Acta Cryst.(2001). E57, m475±m477 DOI: 10.1107/S1600536801015276 Guido Kickelbick [Cu2(C10H8N2)4(CO3)]Br22CH4NH2O
m475
metal-organic papers
Acta Crystallographica Section E Structure Reports
Online
ISSN 1600-5368
The unexpected fixation of CO
2employing a
Cu
IBr(bpy)
2complex
Guido Kickelbick
Institut fuÈr Anorganische Chemie, Technische UniversitaÈt Wien, Getreidemarkt 9/153, A-1060 Wien, Austria
Correspondence e-mail: kickelgu@mail.zserv.tuwien.ac.at
Key indicators Single-crystal X-ray study T= 293 K
Mean(C±C) = 0.012 AÊ Rfactor = 0.047 wRfactor = 0.110 Data-to-parameter ratio = 8.2
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2001 International Union of Crystallography Printed in Great Britain ± all rights reserved
The attempt to crystallize CuIBr(bpy)
2, where bpy is
bi-pyridine, from an acetonitrile solution in a glove-box led unexpectedly to a dimeric carbonato-bridged CuIIprecipitate,
viz. -carbonato-bis[bis(bipyridine)copper(II)] dibromide diacetonitrile monohydrate, [Cu2(C10H8N2)4(CO3)]Br2
-2CH3CNH2O. The2-carbonato ligand is probably the result
of an oxidation of traces of CO2by CuI. The resulting structure
contains two square-pyramidally coordinated CuII atoms
bridged by a CO32ÿunit.
Comment
The ®xation of atmospheric CO2 by copper complexes is a
widely known phenomenon (Van Albadaet al., 2000; Sertucha
et al., 1999; Escueret al., 1997; Kitajimaet al., 1993). However, it is still not easy to predict whether ®xation will occur or not. The crystals studied in this report were obtained from a reaction in an argon-®lled glove-box where CO2 should not
usually be present. Currently, we do not know the source of the CO2. Either there were small impurities in the argon
atmosphere or the chemicals, such as the acetonitrile, were carriers of CO2 impurities. A possible explanation for the
reaction is an oxidation of traces of CO2by CuIresulting in
CO32ÿions. In addition, H2O was also found in the structure;
therefore, probably traces of Cu(OH)2were also present in
the acetonitrile solution. This has been described already in the literature as promoting CO2®xation (Krugeret al., 1995;
Kitajimaet al., 1991; Menifet al., 1991).
In the title complex, (I), each copper has a square-pyra-midal environment with a basal plane formed by three N atoms of the two chelating bipyridine ligands and the O atom of the bridging carbonato group (Fig. 1). The axial position is
occupied by the remaining N atom of the bipyridine ligand. The same structural motif was also obtained by the recrys-tallization of a hexanuclear (2-carbonato)copper(II)
bi-pyridine complex (Kruger et al., 1995). However, in this related compound, the counter-ion is PF6ÿand the structure
crystallized in space groupP1. Additionally, the latter struc-ture shows disorder over two sites of the bridging carbonate ligand, which seems to be a reasonably common phenomenon in bridged carbonate structures but was not observed in the current structure (Einstein & Willis, 1981; Palmer & van Eldik, 1983). CuÐO distances [Cu1ÐO62 1.944 (4) AÊ, Cu2ÐO63 1.928 (4) AÊ] are similar to those in other (2
-carbonato)-copper complexes (Kruger et al., 1995). The Cu1 Cu2 separation is 5.339 AÊ, with the non-coordinated carbonate O atom sitting almost centrally between the two Cu atoms (Cu1ÐO61 2.808 AÊ and Cu2ÐO61 2.779 AÊ). The2
-carbo-nato ligand shows an internal asymmetry with three different CÐO bond lengths [C60ÐO61 1.245 (8) AÊ, C60ÐO62 1.293 (8) AÊ and C60ÐO63 1.311 (8) AÊ]. These bond lengths also reveal the different bonding modes of the carbonato O atoms; two atoms are coordinated to the copper centers, which reduces the electron density on these atoms and leads to a lower CÐO bond order with a longer bond, while the third O atom shows obviously no, or only a small, interaction with the copper centers resulting in a shorter CÐO bond distance. Bipyridyl±bipyridyl -stacking interactions in the crystal lattice may be a reason for the crystallization of this compound (Fig. 2).
Experimental
0.57 g (4.0 mmol) CuBr and 1.25 g (8.0 mmol) bipyridine were placed in a 50 ml round-bottomed ¯ask in a glove-box containing an argon atmosphere. 10 ml of acetonitrile were slowly added with stirring. The mixture was heated for a short time until all of the solid completely dissolved. The maroon solution was cooled to room temperature and was allowed to stand for one week. The solvent was evaporated and
Crystal data
[Cu2(C10H8N2)4(CO3)]Br2 -2CH3CNH2O
Mr= 1071.77 Monoclinic,P21/c
a= 11.0977 (2) AÊ
b= 23.7744 (2) AÊ
c= 17.1024 (1) AÊ
= 94.347 (1)
V= 4499.34 (9) AÊ3
Z= 4
Dx= 1.579 Mg mÿ3 MoKradiation Cell parameters from 5127
re¯ections
= 2.3±24.3 = 2.78 mmÿ1
T= 293 (2) K Irregular, green 0.160.140.14 mm
Data collection
Siemens SMART diffractometer with CCD area detector
!scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996)
Tmin= 0.665,Tmax= 0.697 10 354 measured re¯ections 4681 independent re¯ections
4013 re¯ections withI> 2(I)
Rint= 0.149 max= 20.8
h=ÿ11!11
k= 0!23
l= 0!17
Intensity decay: none
Re®nement
Re®nement onF2
R[F2> 2(F2)] = 0.047
wR(F2) = 0.110
S= 1.17 4681 re¯ections 569 parameters
H atoms treated by a mixture of independent and constrained re®nement
w= 1/[2(F
o2) + (0.0245P)2 + 20.6621P]
whereP= (Fo2+ 2Fc2)/3 (/)max= 0.001
max= 1.02 e AÊÿ3
min=ÿ0.56 e AÊÿ3
Extinction correction:SHELXL97 Extinction coef®cient: 0.00015 (6)
Table 1
Selected geometric parameters (AÊ,). Cu1ÐO62 1.944 (4) Cu1ÐN24 2.011 (5) Cu1ÐN13 2.035 (5) Cu1ÐN1 2.036 (6) Cu1ÐN12 2.191 (6)
Cu2ÐO63 1.928 (4) Cu2ÐN36 2.005 (5) Cu2ÐN25 2.036 (6) Cu2ÐN48 2.037 (5) Cu2ÐN37 2.229 (5)
O62ÐCu1ÐN24 92.2 (2) O62ÐCu1ÐN13 163.4 (2) N24ÐCu1ÐN13 80.5 (2) O62ÐCu1ÐN1 92.2 (2) N24ÐCu1ÐN1 175.5 (2) N13ÐCu1ÐN1 95.3 (2) O62ÐCu1ÐN12 98.1 (2) N24ÐCu1ÐN12 100.9 (2) N13ÐCu1ÐN12 98.0 (2) N1ÐCu1ÐN12 78.1 (2) O63ÐCu2ÐN36 93.4 (2) O63ÐCu2ÐN25 159.4 (2)
N36ÐCu2ÐN25 80.6 (2) O63ÐCu2ÐN48 90.73 (19) N36ÐCu2ÐN48 172.3 (2) N25ÐCu2ÐN48 97.7 (2) O63ÐCu2ÐN37 101.39 (19) N36ÐCu2ÐN37 94.8 (2) N25ÐCu2ÐN37 98.7 (2) N48ÐCu2ÐN37 78.0 (2) O61ÐC60ÐO62 122.8 (6) O61ÐC60ÐO63 122.2 (6) O62ÐC60ÐO63 115.1 (6)
The data set was limited to a resolution of 1.0 AÊ, since only very weak diffraction was observed at higher angles. H atoms were located by difference Fourier maps and re®ned with a riding model, with the exception of the H atoms of the water solvate, which were not included.
Data collection:SMART(Bruker, 1997); cell re®nement:SAINT
(Bruker, 1997); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 1997); program(s) used to re®ne structure: SHELXL97 (Sheldrick, 1997); molecular graphics:
SHELXTL (Bruker, 1998); software used to prepare material for publication:SHELXL97.
Figure 1
References
Bruker (1997). SMART and SAINT. Bruker AXS GmbH, Karlsruhe, Germany.
Bruker (1998). SHELXTL. Version 5.1. Bruker AXS GmbH, Karlsruhe, Germany.
Einstein, F. W. & Willis, A. C. (1981).Inorg. Chem.20, 609±614.
Escuer, A., Penalba, E., Vicente, R., Solans, X. & Font-Bardia, M. (1997).J. Chem. Soc. Dalton Trans.pp. 2315±2319.
Kitajima, N., Koda, T., Hashimoto, S., Kitagawa, T. & Moro-oka, Y. (1991).J. Am. Chem. Soc.113, 5664±5671.
Kitajima, N., Hikichi, S., Tanaka, M. & Moro-oka, Y. (1993).J. Am. Chem. Soc.
115, 5496±5508.
Kruger, P. E., Fallon, G. D., Moubaraki B., Berry, K. J. & Murray, S. K. (1995).
Inorg. Chem.34, 4808±4814.
Menif, R., Reibenspies, J. & Martell, A. E. (1991).Inorg. Chem.30, 3446±3454. Palmer, D. A. & van Eldik, R. (1983).Chem. Rev.83, 651±731.
Sertucha, J., Luque, A., Castillo, O., Roman, P., Lloret, F. & Julve, M. (1999).
Inorg. Chem. Commun.2, 14±16.
Sheldrick, G. M. (1996).SADABS. University of GoÈttingen, Germany. Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of
GoÈttingen, Germany.
Van Albada, G. A., Mutikainen, I., Roubeau, O. S., Turpeinen, U. & Reedijk, J. (2000).Eur. J. Inorg. Chem.10, 2179±2184.
Acta Cryst.(2001). E57, m475±m477 Guido Kickelbick [Cu2(C10H8N2)4(CO3)]Br22CH4NH2O
m477
metal-organic papers
Figure 2
supporting information
Acta Cryst. (2001). E57, m475–m477 [doi:10.1107/S1600536801015276]
The unexpected fixation of CO
2employing a Cu
IBr(bpy)
2complex
Guido Kickelbick
S1. Comment
The fixation of atmospheric CO2 by copper complexes is a widely known phenomenon (Van Albada et al., 2000; Sertucha
et al., 1999; Escuer et al., 1997; Kitajima et al., 1993). However, it is still not easy to predict whether fixation will occur or not. The crystals studied in this report were obtained from a reaction in an argon-filled glove-box where CO2 should
not usually be present. Currently, we do not know the source of the CO2. Either there were small impurities in the argon
atmosphere or the chemicals, such as the acetonitrile, were carriers of CO2 impurities. A possible explanation for the
reaction is an oxidation of traces of CO2 by CuI resulting in CO32- ions. In addition, H2O was also found in the structure;
therefore, probably traces of Cu(OH)2 were also present in the acetonitrile solution. This has been described already in the
literature as promoting CO2 fixation (Kruger et al., 1995; Kitajima et al., 1991; Menif et al., 1991).
In the title complex, (I), each copper has a square-pyramidal environment with a basal plane formed by three N atoms
of the two chelating bipyridine ligands and the O atom of the bridging carbonato group (Fig. 1). The axial position is
occupied by the remaining N atom of the bipyridine ligand. The same structural motif was also obtained by the
recrystallization of a hexanuclear (µ2-hydroxo)(µ2-carbonato)copper(II) bipyridine complex (Kruger et al., 1995).
However, in this related compound, the counter-ion is PF6- and the structure crystallized in space group P1. Additionally,
the latter structure shows disorder over two sites of the bridging carbonate ligand, which seems to be a reasonably
common phenomenon in bridged carbonate structures but was not observed in the current structure (Einstein & Willis,
1981; Palmer & van Eldik, 1983). Cu—O distances [Cu1—O62 1.944 (4) Å, Cu2—O63 1.928 (4) Å] are similar to those
in other (µ2-carbonato)copper complexes (Kruger et al., 1995). The Cu1···Cu2 separation is 5.339 Å, with the
non-coordinated carbonate O atom sitting almost centrally between the two Cu atoms (Cu1—O61 2.808 Å and Cu2—O62
2.779 Å). The µ2-carbonato ligand shows an internal asymmetry with three different C—O bond lengths [C60—O61
1.245 (8) Å, C60—O62 1.293 (8) Å and C60—O63 1.311 (8) Å]. These bond lengths also reveal the different bonding
modes of the carbonato O atoms; two atoms are coordinated to the copper centers, which reduces the electron density on
these atoms and leads to a lower C—O bond order with a longer bond, while the third O atom shows obviously no, or
only a small, interaction with the copper centers resulting in a shorter C—O bond distance. Bipyridyl–bipyridyl π
-stacking interactions in the crystal lattice may be a reason for the crystallization of this compound (Fig. 2).
S2. Experimental
0.57 g (4.0 mmol) CuBr and 1.25 g (8.0 mmol) bipyridine were placed in a 50 ml round-bottomed flask in a glove-box
containing an argon atmosphere. 10 ml of acetonitrile were slowly added with stirring. The mixture was heated for a short
time until all of the solid completely dissolved. The maroon solution was cooled to room temperature and was allowed to
supporting information
sup-2
Acta Cryst. (2001). E57, m475–m477 S3. Refinement
The data set was limited to a resolution of 1.0 Å, since only very weak diffraction was observed at higher angles. H atoms
were located by difference Fourier maps and refined with a riding model, with the exception of the H atoms of the water
[image:5.610.123.481.146.370.2]solvate, which were not included.
Figure 1
The structure of (I) showing displacement ellipsoids at the 50% probability level. For clarity, H atoms have been omitted.
The asymmetric unit also contains (not shown) two molecules of acetonitrile, one molecule of water and two bromide
counter-ions.
Figure 2
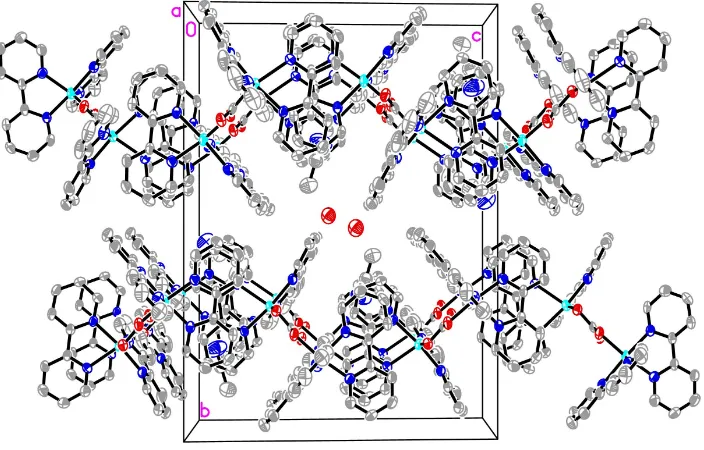
Projection of the structure of (I) along [100], showing displacement ellipsoids at the 50% probability level. For clarity, H
[image:5.610.131.483.442.667.2](I)
Crystal data
[Cu2(C10H8N2)4(CO3)]Br2·2CH3CN·H2O Mr = 1071.77
Monoclinic, P21/c a = 11.0977 (2) Å
b = 23.7744 (2) Å
c = 17.1024 (1) Å
β = 94.347 (1)°
V = 4499.34 (9) Å3 Z = 4
F(000) = 2152
Dx = 1.579 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 5127 reflections
θ = 2.3–24.3°
µ = 2.78 mm−1 T = 293 K Irregular, green 0.16 × 0.14 × 0.14 mm
Data collection
Siemens SMART
diffractometer with CCD area detector Radiation source: fine-focus sealed tube Graphite monochromator
ω scans
Absorption correction: multi-scan (SADABS; Sheldrick, 1996)
Tmin = 0.665, Tmax = 0.697
10354 measured reflections 4681 independent reflections 4013 reflections with I > 2σ(I)
Rint = 0.149
θmax = 20.8°, θmin = 1.8°
h = −11→11
k = 0→23
l = 0→17
Refinement
Refinement on F2
Least-squares matrix: full
R[F2 > 2σ(F2)] = 0.047 wR(F2) = 0.110 S = 1.17 4681 reflections 569 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H atoms treated by a mixture of independent and constrained refinement
w = 1/[σ2(Fo2) + (0.0245P)2 + 20.6621P]
where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.001
Δρmax = 1.02 e Å−3
Δρmin = −0.56 e Å−3
Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4
Extinction coefficient: 0.00015 (6)
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used
only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2
are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
supporting information
sup-4
Acta Cryst. (2001). E57, m475–m477
Cu2 0.50038 (7) 0.70377 (3) 0.26131 (4) 0.0301 (2) Br2 0.42833 (8) 0.95427 (3) 0.28040 (5) 0.0572 (3) C2 0.7538 (7) 0.9085 (3) 0.3216 (4) 0.045 (2)
H2A 0.6785 0.8965 0.3354 0.054*
C3 0.7570 (8) 0.9527 (3) 0.2692 (4) 0.057 (2)
H3A 0.6869 0.9698 0.2476 0.068*
C4 0.8708 (9) 0.9700 (4) 0.2508 (5) 0.058 (2)
H4A 0.8781 1.0001 0.2167 0.069*
C5 0.9723 (8) 0.9434 (3) 0.2822 (4) 0.050 (2)
H5A 1.0482 0.9548 0.2689 0.060*
C6 0.9611 (7) 0.8986 (3) 0.3347 (4) 0.0391 (19) C7 1.0651 (6) 0.8670 (3) 0.3699 (4) 0.0398 (19) C8 1.1848 (7) 0.8767 (4) 0.3530 (5) 0.055 (2)
H8A 1.2029 0.9054 0.3190 0.066*
C9 1.2747 (8) 0.8437 (4) 0.3868 (5) 0.065 (3)
H9A 1.3541 0.8491 0.3746 0.078*
C10 1.2479 (7) 0.8021 (4) 0.4390 (5) 0.062 (2)
H10A 1.3082 0.7795 0.4630 0.074*
C11 1.1276 (7) 0.7952 (4) 0.4544 (5) 0.052 (2)
H11A 1.1084 0.7675 0.4898 0.062*
N12 1.0396 (5) 0.8262 (3) 0.4212 (3) 0.0408 (15) N13 0.8303 (5) 0.8658 (2) 0.5267 (3) 0.0359 (14) C14 0.8255 (7) 0.9219 (3) 0.5293 (4) 0.049 (2)
H14A 0.8327 0.9418 0.4831 0.059*
C15 0.8104 (8) 0.9519 (3) 0.5972 (5) 0.061 (2)
H15A 0.8065 0.9910 0.5965 0.073*
C16 0.8015 (8) 0.9228 (3) 0.6649 (5) 0.057 (2)
H16A 0.7904 0.9417 0.7113 0.068*
C17 0.8091 (6) 0.8647 (3) 0.6641 (4) 0.0412 (19)
H17A 0.8043 0.8445 0.7103 0.049*
C18 0.8238 (6) 0.8370 (3) 0.5948 (4) 0.0324 (17) C19 0.8327 (6) 0.7752 (3) 0.5868 (4) 0.0312 (17) C20 0.8314 (6) 0.7380 (3) 0.6490 (4) 0.0377 (18)
H20A 0.8229 0.7512 0.6995 0.045*
C21 0.8426 (7) 0.6817 (3) 0.6355 (4) 0.045 (2)
H21A 0.8418 0.6563 0.6769 0.054*
C22 0.8550 (7) 0.6626 (3) 0.5608 (4) 0.046 (2)
H22A 0.8644 0.6245 0.5510 0.055*
C23 0.8531 (6) 0.7014 (3) 0.5008 (4) 0.0374 (18)
H23A 0.8594 0.6885 0.4499 0.045*
N24 0.8425 (4) 0.7565 (2) 0.5128 (3) 0.0301 (13) N25 0.3203 (5) 0.7086 (3) 0.2758 (3) 0.0427 (16) C26 0.2440 (7) 0.7463 (4) 0.2430 (5) 0.056 (2)
H26A 0.2735 0.7735 0.2104 0.067*
C27 0.1211 (8) 0.7467 (5) 0.2557 (6) 0.078 (3)
H27A 0.0692 0.7734 0.2320 0.094*
C28 0.0797 (8) 0.7065 (5) 0.3042 (6) 0.079 (3)
C29 0.1588 (8) 0.6669 (4) 0.3390 (5) 0.068 (3)
H29A 0.1311 0.6399 0.3726 0.081*
C30 0.2791 (7) 0.6682 (4) 0.3230 (4) 0.048 (2) C31 0.3712 (7) 0.6273 (3) 0.3524 (4) 0.042 (2) C32 0.3460 (9) 0.5777 (4) 0.3906 (5) 0.065 (3)
H32A 0.2676 0.5699 0.4032 0.078*
C33 0.4376 (12) 0.5400 (4) 0.4097 (5) 0.074 (3)
H33A 0.4212 0.5063 0.4342 0.089*
C34 0.5529 (10) 0.5525 (3) 0.3925 (4) 0.065 (3)
H34A 0.6161 0.5277 0.4052 0.078*
C35 0.5731 (8) 0.6032 (3) 0.3556 (4) 0.0427 (19)
H35A 0.6517 0.6123 0.3449 0.051*
N36 0.4853 (5) 0.6390 (2) 0.3351 (3) 0.0349 (14) N37 0.4918 (5) 0.6506 (2) 0.1537 (3) 0.0333 (14) C38 0.4829 (7) 0.5953 (3) 0.1462 (4) 0.046 (2)
H38A 0.4709 0.5740 0.1906 0.055*
C39 0.4905 (8) 0.5678 (3) 0.0764 (5) 0.059 (2)
H39A 0.4811 0.5289 0.0732 0.070*
C40 0.5124 (8) 0.5988 (3) 0.0113 (5) 0.055 (2)
H40A 0.5190 0.5811 −0.0367 0.065*
C41 0.5245 (6) 0.6561 (3) 0.0177 (4) 0.0394 (18)
H41A 0.5406 0.6777 −0.0257 0.047*
C42 0.5125 (5) 0.6813 (3) 0.0900 (4) 0.0280 (16) C43 0.5189 (5) 0.7434 (3) 0.1012 (4) 0.0291 (16) C44 0.5366 (6) 0.7808 (3) 0.0418 (4) 0.0347 (17)
H44A 0.5459 0.7678 −0.0087 0.042*
C45 0.5405 (7) 0.8374 (3) 0.0574 (4) 0.044 (2)
H45A 0.5528 0.8629 0.0175 0.053*
C46 0.5260 (7) 0.8567 (3) 0.1325 (4) 0.046 (2)
H46A 0.5272 0.8950 0.1439 0.055*
C47 0.5099 (6) 0.8177 (3) 0.1891 (4) 0.0391 (18)
H47A 0.5013 0.8301 0.2399 0.047*
N48 0.5059 (5) 0.7626 (2) 0.1750 (3) 0.0301 (13) C60 0.6989 (6) 0.7512 (3) 0.3344 (4) 0.0278 (16) O61 0.6196 (4) 0.7778 (2) 0.3665 (3) 0.0395 (12) O62 0.8128 (4) 0.76267 (19) 0.3460 (2) 0.0371 (12) O63 0.6725 (4) 0.71026 (18) 0.2849 (2) 0.0325 (11) C102 0.8523 (11) 0.1174 (6) 0.3569 (8) 0.142 (6)
H10B 0.9342 0.1245 0.3454 0.171*
H10C 0.8156 0.1519 0.3723 0.171*
H10D 0.8077 0.1026 0.3112 0.171*
C101 0.8512 (10) 0.0783 (5) 0.4181 (7) 0.095 (4) N100 0.8547 (12) 0.0494 (5) 0.4703 (7) 0.137 (4) N103 0.2028 (10) 0.8144 (5) 0.0675 (7) 0.128 (4) C104 0.1948 (9) 0.8581 (5) 0.0839 (5) 0.065 (3) C105 0.1878 (11) 0.9153 (4) 0.1074 (7) 0.109 (4)
H10E 0.1615 0.9172 0.1595 0.130*
supporting information
sup-6
Acta Cryst. (2001). E57, m475–m477
H10G 0.2660 0.9325 0.1065 0.130*
O110 0.1553 (6) 0.4851 (3) 0.4541 (4) 0.0798 (19)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
C39 0.094 (7) 0.035 (5) 0.047 (6) −0.007 (5) 0.004 (5) −0.001 (5) C40 0.079 (6) 0.049 (5) 0.034 (5) 0.006 (5) −0.006 (4) −0.010 (4) C41 0.049 (5) 0.046 (5) 0.021 (4) 0.006 (4) −0.005 (3) −0.001 (4) C42 0.024 (4) 0.037 (4) 0.023 (4) 0.001 (3) −0.002 (3) 0.001 (3) C43 0.022 (4) 0.039 (4) 0.026 (4) 0.001 (3) 0.000 (3) 0.001 (4) C44 0.034 (4) 0.048 (5) 0.021 (4) 0.002 (4) −0.003 (3) 0.002 (4) C45 0.056 (5) 0.044 (5) 0.032 (5) −0.001 (4) 0.000 (4) 0.012 (4) C46 0.065 (5) 0.030 (4) 0.041 (5) 0.001 (4) 0.002 (4) 0.004 (4) C47 0.047 (5) 0.036 (5) 0.034 (4) 0.002 (4) 0.003 (4) −0.006 (4) N48 0.034 (3) 0.035 (4) 0.021 (3) 0.003 (3) −0.002 (3) 0.003 (3) C60 0.030 (5) 0.037 (4) 0.017 (4) −0.006 (4) −0.001 (3) 0.007 (3) O61 0.042 (3) 0.050 (3) 0.027 (3) 0.005 (3) 0.006 (2) −0.006 (2) O62 0.038 (3) 0.051 (3) 0.022 (3) −0.012 (2) 0.001 (2) −0.003 (2) O63 0.029 (3) 0.040 (3) 0.028 (3) −0.003 (2) 0.003 (2) −0.003 (2) C102 0.113 (10) 0.176 (14) 0.132 (11) −0.017 (10) −0.026 (9) 0.094 (11) C101 0.098 (9) 0.102 (9) 0.085 (9) 0.002 (7) 0.005 (7) 0.023 (7) N100 0.210 (13) 0.098 (8) 0.104 (9) −0.002 (8) 0.019 (8) 0.030 (7) N103 0.126 (9) 0.095 (8) 0.153 (10) 0.004 (7) −0.061 (7) −0.034 (8) C104 0.073 (7) 0.061 (7) 0.059 (6) 0.007 (6) −0.014 (5) −0.003 (5) C105 0.147 (11) 0.078 (8) 0.100 (9) 0.023 (8) 0.003 (8) 0.008 (7) O110 0.080 (4) 0.093 (5) 0.070 (4) −0.018 (4) 0.026 (3) 0.000 (4)
Geometric parameters (Å, º)
Cu1—O62 1.944 (4) C23—N24 1.333 (9)
Cu1—N24 2.011 (5) N25—C26 1.328 (10)
Cu1—N13 2.035 (5) N25—C30 1.355 (9)
Cu1—N1 2.036 (6) C26—C27 1.398 (12)
Cu1—N12 2.191 (6) C27—C28 1.366 (14)
N1—C2 1.333 (9) C28—C29 1.391 (13)
N1—C6 1.346 (9) C29—C30 1.384 (11)
Cu2—O63 1.928 (4) C30—C31 1.471 (11)
Cu2—N36 2.005 (5) C31—N36 1.351 (9)
Cu2—N25 2.036 (6) C31—C32 1.388 (11)
Cu2—N48 2.037 (5) C32—C33 1.375 (13)
Cu2—N37 2.229 (5) C33—C34 1.367 (13)
C2—C3 1.382 (10) C34—C35 1.385 (11)
C3—C4 1.387 (11) C35—N36 1.322 (9)
C4—C5 1.366 (11) N37—C38 1.325 (9)
C5—C6 1.404 (10) N37—C42 1.345 (8)
C6—C7 1.468 (10) C38—C39 1.369 (10)
C7—N12 1.352 (9) C39—C40 1.373 (11)
C7—C8 1.400 (10) C40—C41 1.373 (10)
C8—C9 1.362 (12) C41—C42 1.390 (9)
C9—C10 1.379 (12) C42—C43 1.490 (9)
C10—C11 1.390 (11) C43—N48 1.359 (8)
C11—N12 1.318 (9) C43—C44 1.376 (9)
supporting information
sup-8
Acta Cryst. (2001). E57, m475–m477
N13—C18 1.357 (8) C45—C46 1.385 (10)
C14—C15 1.384 (10) C46—C47 1.362 (9)
C15—C16 1.359 (11) C47—N48 1.333 (8)
C16—C17 1.383 (10) C60—O61 1.244 (7)
C17—C18 1.376 (9) C60—O62 1.294 (7)
C18—C19 1.479 (10) C60—O63 1.310 (8)
C19—N24 1.353 (8) C102—C101 1.400 (15)
C19—C20 1.385 (9) C101—N100 1.125 (13)
C20—C21 1.365 (10) N103—C104 1.082 (12)
C21—C22 1.372 (10) C104—C105 1.422 (14)
C22—C23 1.378 (10)
O62—Cu1—N24 92.2 (2) C20—C21—C22 119.8 (7) O62—Cu1—N13 163.4 (2) C21—C22—C23 118.5 (7) N24—Cu1—N13 80.5 (2) N24—C23—C22 122.5 (6)
O62—Cu1—N1 92.2 (2) C23—N24—C19 118.9 (6)
N24—Cu1—N1 175.5 (2) C23—N24—Cu1 125.8 (4)
N13—Cu1—N1 95.3 (2) C19—N24—Cu1 115.3 (4)
O62—Cu1—N12 98.1 (2) C26—N25—C30 119.8 (7) N24—Cu1—N12 100.9 (2) C26—N25—Cu2 125.9 (5) N13—Cu1—N12 98.0 (2) C30—N25—Cu2 114.3 (5)
N1—Cu1—N12 78.1 (2) N25—C26—C27 122.5 (9)
C2—N1—C6 118.8 (6) C28—C27—C26 117.8 (10)
C2—N1—Cu1 124.1 (5) C27—C28—C29 120.2 (9)
C6—N1—Cu1 117.1 (5) C30—C29—C28 119.2 (9)
O63—Cu2—N36 93.4 (2) N25—C30—C29 120.5 (8) O63—Cu2—N25 159.4 (2) N25—C30—C31 114.5 (6) N36—Cu2—N25 80.6 (2) C29—C30—C31 125.0 (8) O63—Cu2—N48 90.73 (19) N36—C31—C32 120.3 (8) N36—Cu2—N48 172.3 (2) N36—C31—C30 115.3 (6) N25—Cu2—N48 97.7 (2) C32—C31—C30 124.2 (8) O63—Cu2—N37 101.39 (19) C33—C32—C31 119.5 (8) N36—Cu2—N37 94.8 (2) C34—C33—C32 119.7 (8) N25—Cu2—N37 98.7 (2) C33—C34—C35 118.3 (9) N48—Cu2—N37 78.0 (2) N36—C35—C34 122.7 (8)
N1—C2—C3 124.5 (7) C35—N36—C31 119.5 (6)
C2—C3—C4 116.3 (8) C35—N36—Cu2 124.6 (5)
C5—C4—C3 120.6 (8) C31—N36—Cu2 114.9 (5)
C4—C5—C6 119.5 (8) C38—N37—C42 118.5 (6)
N1—C6—C5 120.2 (7) C38—N37—Cu2 129.9 (5)
N1—C6—C7 116.7 (6) C42—N37—Cu2 111.2 (4)
C5—C6—C7 123.0 (7) N37—C38—C39 123.3 (7)
N12—C7—C8 120.0 (7) C38—C39—C40 118.5 (7)
N12—C7—C6 115.9 (6) C39—C40—C41 119.4 (7)
C8—C7—C6 124.1 (7) C40—C41—C42 118.9 (7)
C9—C8—C7 119.6 (8) N37—C42—C41 121.4 (6)
C8—C9—C10 120.0 (8) N37—C42—C43 116.3 (6)