PREPARATION AND CHARACTERIZATION OF MIXED LIGAND
COMPLEXES OF Co(II)
P. R. Shirode*
Department of Chemistry, Pratap College, Amalner (India) Affiliated to North Maharashtra
University, Jalgaon.
ABSTRACT
There is great interest in the study of mixed ligand complexes of
transition metals with oxygen, nitrogen and sulphur donor ligands.
Coordination complexes of Co(II)) of the type Co(L1L2)Cl2 Where
L1=salicylaldehyde semicarbazone and L2=salicylaldehyde oxime,
ortho hydroxy acetophenone oxime, anthranilic acid, benzaldehyde
semicarbazone, acetophenone semicarbazone and acetone
semicarbazone have been prepared by the reaction of metal chloride
with two ligands L1 and L2 in the ratio 1:1:1 molar ratio. The resulting
complexes have been characterized on the basis of elemental analysis,
magnetic measurement, IR and UV spectral analysis, conductivity
measurement, thermal analysis, antimicrobial activities. Analysis of
results reviles that the complexes shows octahedral geometry, electrolytic nature and having
more antimicrobial activity than the ligands. The ligands are bonded through oxygen and
nitrogen.
KEYWORDS: Mixed ligand, salicylaldehyde semicarbazone, salicylaldehyde oxime,
anthranilic acid, transition metals.
INTRODUCTION
Transition metal complexes of oxime, semicarbazone and thiosemicarbazone shows
remarkable antitumor, antiviral, anticancer, anti-malarial, anti-fungal, anti-bacterial and
catalytic activities. By considering these applications, we have carried out synthesis and
characterization and biochemical study of mixed ligand complexes of Co(II) with
salicylaldehyde semicarbazone(L1) and salicylaldehyde oxime, ortho-hydroxyacetophenone
oxime, benzaldehyde semicarbazone, acetophenone semicarbazone and acetone
Volume 6, Issue 8, 1494-1504. Research Article ISSN 2277– 7105
*Corresponding Author Prof. P. R. Shirode
Department of Chemistry, Pratap College, Amalner (India) Affiliated to North Maharashtra University, Jalgaon.
Article Received on 10 June 2017,
Revised on 30 June 2017, Accepted on 21 July 2017
semicarbazone(L2). In this complexation reaction ortho-hydroxyacetophenone oxime,
benzaldehyde semicarbazone and anthranilic acid are acting as a bidentet while
salicylaldehyde semicarbazone and salicylaldehyde thio-semicarbazone are acting as a
tridentate ligand with oxygen, nitrogen and nitrogen as the donor species. Multidentate
organic ligands forming complexes with transition metal ions have been the subject of
intensive research because they not only have interesting spectral and magnetic properties,
but they also possess a diverse spectrum of biological activities.[1,6]
Depending on the widely diverse coordination environment of the transition metal complexes
and variation in the identities of the coordinating ligands, synthesis of such complexes with
desired molecular geometry can be determined.
MATERIALS AND METHODS
All the reagents /chemicals were of reagent grade. Solvents were dried and distilled before
use according to standard procedures.[8] The metal chlorides / salts used were in their
hydrated form.
The amount of metals are determined volumetrically by EDTA using double burette
technique for optimum utilization of reagents. Indicator xylenol orange is used for Co(II.
Carbon, hydrogen, nitrogen and sulphur analysis were carried from Central Instrumentation
Laboratory, Pratap College, Amalner. IR spectra and UV spectra of the complexes were
recorded on JASCO instrument in the region 250-4000 cm-1 and 200-1400nm respectively
from Central Instrumentation Laboratory, Pratap College, Amalner. Thermo gravimetric
analysis was carried out on Perkin Elmer STA 6000 at the rate of 100C per minute.
Antimicrobial activities are determined by using three microbial nutrients.
Ligands L1 and L2 were prepared and general methods of their preparation are given below.
Synthesis of Ligand (L1).
Salicylaldehyde semicarbazone
A mixture of semicarbazide hydrochloride and salicyladehyde in 1:1 molar proportions in
alcoholic medium containing small amount of sodium acetate, was refluxed for15-20minutes
in a water bath. The reaction mixture was cooled to room temperature, white solid product
+ H2N HN C NH2
N
H H
N C NH2
O H
O
O
Salicylaldehyde semicarbazone OH
OH
Synthesis of Ligand (L2)
1. Salicylaldehyde oxime
Equimolar quantities of hydroxylamine hydrochloride and salicylaldehyde in alcoholic
medium containing small amount of pyridine was refluxed on water bath half an hour .White
solid product separates out from solution. On Cooling filter and wash with cold water and
recrystallized from ethyl alcohol and record the melting point.
OH O H
+
H2N OHOH N H
OH
EtOH
2. Ortho-hydroxyacetophenone oxime
Mixture of alcoholic solution of o-hydroxyacetophenone and aqueous solution
hydroxylamine hydrochloride in 1:1 molar proportions were mixed together. The reaction
mixture is acidified by 3-4 drops of glacial acetic acid and refluxed on water bath for two
hour. The precipitated compound was filtered, washed with cold water, dried and melting
point was recorded.
OH
O
CH3
o-hydroxy
acetophenone oxime +H2N OH
OH N
OH
3. Anthranilic acid
Anthranilic acid is used directly by recrystallisation.
4. Benzaldehyde semicarbazone
Aqueous solution of semicarbazide hydrochloride and sodium acetate was mixed with
alcoholic solution of benzaldehyde in 1:1 molar proportions. Heat the mixture on water bath
for 10 to 15 minutes. Shake well and allow standing for 10 minutes. The semicarbazone
derivative crystallizes from the cold solution on standing. Therefore cool the reaction mixture
ice water. Filter the crystal on buchner funnel; wash the precipitate with cold water. The solid
product was recrystallized from hot ethanol and record the melting point.
+ H2N
H
N C NH2
N
H H
N C NH2
O H
O
O
Benzaldehyde semicarbazone
5. Acetophenone semicarbazone( L2)
Dissolve one gram semicarbazide hydrochloride and 1.5 gram of crystallized sodium acetate
in 8-10 ml distilled water, Then add 0.5 gram of salicylaldehyde and shake well. If the
solution is turbid, add Alcohol or water until a clear solution is obtained .Heat the mixture on
water bath for 10 to 15 minutes. Shake well and allow to stand for 10 minutes. The
semicarbazone crystallizes from the cold solution on standing. Therefore cool the reaction
mixture ice water. Filter the crystal on Buchner funnel; wash the precipitate with cold water.
The solid product was recrystallized from hot ethanol and record the melting point.
OH O CH3
+
OH N CH3
H N
EtOH H2N
N
H NH2
O
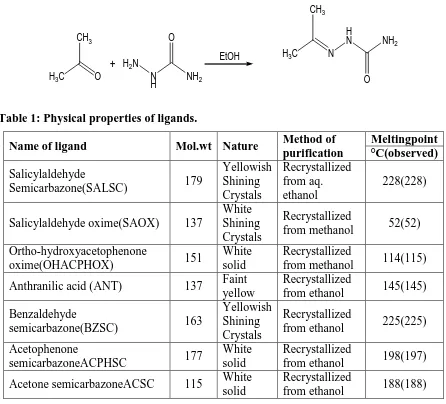
6. Acetone semicarbazone (L2)
Equimolar quantities of aqueous solution semicarbazide hydrochloride and acetone are mixed
together in presence of sodium acetate. Then the reaction mixture was thoroughly cooled with
constant stirring. White solid product separate out from solution. The solid product was
filtered wash with ethyl alcohol and recrystallized from ethyl alcohol.
H3C O CH3
+
H3C N CH3
H N
EtOH H2N
N
H NH2 O
[image:5.595.74.520.186.590.2]O NH2
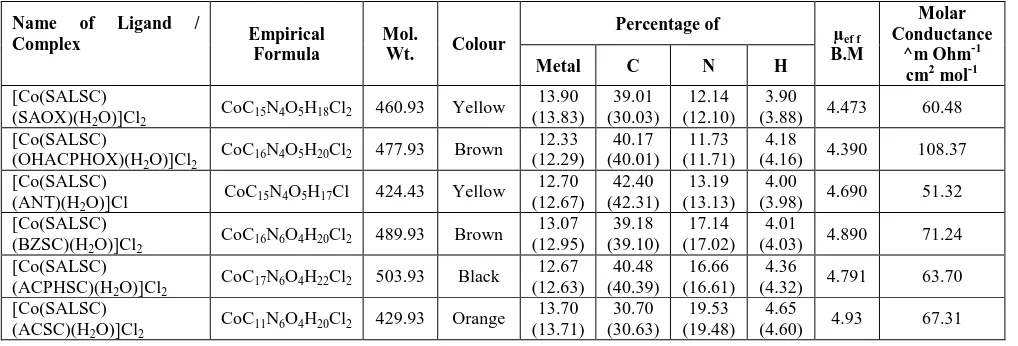
Table 1: Physical properties of ligands.
Name of ligand Mol.wt Nature Method of
purification
Meltingpoint °C(observed)
Salicylaldehyde
Semicarbazone(SALSC) 179
Yellowish Shining Crystals
Recrystallized from aq. ethanol
228(228)
Salicylaldehyde oxime(SAOX) 137
White Shining Crystals
Recrystallized
from methanol 52(52)
Ortho-hydroxyacetophenone
oxime(OHACPHOX) 151
White solid
Recrystallized
from methanol 114(115)
Anthranilic acid (ANT) 137 Faint yellow
Recrystallized
from ethanol 145(145)
Benzaldehyde
semicarbazone(BZSC) 163
Yellowish Shining Crystals
Recrystallized
from ethanol 225(225)
Acetophenone
semicarbazoneACPHSC 177
White solid
Recrystallized
from ethanol 198(197)
Acetone semicarbazoneACSC 115 White solid
Recrystallized
from ethanol 188(188)
Synthesis of transition metal complexes of the type M(L1)2
Alcoholic solution of salicylaldehyde was added to methanolic solution of Co(II) chloride in
1:2 molar praporation with continuous stirring at room temp, till clear solution was obtained.
Then the solution was refluxed on a heating mental at about 60-700C for four hours. The
colored solid complex separates out from solution. The product was filtered on cooling,
washed with methanol, dried under inert atmosphere and practical yield of complex is
Synthesis of transition metal complexes of the type M(L2)2
This complex is prepared by mixing methanolic solution of metal chloride and alcoholic
solution of ligand L2 in the 1:2 molar preporation with continuous stirring at room
temperature., The clear shown was refluxed on a heating mental .Then the solution was
refluxed on a heating mental at about 60-700C for four hours. The colored solid complex
separates out from solution. The solid product is filtered on cooling, washed with methanol,
dried in inert atmosphere.
Synthesis of transition metal complexes of the type ML1L2
In a round bottom flask containing alcoholic solution of metal chloride, hot methanolic
solution of mixture of salicylaldehyde semicarbazone and salicylaldehyde oxime in the molar
preporation 1:1:1 was added with continuous stirring at room temp, till clear solution was
obtained. Then the solution was refluxed on a water bath for four hours. The colored solid
complex separates out from solution. The solid product is filtered on cooling, washed with
methanol, dried and weight of the complex obtained is taken to determine practical yield.
Similarly, complexes of salicylaldehyde semicarbazone with ortho-hydroxyacetophanone
oxime, anthranilic acid, benzaldehyde semicarbazone, acetophenone semicarbazone with
Co(II) chlorides were prepared by mixing equimolar quantities in 1:1:1 proportions.
RESULTS AND DISCUSSION
Analytical Properties
The mixed ligand complexes formed were having different colors as shown in the table 2.
These complexes were insoluble in chloroform, carbon tetrachloride, methanol, ethanol but
soluble in DMF. The properties of complexes were indicated in table 2. The TLC of the
mixed ligand complexes with M(L1)2 and M(L2)2 was taken. It exhibit single spot with Rf
values being intermediate of the two corresponding symmetrical bis-complexes. From the
magnetic moments these complexes shows octahedral geometry. The amount of metals
present in complexes were determined volumetrically by EDTA using double burette
technique for optimum utilization of reagents. Indicator xylenol orange for Co(II). Specific
conductance were measured at room temperature in DMF by a Systronics direct reading 304
conductivity meter using a glass conductivity cell having a cell constant L1 and L2.
Antimicrobial activities were determined in the PG department of Microbiology, Pratap
The elemental analysis shown in Table 2 indicates that, all the metal complexes have 1:1:1
stoichiometry and are, soluble only in DMF and DMSO. The molar conductance values
obtained for these complexes at the concentration of 10-3 M are in the range of 52-109 ohm-1
mol-1 cm2. The magnetic moments these mixed ligand complexes shows that these complexes
[image:7.595.46.555.230.403.2]were having octahedral geometry.
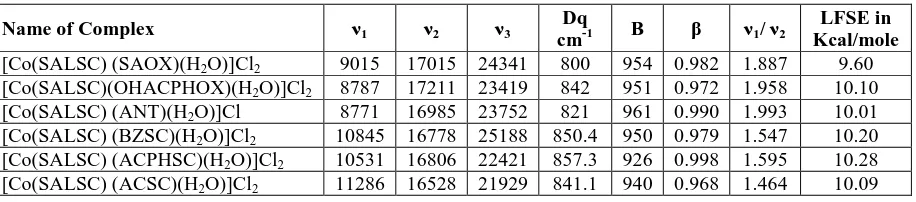
Table 2: Physical, analytical, magnetic susceptibility and molar conductance data of the
mixed ligand complexes:
Name of Ligand /
Complex Empirical
Formula
Mol.
Wt. Colour
Percentage of
µef f B.M
Molar Conductance
^m Ohm-1 cm2 mol-1
Metal C N H
[Co(SALSC) (SAOX)(H2O)]Cl2
CoC15N4O5H18Cl2 460.93 Yellow
13.90 (13.83) 39.01 (30.03) 12.14 (12.10) 3.90
(3.88) 4.473 60.48 [Co(SALSC)
(OHACPHOX)(H2O)]Cl2
CoC16N4O5H20Cl2 477.93 Brown
12.33 (12.29) 40.17 (40.01) 11.73 (11.71) 4.18
(4.16) 4.390 108.37 [Co(SALSC)
(ANT)(H2O)]Cl
CoC15N4O5H17Cl 424.43 Yellow
12.70 (12.67) 42.40 (42.31) 13.19 (13.13) 4.00
(3.98) 4.690 51.32 [Co(SALSC)
(BZSC)(H2O)]Cl2
CoC16N6O4H20Cl2 489.93 Brown
13.07 (12.95) 39.18 (39.10) 17.14 (17.02) 4.01
(4.03) 4.890 71.24 [Co(SALSC)
(ACPHSC)(H2O)]Cl2
CoC17N6O4H22Cl2 503.93 Black
12.67 (12.63) 40.48 (40.39) 16.66 (16.61) 4.36
(4.32) 4.791 63.70 [Co(SALSC)
(ACSC)(H2O)]Cl2
CoC11N6O4H20Cl2 429.93 Orange
13.70 (13.71) 30.70 (30.63) 19.53 (19.48) 4.65
(4.60) 4.93 67.31
Magnetic susceptibility
The magnetic moment values for Co(II) complexes with SALSC as one of the ligand and
SAOX,OHACPHOX, ANT,BZSC, ACPHSC AND ACSC as one of the other ligand are
shown in Table2. The mixing of the singlet states lowers the magnetic moments of Co(II)
complexes are in the range of 4.390-4.93 BM indicating that the Co(II) complexes are
typically high spin complexes and having octahedral structure.[7]
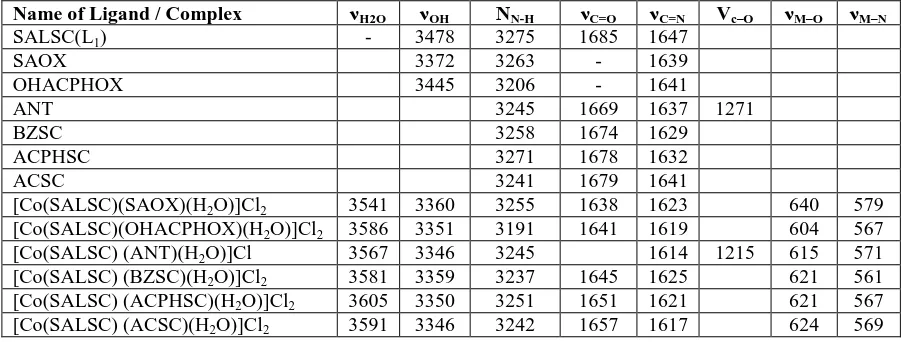
Electronic spectra
The electronic spectral data of The magnetic moment values for Co(II) complexes with
SALSC as one of the ligand and SAOX,OHACPHOX, ANT,BZSC, ACPHSC and ACSC as
one of the other ligand shown in Table 3. In the electronic spectra of Co(II) complex the three
absorption bands ARE observed in the range 8771-10745cm-1, 16778-16211 cm-1 and
23419-25488 cm-1, due to 4T2g (F) --> 4A2g (ν1) , 4T1g (F) --> 4A2g (F)(ν2) and 4T1g (F) --> 4T1g (P)
(ν3) transitions respectively. These transition suggest octahedral geometry for Co(II)
complex.[8-9] These assignments are in good agreement with the reported value.[10,11] The
ligand field parameters such as Dq, B' and β have been calculated by using band-fitting
were found to be in the range 800-857.3 cm-1. These values are well within the range reported
are most of the octahedral Co(II) complexes. The Co(II) complex under present investigation
process interelectronic repulsion parameter (B') is in the range 926-961cm-1. The Racha
parameter (B') is less than free ion value 971 suggesting a considerable orbital overlap and
delocalization of electrons on the metal ion. The nephelauxetic ratio (β) for the present Co(II)
complexes is 0.98 which is less than one, suggesting partial covalency in the metal ligand
bond. The values Dq, β, LFSE, ν2 / ν1 (Table 2) suggest the octahedral geometry for Co(II)
[image:8.595.72.530.270.371.2]complex.[13]
Table 3: Electronic spectral data and ligand field parameters of Co(II) complexes.
Name of Complex ν1 ν2 ν3
Dq
cm-1 B β ν1/ ν2
LFSE in Kcal/mole
[Co(SALSC) (SAOX)(H2O)]Cl2 9015 17015 24341 800 954 0.982 1.887 9.60
[Co(SALSC)(OHACPHOX)(H2O)]Cl2 8787 17211 23419 842 951 0.972 1.958 10.10
[Co(SALSC) (ANT)(H2O)]Cl 8771 16985 23752 821 961 0.990 1.993 10.01
[Co(SALSC) (BZSC)(H2O)]Cl2 10845 16778 25188 850.4 950 0.979 1.547 10.20
[Co(SALSC) (ACPHSC)(H2O)]Cl2 10531 16806 22421 857.3 926 0.998 1.595 10.28
[Co(SALSC) (ACSC)(H2O)]Cl2 11286 16528 21929 841.1 940 0.968 1.464 10.09
Infrared spectra
The important infrared bands for the ligands and their complexes together with their
assignments are listed in (Table 4). The IR spectra of the ligands show characteristic bands at
1685–1669 and 1647–1629 cm−1 due to the ν (C=O), ν (C=N) functional groups, respectively.
The bands at 3478-3372, and 3275- 3241 cm−1assigned to the ν (O–H) and ν (N–H) stretching
group for ligands L1 and L2, respectively.[14-16] The IR spectra of the complexes exhibited
ligand bands with the appropriate shifts due to complex formation (Table 2). The ν (C=O)
and ν (C=N) stretching bands that appeared in the free ligands in the range 1685–1669 and
1647–1629 cm−11, respectively, are shifted to lower frequency in the complexes and observed
in the ranges 1657–1638 cm−1 and 1614–1625 cm−1 for ν (C=O) and ν (C=N), respectively.
These bands are assigned to a ν (C=O) and ν (C=N) stretches of reduced bond order. This can
be attributed to delocalisation of metal electron density (t2g) to the π-system of the
ligand.[14,15] indicating coordination of oxygen of C=O and nitrogen of the C=N moieties to
the metal atoms.[16] The bands of ν (C–O) at ca. 1271 cm−1 in the free ligands are shifted to
lower frequencies and appeared at 1215 cm−1 for the complexes. At lower frequency the
complexes exhibited bands around 615–640 and 561–579 cm−1 which could be assigned
to ν (M–O) and ν (M–N) vibration mode.[17,18] These bands indicated that the imine, nitrogens
and the oxygen of carbonyl group of the ligands are involved in coordination with metal ion.
3255 cm−1 assigned for the free O–H and N–H functional groups. A broad band appeared in
the region 3541-3591cm-1 in all complexes complexes indicates the presence of coordinated
water or lattice water.[19]
Table 4.Characteristic IR frequencies (cm-1) of the ligands and its complexes Ligand/
complex.
Name of Ligand / Complex νH2O νOH ΝN-H νC=O νC=N Vc–O νM–O νM–N
SALSC(L1) - 3478 3275 1685 1647
SAOX 3372 3263 - 1639
OHACPHOX 3445 3206 - 1641
ANT 3245 1669 1637 1271
BZSC 3258 1674 1629
ACPHSC 3271 1678 1632
ACSC 3241 1679 1641
[Co(SALSC)(SAOX)(H2O)]Cl2 3541 3360 3255 1638 1623 640 579
[Co(SALSC)(OHACPHOX)(H2O)]Cl2 3586 3351 3191 1641 1619 604 567
[Co(SALSC) (ANT)(H2O)]Cl 3567 3346 3245 1614 1215 615 571
[Co(SALSC) (BZSC)(H2O)]Cl2 3581 3359 3237 1645 1625 621 561
[Co(SALSC) (ACPHSC)(H2O)]Cl2 3605 3350 3251 1651 1621 621 567
[Co(SALSC) (ACSC)(H2O)]Cl2 3591 3346 3242 1657 1617 624 569
Antimicrobial activity
The synthesized ligands and its complexes were screened for their antibacterial activity.[20]
against E. coli, Baciullus Sp., Staphylococcus Sp,. Pseudomonas Sp. and Proteus Sp.at 100
µg/0.1 cm3 concentration. The zones of inhibitions of the antimicrobial activity have been
presented in Table 5. The results of antibacterial activity shows that complexes with Co(II)
shows weak activity.
Table 5: The results of antibacterial activity of ligands and its complexes.
Name of Ligand / Complex
Antibacterial Activity of zone of inhibition (in mm)
E.coli Baciullus Sp.
Staphylococcus Sp.
Pseudomonas
Sp. ProtusSp
SALSC(L1) 11 13 10 - -
SAOX 11 - - 12 11
OHACPHOX 12 10 13 11 13
ANT 13 10 12 09 10
BZSC 13 12 11 10 09
ACPHSC 09 10 09 10 10
ACSC 10 11 10 09 11
[Co(SALSC) (SAOX)(H2O)]Cl2 12 16 15 13 14
[Co(SALSC) (OHACPHOX)(H2O)]Cl2 14 13 14 12 12
[Co(SALSC) (ANT)(H2O)]Cl 17 15 17 18 17
[Co(SALSC) (BZSC)(H2O)]Cl2 15 15 13 15 14
[Co(SALSC) (ACPHSC)(H2O)]Cl2 14 13 15 15 14
[image:9.595.72.523.189.360.2]CONCLUSIONS
The ligand salicylaldehyde semicarbazone is tridentate coordinating through ‘N’ and ‘O’
while ligands ortho hydroxy acetophenone oxime, anthranilic acid, benzaldehyde
semicarbazone, acetophenone semicarbazone and acetone semicarbazone are bidentate
coordinating through ‘N’ and ‘O’. Analytical data, electronic spectra, magnetic susceptibility,
IR spectral data reveal octahedral geometry for all the complexes. The conductance values
show electrolytic behavior of the complexes. The ligands and its all complexes were tested
for antimicrobial activity. The complexes are shows moderate to good antibacterial and
antifungal activity compared to its ligand. On the basis of spectral evidence, the following
probable structures have been assigned for synthesized compounds.
Structure of complexes
1. [Co(SALSC) (SAOX)(H2O)]Cl2 2.[Co(SALSC)(OHACPHOX)(H2O)]Cl2 3. [Co(SALSC) (ATN)(H2O)]Cl
OH N HN O NH2 Co H 2 HO N H H2O
HO
2Cl- OH
N HN O NH2 Co H 2 HO N CH3
H2O
HO
2Cl
-N H3C HN
O NH2
OH Co
O
O NH2
H2O
+
Cl
-4. [Co(SALSC)(BZSC)(H2O)]Cl2 5. [Co(SALSC)(ACPHSC)(H2O)]Cl26. [Co(SALSC) (ACSC)(H2O)]Cl2
N HN O NH2
Co
H HO N NH OH2N
H
H2O
2+
2Cl- H3C
N HN
O NH2
Co
H3C
HO
N NH O
H2N H
H2O
2+
2Cl
-H3C
N HN
O NH2
Co
H3CHO
N NH O
H2N
H
H2O
2+
2Cl
-ACKNOWLEDGEMENT
Auther is very thankful to Principal, Pratap college, Amalner for providing necessary
REFERENCES
1. Prem Mohan Mishra1, Manoj Kumarjha2, Ram Subhag Chaudhari3 and Chandan Kumar 4
Oriental Journal of Chemistry, 2013; 29(4): 1651-1656.
2. P. A. Stabnikov, I. V. Korolkov, V. V. Krisyuk and I. A. Baidina, Journal of Structural
Chemistry, 2013; 54(1): 123-128.
3. Dina M. Fouad, Ahmed Bayoumi, Mohamed A. El-GAhami, Said A Ibrahim, Abbs
Hammmam Natural Science, 2010; 2(8): 817-827.
4. Shashi B, Kalia, Geetanjli Kaushal, Rojila, Dharvinder Kumar, J Therm Anal Calorim,
2012; 109: 14630-1471. DOI 10.1007/10973-011-1925-7.
5. A. I. Ei-Said Journal of thermal Analysis and calorimetry, 2002; 68: 917-929.
6. H. M. Parekh amd M. N. Patel Pharmaceutical Chemistry Journal. 2006; 40(12).
7. Figgis B. N. and Lewis J, Progress in Inorganic Chemistry Edited by Cotton, F. A. 6th
Ed., Interscience, New York, 1964.
8. Sulekha Chandra and Gupta K, Indian J. Chem. 2001; 40: 775.
9. (a) Verma J K and Verma G S P, Indian J. Chem, 1982; 21: 825.
10.(b) Lakshumi and Rai R A, J. Inorg. Nucl. Chem, 1984; 42: 450.
11.Satpathy K C, Jal B B and Mishra R, Trans. Met. Chem, 1984; 9: 8.
12.Underhill A E and Billing D, Nature, 1996; 210: 834.
13.Lever A B P, Inorganic Spectroscopy; Elsevier, Amsterdam, 1968.
14.Figgs B N, Introduction to Ligand Fields; Interscience John-wiley and Sons, New York,
196.
15.Sulekha Chandra and Gupta K, Indian J. Chem, 2001; 40: 775.
16.(a) Verma J K and Verma G S P, Indian J. Chem, 1982; 21: 825.
17.(b) Lakshumi and Rai R A, J. Inorg. Nucl. Chem, 1984; 42: 450.
18.Satpathy K C, Jal B B and Mishra R, Trans. Met. Chem, 1984; 9: 8.
19.Underhill A E and Billing D, Nature, 1996; 210: 834.
20.Lever A B P, Inorganic Spectroscopy; Elsevier, Amsterdam, 1968.
21.Chetan K M, Ashwin S P and Bharat T T, E- J. Chem., 2005; 2(6): 2.
22.Simoncini F, Rangone R and Calanni C, Farnance Ed., Prat, 1968; 23(10): 559. Chem.
Abstr, 1968; 69: 109-851.
23.Vogel A I, A Text book of Quantitative Organic Analysis; 3rd Ed., ELBS Longmans