FORMULATION AND EVALUATION OF ORAL MOUTH DISSOLVING FILM OF ANTIEMETICS DRUGS
Full text
Figure


Related documents
KEYWORD: Mouth dissolving tablets, Dimenhydrinate, Crospovidone, Sodium Starch Glycolate, Direct compression... 1124
study the IR study of pure drug levosalbutamol sulphate, polymer HPMC K-15, drug with. HPMC K-15, HPMC–K100, HPMC E-15 & sodium lauryl sulphate were carried out
Vol 12, Issue 9, 2019 Online 2455 3891 Print 0974 2441 FORMULATION OPTIMIZATION AND EVALUATION OF MOUTH DISSOLVING FILM OF APREPITANT ADITI D BAVISKAR*, BARI MM, BARHATE SD Department
Development of Pulsincap formulation The developed system contained hydrogel plug prepared with swellable polymer such as hydroxypropyl methyl cellulose HPMC 10K, Guar Gum, Xanthan
Evaluation of mouth dissolving tablet: The formulated mouth dissolving tablets were evaluated for different parameters like general characteristic, uniformity of
Placebo and Perindopril Erbumine containing mouth dissolving film (MDF) were prepared with Pullulan, HPMC E-15 as film forming agent and other excipient by solvent casting method
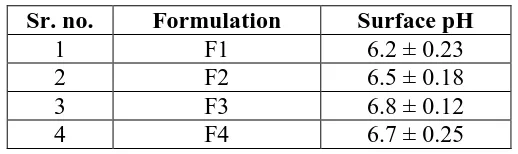
The fast dissolving oral film evaluated for folding endurance, swelling index, surface pH, in-vitro disintegration time, drug content, drug polymer compatibility
Dissolution profiles of the mouth dissolving films containing rofecoxib formulations were compared with pure drug.. No significant differences were observed from in-vitro