gruber, M., Jones, P. G. & Schmutzler, R. (1990).Chem. Ber.123, 1313±1317. Nicolet (1987). P3, XDISK and XEMP. Nicolet Instrument Corporation,
Madison, Wisconsin, USA.
Sheldrick, G. M. (1990).Acta Cryst.A46, 467±473.
Sheldrick, G. M. (1997).SHELXL97. University of GoÈttingen, Germany. Siemens (1994). XP. Version 5.03. Siemens Analytical X-ray Instruments,
Madison, Wisconsin, USA. Figure 1
supporting information
sup-1
Acta Cryst. (2002). E58, m478–m479
supporting information
Acta Cryst. (2002). E58, m478–m479 [doi:10.1107/S1600536802014125]
Pentacarbonyl(tetraphenyldiphosphine monosulfide-
P
)chromium(0)
Peter G. Jones, Axel K. Fischer, Michael Farkens and Reinhard Schmutzler
S1. Comment
The title compound, (I), arose during studies (see Experimental) of thioureas substituted with phosphorus-containing
groups (Farkens, 1991). It was characterized by this structure determination.
The molecule of (I) is shown in Fig. 1. A search of the Cambridge Structural Database (CSD, Version of April 2002;
Allen & Kennard, 1993) revealed no other complexes of any tetra(organyl)diphosphine monosulfide (in the strict sense of
a compound containing a P—P bond). The key bond lengths are P1—P2 2.2659 (15), P2—S 1.9531 (15), P1—C 1.830,
1.833 (3) and P2—C 1.821 1.822 (3) Å. These may be compared, although the two systems clearly differ chemically in
many respects, with values for uncomplexed tetramethyldiphosphane monosulfide (Gruber et al., 1990): P—P 2.201 (1),
P—S 1.970 (1), PIII—C 1.830 and 1.834 (1), and P(S)—C 1.803 and 1.803 (1) Å. The P—P bond in (I) may be regarded
as long, and it is reasonable to propose steric effects as the cause. A CSD search revealed 57 structures with (C)2P—P(C)2
single bonds, involving 69 bonds in the range 2.104–2.310 Å (average 2.216 Å).
The P—Cr distance is 2.3921 (13) Å, and the Cr—C bond trans to Cr—P is by far the shortest at 1.865 (4) Å, cf.
average 1.903 Å for the other four Cr—C bonds. The conformation about the P—P bond is defined by the torsion angle S
—P2—P1—Cr of −58.60 (7)° (in the above-mentioned diphosphine monosulfide, the S atom is trans to the lone pair at
the other phosphorus). As expected, the largest angles at phosphorus are those involving the Cr atom at P1 and the S atom
at P2.
Two short H···O contacts could reasonably be interpreted as weak hydrogen bonds (Table 2).
S2. Experimental
The reaction between (norbornadiene)Cr(CO)4 and N,N′-bis(diphenylphosphino)-N,N′-dimethylthiourea in
dichloro-methane led to a complex mixture of products. The mixture was dissolved in diethyl ether, from which the title
compound crystallized in 13% yield (Farkens, 1991). The decomposition of the thiourea to tetraphenyldiphosphine
mono-sulfide has been observed before in our laboratory (Gruber, 1989).
S3. Refinement
H atoms were included using a riding model with fixed C—H bond lengths of 0.95 Å; Uiso(H) values were fixed at 1.2
Figure 1
The molecule of compound (I) in the crystal. Displacement ellipsoids represent 30% probability levels. H-atom radii are
arbitrary.
(Tetraphenyldiphosphinmonosulfide-P)pentacarbonylchromium(0)
Crystal data
[Cr(C24H20P2S)(CO)5]
Mr = 594.45 Triclinic, P1 a = 9.840 (3) Å b = 10.089 (5) Å c = 14.967 (6) Å α = 77.90 (3)° β = 84.43 (3)° γ = 68.02 (3)° V = 1347.0 (10) Å3
Z = 2 F(000) = 608 Dx = 1.466 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 50 reflections θ = 10–11.5°
µ = 0.66 mm−1
supporting information
sup-3
Acta Cryst. (2002). E58, m478–m479 Data collection
Nicolet R3 diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
Absorption correction: ψ scan (XEMP; Nicolet, 1987) Tmin = 0.801, Tmax = 0.991
5120 measured reflections
4578 independent reflections 3476 reflections with I > 2σ(I) Rint = 0.038
θmax = 25.1°, θmin = 3.1°
h = −11→11 k = −11→11 l = −12→17
3 standard reflections every 147 reflections intensity decay: none
Refinement
Refinement on F2
Least-squares matrix: full R[F2 > 2σ(F2)] = 0.040
wR(F2) = 0.108
S = 1.03 4578 reflections 343 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.0464P)2 + 1.6663P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max < 0.001
Δρmax = 0.48 e Å−3
Δρmin = −0.47 e Å−3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
Cr 0.12119 (5) 0.22328 (5) 0.40722 (3) 0.02628 (15)
S 0.06062 (9) 0.57737 (10) 0.15723 (6) 0.0382 (2)
P1 0.23659 (8) 0.21117 (8) 0.25938 (5) 0.02230 (19)
P2 0.25228 (8) 0.42161 (8) 0.17823 (5) 0.02327 (19)
O1 0.3039 (3) −0.0945 (3) 0.47601 (17) 0.0481 (7)
O2 0.3385 (3) 0.3212 (3) 0.48080 (17) 0.0508 (7)
O3 −0.0302 (3) 0.2233 (3) 0.59188 (18) 0.0514 (7)
O4 −0.1003 (3) 0.1140 (3) 0.35052 (18) 0.0489 (7)
O5 −0.0987 (3) 0.5345 (3) 0.3672 (2) 0.0626 (8)
C1 0.4255 (3) 0.0849 (3) 0.2502 (2) 0.0240 (6)
C2 0.4827 (3) 0.0388 (3) 0.1683 (2) 0.0299 (7)
H2 0.4217 0.0691 0.1168 0.036*
C3 0.6278 (4) −0.0508 (4) 0.1620 (3) 0.0378 (8)
H3 0.6666 −0.0801 0.1056 0.045*
H4 0.8160 −0.1620 0.2327 0.051*
C5 0.6619 (4) −0.0527 (4) 0.3175 (2) 0.0400 (9)
H5 0.7235 −0.0837 0.3688 0.048*
C6 0.5170 (3) 0.0381 (4) 0.3248 (2) 0.0321 (7)
H6 0.4799 0.0687 0.3810 0.038*
C7 0.1355 (3) 0.1573 (3) 0.1860 (2) 0.0237 (6)
C8 0.0092 (4) 0.2571 (4) 0.1432 (2) 0.0365 (8)
H8 −0.0171 0.3576 0.1435 0.044*
C9 −0.0793 (4) 0.2120 (4) 0.0998 (3) 0.0450 (9)
H9 −0.1650 0.2815 0.0699 0.054*
C10 −0.0430 (4) 0.0660 (4) 0.1002 (2) 0.0407 (9)
H10 −0.1036 0.0350 0.0704 0.049*
C11 0.0804 (4) −0.0344 (4) 0.1435 (2) 0.0405 (9)
H11 0.1046 −0.1350 0.1442 0.049*
C12 0.1698 (3) 0.0107 (4) 0.1862 (2) 0.0322 (7)
H12 0.2554 −0.0594 0.2159 0.039*
C13 0.3465 (3) 0.3827 (3) 0.0705 (2) 0.0245 (6)
C14 0.2578 (4) 0.3983 (4) −0.0014 (2) 0.0325 (7)
H14 0.1543 0.4310 0.0070 0.039*
C15 0.3201 (4) 0.3664 (4) −0.0847 (2) 0.0397 (8)
H15 0.2593 0.3772 −0.1334 0.048*
C16 0.4705 (4) 0.3189 (4) −0.0978 (2) 0.0358 (8)
H16 0.5133 0.2968 −0.1551 0.043*
C17 0.5573 (4) 0.3040 (4) −0.0273 (2) 0.0378 (8)
H17 0.6607 0.2715 −0.0362 0.045*
C18 0.4972 (3) 0.3354 (4) 0.0562 (2) 0.0324 (7)
H18 0.5592 0.3245 0.1042 0.039*
C19 0.3649 (3) 0.4636 (3) 0.2496 (2) 0.0265 (7)
C20 0.5115 (3) 0.3782 (4) 0.2695 (2) 0.0311 (7)
H20 0.5586 0.2917 0.2455 0.037*
C21 0.5876 (4) 0.4198 (4) 0.3243 (3) 0.0435 (9)
H21 0.6875 0.3623 0.3370 0.052*
C22 0.5201 (4) 0.5442 (4) 0.3606 (3) 0.0466 (10)
H22 0.5737 0.5726 0.3976 0.056*
C23 0.3750 (4) 0.6270 (4) 0.3433 (2) 0.0412 (9)
H23 0.3280 0.7119 0.3689 0.049*
C24 0.2971 (4) 0.5864 (4) 0.2883 (2) 0.0336 (8)
H24 0.1966 0.6432 0.2771 0.040*
C25 0.2382 (4) 0.0257 (4) 0.4483 (2) 0.0333 (8)
C26 0.2590 (4) 0.2869 (4) 0.4496 (2) 0.0346 (8)
C27 0.0275 (4) 0.2238 (4) 0.5217 (2) 0.0365 (8)
C28 −0.0164 (4) 0.1567 (4) 0.3695 (2) 0.0334 (8)
C29 −0.0118 (4) 0.4205 (4) 0.3756 (2) 0.0376 (8)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-5
Acta Cryst. (2002). E58, m478–m479
S 0.0323 (5) 0.0326 (5) 0.0487 (5) −0.0077 (4) −0.0059 (4) −0.0106 (4)
P1 0.0210 (4) 0.0235 (4) 0.0235 (4) −0.0079 (3) −0.0023 (3) −0.0061 (3)
P2 0.0222 (4) 0.0219 (4) 0.0263 (4) −0.0072 (3) −0.0029 (3) −0.0064 (3)
O1 0.0527 (16) 0.0344 (15) 0.0443 (15) −0.0035 (13) 0.0030 (12) −0.0054 (12)
O2 0.0583 (17) 0.076 (2) 0.0383 (14) −0.0420 (16) 0.0004 (12) −0.0207 (14)
O3 0.0471 (16) 0.0629 (18) 0.0383 (15) −0.0131 (14) 0.0133 (13) −0.0162 (13)
O4 0.0420 (15) 0.0630 (18) 0.0549 (17) −0.0323 (14) 0.0076 (12) −0.0182 (14)
O5 0.0576 (18) 0.0338 (16) 0.078 (2) −0.0006 (14) 0.0144 (15) −0.0092 (14)
C1 0.0206 (15) 0.0214 (16) 0.0314 (17) −0.0087 (13) −0.0002 (12) −0.0059 (13)
C2 0.0304 (17) 0.0277 (17) 0.0353 (18) −0.0139 (14) 0.0006 (14) −0.0077 (14)
C3 0.038 (2) 0.0325 (19) 0.045 (2) −0.0148 (16) 0.0101 (16) −0.0133 (16)
C4 0.0242 (17) 0.0287 (19) 0.065 (3) −0.0028 (15) 0.0035 (17) −0.0037 (18)
C5 0.0281 (18) 0.040 (2) 0.042 (2) −0.0064 (16) −0.0068 (15) 0.0037 (17)
C6 0.0257 (16) 0.0367 (19) 0.0304 (18) −0.0091 (15) −0.0002 (13) −0.0033 (15)
C7 0.0258 (15) 0.0263 (16) 0.0232 (15) −0.0137 (13) −0.0018 (12) −0.0049 (13)
C8 0.0358 (19) 0.0293 (18) 0.047 (2) −0.0136 (15) −0.0133 (16) −0.0035 (16)
C9 0.044 (2) 0.049 (2) 0.048 (2) −0.0264 (19) −0.0226 (17) 0.0054 (18)
C10 0.049 (2) 0.057 (2) 0.0329 (19) −0.036 (2) −0.0030 (16) −0.0122 (17)
C11 0.047 (2) 0.037 (2) 0.050 (2) −0.0229 (18) 0.0062 (17) −0.0222 (17)
C12 0.0280 (17) 0.0294 (18) 0.0408 (19) −0.0107 (14) 0.0000 (14) −0.0102 (15)
C13 0.0306 (16) 0.0176 (15) 0.0275 (16) −0.0090 (13) −0.0035 (13) −0.0071 (12)
C14 0.0287 (17) 0.0345 (19) 0.0322 (18) −0.0090 (15) −0.0038 (14) −0.0053 (15)
C15 0.048 (2) 0.044 (2) 0.0318 (19) −0.0170 (18) −0.0074 (16) −0.0127 (16)
C16 0.046 (2) 0.0350 (19) 0.0299 (18) −0.0184 (17) 0.0071 (15) −0.0095 (15)
C17 0.0317 (18) 0.044 (2) 0.043 (2) −0.0179 (16) 0.0079 (15) −0.0153 (17)
C18 0.0284 (17) 0.041 (2) 0.0309 (18) −0.0162 (15) −0.0004 (14) −0.0076 (15)
C19 0.0298 (16) 0.0250 (16) 0.0279 (17) −0.0120 (14) −0.0020 (13) −0.0078 (13)
C20 0.0294 (17) 0.0322 (18) 0.0329 (18) −0.0104 (14) −0.0046 (14) −0.0088 (14)
C21 0.0330 (19) 0.051 (2) 0.048 (2) −0.0134 (17) −0.0117 (16) −0.0115 (18)
C22 0.051 (2) 0.061 (3) 0.043 (2) −0.029 (2) −0.0081 (18) −0.0207 (19)
C23 0.049 (2) 0.048 (2) 0.038 (2) −0.0228 (19) 0.0023 (17) −0.0228 (17)
C24 0.0311 (17) 0.0357 (19) 0.0377 (19) −0.0140 (15) 0.0016 (14) −0.0123 (15)
C25 0.0331 (18) 0.041 (2) 0.0270 (17) −0.0135 (17) 0.0063 (14) −0.0108 (16)
C26 0.0401 (19) 0.042 (2) 0.0236 (17) −0.0165 (17) 0.0037 (14) −0.0099 (15)
C27 0.0307 (18) 0.036 (2) 0.040 (2) −0.0062 (15) 0.0007 (16) −0.0128 (16)
C28 0.0320 (18) 0.037 (2) 0.0316 (18) −0.0138 (16) 0.0048 (14) −0.0075 (15)
C29 0.0373 (19) 0.032 (2) 0.042 (2) −0.0111 (17) 0.0073 (16) −0.0123 (16)
Geometric parameters (Å, º)
Cr—C27 1.865 (4) C8—H8 0.9500
Cr—C25 1.893 (4) C9—C10 1.378 (5)
Cr—C28 1.895 (4) C9—H9 0.9500
Cr—C26 1.909 (4) C10—C11 1.369 (5)
Cr—C29 1.916 (4) C10—H10 0.9500
Cr—P1 2.3921 (13) C11—C12 1.386 (5)
S—P2 1.9531 (15) C11—H11 0.9500
P1—C7 1.833 (3) C13—C18 1.387 (4)
P1—P2 2.2659 (15) C13—C14 1.399 (4)
P2—C13 1.821 (3) C14—C15 1.381 (5)
P2—C19 1.822 (3) C14—H14 0.9500
O1—C25 1.146 (4) C15—C16 1.382 (5)
O2—C26 1.135 (4) C15—H15 0.9500
O3—C27 1.144 (4) C16—C17 1.371 (5)
O4—C28 1.144 (4) C16—H16 0.9500
O5—C29 1.138 (4) C17—C18 1.376 (5)
C1—C6 1.391 (4) C17—H17 0.9500
C1—C2 1.394 (4) C18—H18 0.9500
C2—C3 1.382 (5) C19—C24 1.387 (4)
C2—H2 0.9500 C19—C20 1.399 (4)
C3—C4 1.383 (5) C20—C21 1.381 (5)
C3—H3 0.9500 C20—H20 0.9500
C4—C5 1.371 (5) C21—C22 1.380 (5)
C4—H4 0.9500 C21—H21 0.9500
C5—C6 1.384 (5) C22—C23 1.376 (5)
C5—H5 0.9500 C22—H22 0.9500
C6—H6 0.9500 C23—C24 1.389 (5)
C7—C8 1.384 (4) C23—H23 0.9500
C7—C12 1.388 (4) C24—H24 0.9500
C8—C9 1.384 (5)
C27—Cr—C25 88.48 (15) C10—C9—H9 120.1
C27—Cr—C28 88.55 (15) C8—C9—H9 120.1
C25—Cr—C28 87.44 (15) C11—C10—C9 120.1 (3)
C27—Cr—C26 89.65 (15) C11—C10—H10 119.9
C25—Cr—C26 91.50 (15) C9—C10—H10 119.9
C28—Cr—C26 177.94 (14) C10—C11—C12 120.0 (3)
C27—Cr—C29 85.89 (15) C10—C11—H11 120.0
C25—Cr—C29 173.94 (14) C12—C11—H11 120.0
C28—Cr—C29 90.19 (15) C11—C12—C7 120.6 (3)
C26—Cr—C29 90.69 (16) C11—C12—H12 119.7
C27—Cr—P1 176.55 (11) C7—C12—H12 119.7
C25—Cr—P1 89.77 (10) C18—C13—C14 118.7 (3)
C28—Cr—P1 88.40 (10) C18—C13—P2 125.1 (2)
C26—Cr—P1 93.36 (10) C14—C13—P2 116.2 (2)
C29—Cr—P1 95.75 (11) C15—C14—C13 120.2 (3)
C1—P1—C7 103.54 (14) C15—C14—H14 119.9
C1—P1—P2 101.21 (10) C13—C14—H14 119.9
C7—P1—P2 104.56 (10) C14—C15—C16 120.4 (3)
C1—P1—Cr 119.02 (11) C14—C15—H15 119.8
C7—P1—Cr 110.78 (10) C16—C15—H15 119.8
P2—P1—Cr 116.02 (5) C17—C16—C15 119.3 (3)
C13—P2—C19 110.90 (14) C17—C16—H16 120.3
C13—P2—S 111.01 (11) C15—C16—H16 120.3
supporting information
sup-7
Acta Cryst. (2002). E58, m478–m479
C13—P2—P1 107.35 (10) C16—C17—H17 119.4
C19—P2—P1 103.03 (11) C18—C17—H17 119.4
S—P2—P1 112.55 (6) C17—C18—C13 120.2 (3)
C6—C1—C2 118.7 (3) C17—C18—H18 119.9
C6—C1—P1 119.9 (2) C13—C18—H18 119.9
C2—C1—P1 121.3 (2) C24—C19—C20 118.9 (3)
C3—C2—C1 120.1 (3) C24—C19—P2 116.7 (2)
C3—C2—H2 119.9 C20—C19—P2 124.4 (2)
C1—C2—H2 119.9 C21—C20—C19 119.9 (3)
C2—C3—C4 120.6 (3) C21—C20—H20 120.0
C2—C3—H3 119.7 C19—C20—H20 120.0
C4—C3—H3 119.7 C22—C21—C20 120.7 (3)
C5—C4—C3 119.6 (3) C22—C21—H21 119.7
C5—C4—H4 120.2 C20—C21—H21 119.7
C3—C4—H4 120.2 C23—C22—C21 119.9 (3)
C4—C5—C6 120.5 (3) C23—C22—H22 120.1
C4—C5—H5 119.7 C21—C22—H22 120.1
C6—C5—H5 119.7 C22—C23—C24 120.0 (3)
C5—C6—C1 120.5 (3) C22—C23—H23 120.0
C5—C6—H6 119.8 C24—C23—H23 120.0
C1—C6—H6 119.8 C19—C24—C23 120.5 (3)
C8—C7—C12 118.5 (3) C19—C24—H24 119.7
C8—C7—P1 121.0 (2) C23—C24—H24 119.7
C12—C7—P1 119.4 (2) O1—C25—Cr 176.9 (3)
C9—C8—C7 120.8 (3) O2—C26—Cr 175.3 (3)
C9—C8—H8 119.6 O3—C27—Cr 179.6 (3)
C7—C8—H8 119.6 O4—C28—Cr 177.1 (3)
C10—C9—C8 119.9 (3) O5—C29—Cr 171.5 (3)
C25—Cr—P1—C1 −29.32 (15) P2—P1—C7—C12 145.4 (2)
C28—Cr—P1—C1 −116.76 (15) Cr—P1—C7—C12 −88.9 (2)
C26—Cr—P1—C1 62.17 (16) C12—C7—C8—C9 −1.4 (5)
C29—Cr—P1—C1 153.20 (15) P1—C7—C8—C9 −169.7 (3)
C25—Cr—P1—C7 90.49 (15) C7—C8—C9—C10 1.0 (6)
C28—Cr—P1—C7 3.04 (15) C8—C9—C10—C11 0.1 (6)
C26—Cr—P1—C7 −178.02 (15) C9—C10—C11—C12 −0.7 (5)
C29—Cr—P1—C7 −86.99 (15) C10—C11—C12—C7 0.2 (5)
C25—Cr—P1—P2 −150.53 (11) C8—C7—C12—C11 0.8 (5)
C28—Cr—P1—P2 122.02 (11) P1—C7—C12—C11 169.3 (3)
C26—Cr—P1—P2 −59.04 (12) C19—P2—C13—C18 23.4 (3)
C29—Cr—P1—P2 31.99 (12) S—P2—C13—C18 148.1 (3)
C1—P1—P2—C13 48.63 (15) P1—P2—C13—C18 −88.5 (3)
C7—P1—P2—C13 −58.72 (15) C19—P2—C13—C14 −158.7 (2)
Cr—P1—P2—C13 178.96 (10) S—P2—C13—C14 −33.9 (3)
C1—P1—P2—C19 −68.50 (15) P1—P2—C13—C14 89.5 (2)
C7—P1—P2—C19 −175.85 (14) C18—C13—C14—C15 0.2 (5)
Cr—P1—P2—C19 61.83 (11) P2—C13—C14—C15 −177.8 (3)
C7—P1—P2—S 63.72 (11) C14—C15—C16—C17 −0.1 (5)
Cr—P1—P2—S −58.60 (7) C15—C16—C17—C18 0.1 (5)
C7—P1—C1—C6 −146.8 (3) C16—C17—C18—C13 0.1 (5)
P2—P1—C1—C6 105.1 (2) C14—C13—C18—C17 −0.3 (5)
Cr—P1—C1—C6 −23.3 (3) P2—C13—C18—C17 177.6 (3)
C7—P1—C1—C2 36.1 (3) C13—P2—C19—C24 132.2 (3)
P2—P1—C1—C2 −72.0 (2) S—P2—C19—C24 7.9 (3)
Cr—P1—C1—C2 159.5 (2) P1—P2—C19—C24 −113.2 (2)
C6—C1—C2—C3 −0.4 (4) C13—P2—C19—C20 −49.9 (3)
P1—C1—C2—C3 176.8 (2) S—P2—C19—C20 −174.3 (2)
C1—C2—C3—C4 1.3 (5) P1—P2—C19—C20 64.7 (3)
C2—C3—C4—C5 −1.7 (5) C24—C19—C20—C21 −2.4 (5)
C3—C4—C5—C6 1.2 (5) P2—C19—C20—C21 179.8 (3)
C4—C5—C6—C1 −0.2 (5) C19—C20—C21—C22 0.8 (5)
C2—C1—C6—C5 −0.2 (5) C20—C21—C22—C23 0.8 (6)
P1—C1—C6—C5 −177.3 (3) C21—C22—C23—C24 −0.8 (6)
C1—P1—C7—C8 −152.0 (3) C20—C19—C24—C23 2.4 (5)
P2—P1—C7—C8 −46.4 (3) P2—C19—C24—C23 −179.7 (3)
Cr—P1—C7—C8 79.3 (3) C22—C23—C24—C19 −0.8 (5)
C1—P1—C7—C12 39.8 (3)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
C22—H22···O2i 0.95 2.65 3.581 (4) 166
C21—H21···O4ii 0.95 2.57 3.426 (5) 149