HAEMATINIC AND ANTI-ANEMIC EFFECTS OF THE METHANOL
EXTRACT OF
SARACA INDICA
STEM BARK AGAINST PHENYL
HYDRAZINE-INDUCED HEMOLYTIC ANEMIA IN RATS
F. W. Bansode1*, K. R. Arya2, A. K. Meena3 and R. K. Singh3
1
Division of Endocrinology, CSIR-Central Drug Research Institute, Sector- 10, Janakipuram
Extension, Sitapur Road, Lucknow-226031, U. P., India.
2
Division of Ethnobotany, CSIR-Central Drug Research Institute, Sector- 10, Janakipuram
Extension, Sitapur Road, Lucknow-226031, U. P., India.
3
Division of Toxicology & Experimental Medicine, CSIR-Central Drug Research Institute,
Sector- 10, Janakipuram Extension, Sitapur Road, Lucknow-226031, U. P., India.
ABSTRACT
Saraca indica (Caesalpiniaceae) is one of the most renowned and a
religious tree of India. This versatile plant shows cancer,
anti-menorrhagic, anti-oxytocic, anti–microbial activity and has extended
uses in Ayurveda, Unani and Homeopathy System of Medicine. Aims
of the study were to evaluate the hematinic and anti-anemic effects of
the methanol extract of Saraca indica stem bark (SI) against
phenylhydrazine-induced hemolytic anemia in rats. Adult male rats
were divided into 5 groups (Gr.I-V) containing 6 rats each. Rats in Gr.
I served as control. Gr. II rats were treated with SI (250mg/kg) extract
for 14 days. Rats in Gr. III, IV and V were pre-treated with phenyl
hydrazine (PHZ,10mg/kg for 7 consecutive days) so as to induce
anemia, followed by 0, 250 and 500mg/kg doses of SI extract
respectively up to 14 days. To investigate hematinic and anti-anemic profile of plant extract,
blood samples were examined for various hematological parameters viz. Hgb, RBCs, WBC,
MCV, MCHC, MCH, RDW, Platelet count, etc. Biochemical analysis of marker enzymes for
general metabolic, liver and kidney function, and histological examination was done in
control and treated-groups of rats. Antioxidant enzymes and level of lipid peroxidation was
studied in blood samples from treated and control animals. Results showed that PHZ
(10mg/kg) treatment for 7 consecutive days caused a significant increase in MDA activity
Volume 8, Issue 9, 1176-1201. Research Article ISSN 2277– 7105
Article Received on 04 June 2019,
Revised on 25 June 2019, Accepted on 15 July 2019,
DOI: 10.20959/wjpr20199-15507
*Corresponding Author
Dr. F. W. Bansode
Division of Endocrinology,
CSIR-Central Drug
Research Institute, Sector-
10, Janakipuram Extension,
Sitapur Road,
but, declined SOD and Catalase activity in blood serum. Further, PHZ- treatment caused
significant decrease in Hgb (%), RBC, MCV, but increased TLC count in Gr. III-V rats.
There was significant increase in Hgb (%), RBC, MCV and TLC count in Gr. IV and V rats
treated with SI extract at 250 and 500 mg/kg doses from day 8-14, as compared to
PHZ-treated (Gr.III) rats. Rats PHZ-treated with SI extract alone did not alter any haematological/
biochemical/histological aspects and organ weights as compared to controls. In conclusion,
the results of the study clearly indicate antioxidant, hematinic and anti-anemic activity of the
methanolic extract of SI stems bark. Findings provide scientific evidence for traditional use
of SI extract as natural antioxidant and hematoprotective agent.
KEYWORDS: Antioxidant, hematinic, anti-anemic activity, Saraca indica.
INTRODUCTION
Herbal plants and phytomedicines are symbolized for safety of mankind, serving several
purposes like health and protection from diseases and/or nutrition. In the present scenario the
knowledge of traditional medicine has always guided the search for new cures and clues.
Therefore, the demand for herbal products is growing exponentially throughout worldwide
for the discovery of valuable drugs because of its non-toxicity and safety in spite of modern
high throughput drug discovery and screening techniques.[1-3] Nowadays, attention has been
focused on the investigation, isolation and fractionation of drugs from plant origin for
pharmacological basis of traditionally used plants.[4-6]
Ashoka is the most ancient tree of India, generally known as “Ashok briksh”, botanical name
Saraca asoca (Roxb.), De.wild or Saraca indica (SI) belonging to family Caesalpinaceae.[7] It
is found throughout India, especially in Himalaya, Kerala, Bengal and entire southern
region.[8] As a medicinal tree the utility of Ashoka seems to have been recognized first in the
Agnivesa Caraka Samhita which is supposed to have been complied somewhere near 1000
B.C. Ayurvedic physicians of the present century are using Ashoka in different female
diseases especially for uterine infections and pain traditionally as per Charka Samhita (100
A.D.). The leaves of S. asoca have been used in the treatment of diabetes. The plant contains
many flavonoids, sterols and triterpenoids as its main constituents [9, 10], which are known
bioactive principles for antidiabetic potential[4, 11, 12], regenerate the damaged β-cells in
diabetic mice.[13,14] SI used in the Ayurvedic system of medicine as hypothermic and diuretic,
as a blood purifier and in stomach ache.[4,5] Asokarista, Asokaghrta, Asoka decoction and
dysentary. The drug Asoka Aristha is traditionally used in India and Sri Lanka to treat
menorrhagia.[15] The antibacterial activity of the extracts of leaves, stem and flowers of S.
asoca has been reported elsware.[16-18] Petroleum ether extract of SI leaves exhibits potential
antitumor and antioxidant activities.[19] Methanol extracts of SI leaves shows better
antihelmintic and analgesic activity.[20,21] The stem bark of SI shows various pharmacological
actions like antibacterial[22], antiulcer[23], anticancer[24], larvicidal[25], antidepressant[26] and
chemo-protective activities.[27]
The use of antioxidants for inhibition of oxidative damage is one of the important approaches
towards the prevention of health problems. Several studies have been indicated the
antioxidant activities of herbal plants and correlated with their total phenolic contents.[28-30]
The antioxidant activity of phenolic compounds is mainly due to their redox properties,
which can play an important role in absorbing and neutralizing free radicals or their effects,
quenching singlet and triplet oxygen or decomposing peroxides.[31,32] The flavonoids and
terpenoids have multiple biological effects including an antioxidant activity, plays an
important role in the defense against free radicals.[33] The oxidative environment of living
organism possess a range of free radicals including superoxide radical, hydroxyl radical,
hydrogen peroxide, nitric oxide and peroxynitrite, which are essential for the production of
energy for various biological processes. However, excessive production of free radicals
results in the development of carcinogenesis[1], neurodegenerative diseases[2], inflammatory
diseases[34], atherosclerosis, aging, immunosuppression, ischemic heart disease, diabetes, hair
loss, Alzheimer‟s disease, cataract and many other problems.[34-39] The human body possesses
innate defense mechanisms to counter free radicals in the form of enzymes such as
superoxide dismutase, catalase, and glutathione peroxidase. Vitamin C, vitamin E, selenium,
β-carotene, lycopene, lutein and other carotenoids have been used as supplementary
antioxidants.[32, 40-42] The major phytoconstituents of SI stem bark extract are reported to be
the flavonoids, terpenoid, lignin, cardiac glycosides, phenolic compounds, tannins and
leucoanthocyanidins.[43-46] It also shows antioxidants by preventing the oxidation of
low-density Lipoproteins (LDL), platelet aggregation and damage of red blood cells.[47] The ethyl
acetate fraction of Saraca ashoka flowers exhibited free radical scavenging activity against
the 1,1-diphenyl-2-picrylhydrazyl radical and superoxide radical, along with hydroxyl radical
scavenging activity and Lipid peroxidation inhibitory potential and significant xanthine
Phenyl hydrazine (PHZ)-induced hemolytic anemia hereditary or acquired haemolytic anemia
in humans results from reduced life span or destruction of red blood cells (RBCs) and a
failure of the bone marrow compensatory responses. Haemolysis of the RBCs reduces the
efficiency of oxygen delivery which stimulates increased erythropoiesis and because of the
deficit in the Fe supply and haemoglobin (Hgb) levels, the haemopoietic cells are numerically
and morphologically abnormal. Other features include spherocytosis, polychromasia, red-cell
reticulocytosis, an increase in urinary urobilinogen and porphyrins.[49] PHZ induces a reactive
oxygen species formation, peroxidation of lipids and oxidative degradation of spectrin in the
membrane skeleton. PHZ-induced haemolytic injury seems to be derived from oxidative
alternations to RBCs proteins.[50] This compound can modulate immune reactions.[51]
PHZ-induced anemia has been used as a model to study of hematinic effects[52, 53] and for the
evaluation of its influence on therapeutic effectiveness, like antitumor therapy[54] or for
reticulocyte research or erythrocyte senescence under abnormal physiological conditions.[55]
It causes ten folds increase in DNA-dependent RNA polymerase activity of developing
erythropoietic spleen in haemolytic anemia.[56] Our earlier studies have shown amelioration
of such effects by flower extract of Hibiscus rosa sinensis and coconut oil in rats.[57,58]
The present study was conducted to evaluate the antioxidant potential and anti-anemic effects
of the methanol extract of SI stem bark against PHZ-induced hemolytic anemia in rats.
MATERIALS AND METHODS
Chemicals
The kits for biochemical analysis of SOD, Catalase and MDA and phenyl hydrazine were
purchased from Sigma-Aldrich, USA. All other chemicals used in this study were available
locally.
Collection of plant material
The fresh plant material - stem bark of Saraca indica (SI; family-Caesalpiniaceae) was
collected locally nearby Lucknow region, Uttar Pradesh, India. Plant material was identified
by Dr. K. R. Arya, Principal Scientist, Ethnobotany Division, CSIR-Central Drug Research
Institute Lucknow and authenticated in Institutional Herbarium (Voucher Specimen No.
Preparation of plant extract
The stem bark of SI was dried at room temperature (at 370C) and milled into fine powder
using electric Laboratory grinder. Dry powder (2kg) was macerated in 2000 ml Absolute
Methanol for 48 h and subsequently the mixture was filtered, semi-dried under reduced
pressure (Rotor vapor, Buchi, Germany) at 35°C (yield: 8.357% yield(w/w). The
reconstituted extract was used for haemato-protective/ant anemic activity. SI extract was
administered orally using metal oropharyngeal cannula to the animals at different dose
schedule.
Experimental Protocol
Test animals
Sprague–Dawley rats (150-175 gm) of both sexes were obtained from National Laboratory
Animal Center (NLAC), CSIR-Central Drug Research Institute, Lucknow, India. Animals
were allowed to acclimatize to uniform husbandry conditions (22±3 °C, 12h light: 12h dark
cycle) for 1 week prior to the experiment. The animals were fed with a standard pellet diet
(supplied by Hindustan Lever Ltd., Bangalore) and access to water ad libitum. Animal studies
were conducted according to the regulations of the Institutes Animal Ethics Committee and
the protocol was approved by the Committee for the Purpose of Control and Supervision of
Experiments on Animals, New Delhi, India (IAEC No.- IAEC/2012/86).
Phenyl hydrazine (PHZ)-induced haematotoxicity/haematoprotective activity of Saraca
indica (SI)
Rats (Males and females) were randomly divided into five groups (Gr.) containing five
animals each of either sex. Rats in Gr. I (Control) was administered with 1% gum acacia
orally (p.o.). The rats in Gr. II were orally administered with methanol extract of SI
(500mg/kg) for 7day. Rats in Gr. III, IV and V were treated with PHZ (10 mg/kg/day, p.o) for
7 days to induce haematotoxicity.[51,52,59] Group III rats severed as PHZ-induced
haematotoxicity Control (STD Control). Animals in Gr. IV and V (pre-treated with PHZ for
7 days) were administered orally with SI extract at 250mg/kg and 500mg/kg doses
respectively for another 7 consecutive days up to day 14. On day 15, body weights of control
and treated animals were recorded. Blood was collected in pre-coated EDTA-vials for
hematology and then autopsied by anesthetizing with solvent anesthetic ether. The vital
organs (viz. heart, liver, lungs, spleen, kidney and brain) were dissected out, weighed and
Hematological analysis
Blood samples from control and treated groups were collected on days 0, 7 and 14 of
treatment by puncturing tail vein. The hematological parameters were analyzed using MS-9
Fully Automated Hematology Analyzer (Melet Schloesing, France). The parameters included
were hemoglobin (Hgb), total erythrocyte-red blood cells (T-RBCs), hematocrit (Hct), mean
corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), platelet
count (PC) and total leucocytes count (TLC).
Biochemical estimations
Blood samples were collected by cardiac puncture and retro-orbital plexus method from all
animals into the EDTA sprinkled tubes and were centrifuged at 3000 rpm for 20 min. Serum
was separated and stored at -20°C until analysis was performed. Serum samples were
analyzed for general metabolic function viz. Glucose(GLU), cholesterol(CHO),
triglycerides(TG), total proteins(TP) and albumin(ALB)levels. For liver function, serum
biochemistry for alanine trasparase (ALT), Aspartate transferase (AST), alkaline phosphatase
(ALP) and total billirubin (T-BIL) was done. For kidney function, estimations of blood urea
and nitrogen (BUN), creatinin (CRTN), calcium (Ca) and phosphate (P) were carried out
using the diagnostic kit (ERBA Diagnostics Mannheim, Germany) in Auto analyzer.
Enzymatic antioxidant assays
On day 15, blood was collected in pre-coated EDTA-vials, centrifuged at 10000xg for 10 min
and serum was collected and stored at -200C in refrigerator till estimation of antioxidant
enzyme activity.
Superoxide dismutase (SOD) activity: SOD activity was performed by 19160 SOD
determinations Kit (Sigma-Aldrich, USA). Kit contained WST Solution (5ml), Enzyme
solution (100μl), Buffer Solution (100ml) and Dilution buffer (50ml). Preparation of working
solution was done by diluting 1 ml of WST solution with 19 ml Buffer solution. For enzyme
working solution, enzyme solution was centrifuged for 5 sec, mixed thoroughly and diluted
15 μl of enzyme solution with Dilution buffer. SOD was diluted with dilution buffer to
prepare SOD STD solution as 200U/ml, 50U/ml, 10 U/ml, 5 U/ml, 1U/ml, 0.05 U/ml,
0.01U/ml,0.001U/ml.
Methods: 20μl of sample solution was added to each sample and blank well 2 and 20 μl of
working solution was added in each well and mixed. Then added 20 μl of Enzyme working
solution to each sample and blank 1 well, and mixed thoroughly. Incubated the Eliza plate at
370 C for 20 minutes and read the absorbance at 450nm using a micro plate reader. The SOD
activity (inhibition rate %) was calculated using the equation: SOD activity (inhibition rate
%) = {[(Ablank1-Ablank3)-Asample-Ablank 2))]/(Ablank1 – Ablank3)} x 100.
Catalase enzyme activity: Catalase enzyme activity was performed using Catalase Assay
Kit (CAT 100) from Sigma-Aldrich, USA. Tissue samples were prepared in 1X Assay buffer
solution (500mM potassium phosphate buffer, pH 7.0), then diluted with enzyme dilution
buffer(50mM potassium phosphate buffer, pH7.0 containing 0.1% TritonX-100) in a
microcentrfuge tube and added calorimetric assay substrate solution(200mM H2O2) and
mixed and incubated for 5 min in incubator at 370C. Then added 900 μl of stop solution and
inverted the tube. 10ul aliquot of catalase enzyme reaction was taken in another centrifuge
tube and added 1 ml of Color reagent and mixed by inversion and kept for 15 minutes at
room temperature for color development and read the absorbance at 520 nm.
Lipid peroxidation (MDA) activity: MDA activity was assayed by using lipid peroxidation
assay kit (Catalog no. MAK085, Sigma-Aldirch, USA). Briefly, blood serum (10μl) was
gently mixed with 500 μl of 42 mM H2SO4 in a microcentrfuge tube. Then added 125 μl
phosphotungustic acid solution, mixed by vortexing and incubated for 5 minutes and then
centrifuged at 13000xg for 3 minutes. In a separate tube 2μl of BHT (100x) to 100μl H2O.
Resuspended the pellet on ice with water/BHT solution and adjusted the volume for 200 μl
with water.
Assay reaction: To form MDA-TBA adduct, added 600 μl of the TBA solution into each vial
containing standard and sample. Incubated at 950C for 60 min. Cooled to room temperature in
a ice bath for 10 minutes, pipetted 200 μl from each reaction mixture into 96 well plates for
analysis and read the absorbance at 532 nm.
Histology of vital organs
Tissues from liver, kidney and spleen were fixed in Bouins‟ fluid (24 hr) for histology
purpose. Further, they were dehydrated in graded series of ethanol, cleared in xylene and
infiltrated and embedded in paraffin wax (at 580C). Transverse tissue sections (5 μm) were
stained with routine Haematoxylin-eosin, observed the histological changes under Olympus
Statistical analysis
Analysis for significance of differences between control and treated group of animals was
done by Students „t‟ test and one-way ANOVA (one factor analysis of variance). Values for
antioxidant assays represented in a triplicate manner and were expressed as Mean ± SD.
Values with p < 0.05 were considered as significant.
RESULTS
Body and organ weights
There were no significant differences observed in the body weights of treated male rats as
compared to controls. The absolute organ weights viz. adrenal, brain, testis, heart and kidney
of treated rats were comparable to controls. However, PHZ (10mg/kg) treatment for 7 days
caused significant increase in weights of liver (p<0.01), lung (p<0.01) and spleen (p<0.001)
as compared to control rats. Treatment with SI extract alone did not show any significant
difference in body and organ weights. But, oral administration SI stem bark extract at 250 or
500mg/kg doses to PHZ-induced anemic rats, caused significant decrease (recovery) in
[image:8.595.86.513.425.544.2]weights of liver, lung and spleen comparable to that of control rats (Tables 1 & 2).
Table 1. Body and Absolute organ weights (g) in control, PHZ-treated and PHZ+SI-treated rats after 14 days.
Groups Bodyweight Adrenal gland Brain Gonads Heart Kidney Liver Lungs Spleen
(g) (g) (g) (g) (g) (g) (g) (g) (g)
______ _____________________________________________________________________________________________________________________________________
I. Control 301.0±19.13 0.020±0.004 1.50±0.32 1.04±0.43 0.97±0.13 0.86±0.18 6.54±1.71 1.65±0.11 0.65±0.17
II.SI (250) 302.0±18.64 0.022±0.004 1.80±0.26 1.48±0.103 0.96±0.10 0.87±0.10 8.92±1.62 1.80±0.24 0.76±0.11
III.PHZ (10) 288.6±43.40 0.040±0.025 1.99±0.12 1.46±0.33 1.21±0.40NS 1.06±0.07 NS 11.70±0.52** 2.31±0.31* 4.44±0.04***
IV.PHZ+SI(250) 321.2±16.25 0.04±0.08 1.71±0.22 1.50±0.17 0.90±0.08 0.87±0.08 8. 06±1.71 1.77±0.28 1.18±0.31
V. PHZ+SI(500) 308.6±43.40 0.043±0.01 1. 70±0.10 1.54±0.13 0.95±0.09 0.84±0.02 8.04±0.54 1.96±0.29 1.16±0.31
Significance, Control vs. PHZ-treated, *p <0.05; **p < 0.02; ***p < 0.001, NS- Not significant. Gr. I- Control; Gr. II- SI(250mg/kg); Gr.III – PHZ(10mg/kg); Gr. IV- PHZ+ SI(250mg/kg); Gr. V-PHZ+SI(500mg/kg).
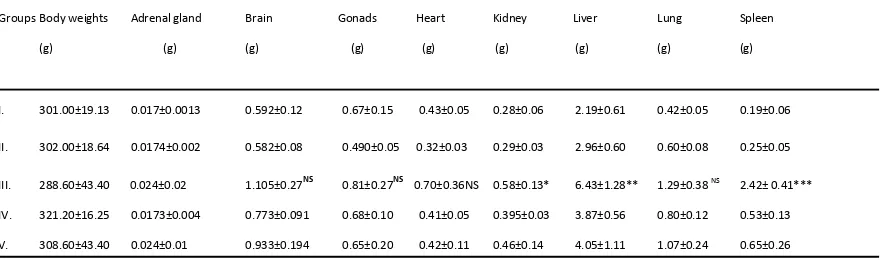
Table 2. Body and Relative organ weights (%) in control, PHZ-treated and PHZ+SI-treated rats after 14 days.
Groups Body weights Adrenal gland Brain Gonads Heart Kidney Liver Lung Spleen
(g) (g) (g) (g) (g) (g) (g) (g) (g)
I. 301.00±19.13 0.017±0.0013 0.592±0.12 0.67±0.15 0.43±0.05 0.28±0.06 2.19±0.61 0.42±0.05 0.19±0.06
II. 302.00±18.64 0.0174±0.002 0.582±0.08 0.490±0.05 0.32±0.03 0.29±0.03 2.96±0.60 0.60±0.08 0.25±0.05
III. 288.60±43.40 0.024±0.02 1.105±0.27NS 0.81±0.27NS 0.70±0.36NS 0.58±0.13* 6.43±1.28** 1.29±0.38 NS 2.42± 0.41***
IV. 321.20±16.25 0.0173±0.004 0.773±0.091 0.68±0.10 0.41±0.05 0.395±0.03 3.87±0.56 0.80±0.12 0.53±0.13
V. 308.60±43.40 0.024±0.01 0.933±0.194 0.65±0.20 0.42±0.11 0.46±0.14 4.05±1.11 1.07±0.24 0.65±0.26
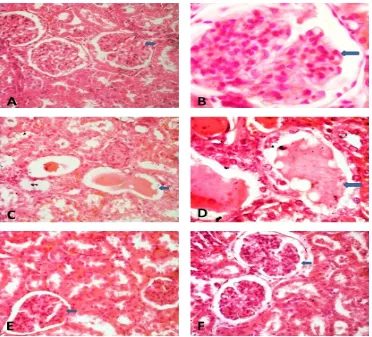
[image:8.595.78.522.605.735.2]Histology of vital organs
Transverse sections of kidney in control rat showed normal distinct glomeruli, Bowman‟s
capsule. PHZ-treated rats showed shrinkage and glomerular and renal damage in kidney of
some animals. SI treatment to PHZ-induced anemic rats showed distinct glomerular and
tubular structure with improvement as compared to PHZ-treated rats but similar to controls.
In control rats, histology of liver was normal showing hepatocytes, portal tract and leucocytic
infiltration. PHZ-treatment caused distortion of hepatocytes, portal tract dilation and
inflammatory infiltration. SI treatment to PHZ-induced anemic rats showed normal structure
similar to in controls. In control rats, spleen showed normal structure. PHZ treatment caused
higher erythroblastic islands in spleen with a higher excessive erythrocytic congestion,
inflammatory infiltration than in liver and kidney in anemic rats. SI treatment showed repair
of all changes which were comparable to normal control rat spleen (Figs.1-3).
Figure 1. A, B. Transverse sections of kidney in control rat showing normal distinct
glomeruli and Bowman’s capsule(Arrow). PHZ-treated rats showing shrinkage and
[image:9.595.115.487.345.682.2]treatment at the dose of 250 mg/kg (E) or 500mg/kg (F) to PHZ-induced anemic rats
showed normal glomerular and tubular structure with improvement as compared to
PHZ-treated rats which is similar to controls. Magnification: x400 (A,C,E, F) & x1000
[image:10.595.144.457.168.388.2]under oil immulsion(B,D) ; H-E stained.
Figure 2. In control rats, histology of liver showing normal hepatocytes, portal tract and
leucocytes infiltration (A). PHZ-treatment caused slight infiltration of leucocytes in liver
(B). SI treatment at 250mg/kg(C) and 500mg/kg(D) to PHZ-induced anemic rats showed
normal liver structure similar to in control rats. Hepatic portal duct-(*), leucocytes
(Arrow). Magnification; x 400 for all figures. H-E stained.
Figure 3. In control rats, spleen showed normal structure(A). PHZ treatment caused
[image:10.595.157.443.515.714.2]inflammatory infiltration(*) in anemic rat (B). SI treatment at 250mg/kg(C) and
500mg/kg (D) showed repair of all changes which were comparable to control rat
spleen. Magnification: x400; H-E stained.
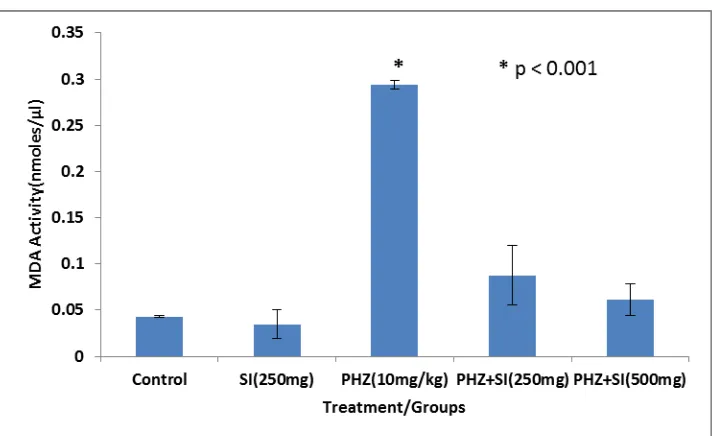
Antioxidants enzymes activity
PHZ (10mg/kg) treatment caused a significant decrease in SOD and CAT enzyme activity,
whereas MDA activity was significantly increased by PHZ treatment as compared to control
rats. SI extract alone did not show any significant change in these enzymes activity compared
to controls. However, there was a significant increase in SOD and CAT enzyme activity in
PHZ-induced anemic rats treated with SI extract at 250 or 500 mg/kg (Figs. 4-6).
Figure 4. Superoxide dismutase enzyme activity in blood serum of control, PHZ-treated
and SI+PHZ treated rats after 14 days treatment (Mean±SD, n=5 number of animals).
Figure 5: Catalase enzyme activity in blood serum of control, PHZ-treated and SI+PHZ
Figure 6: MDA activity in blood serum of control, PHZ-treated and SI+PHZ treated
rats after 14 days treatment (Mean±SD, n=5 number of animals).
Haematology
Adult male rats treated with SI (250mg/kg) for 14 consecutive days (Gr. II) did not show any
significant change in Hgb, RBC and MCV, Platelets(x103/mm3) and TLC(x103/mm3)
concentration in blood as compared to Control rats (Gr.I). Rats in Gr. III treated with PHZ
(10mg/kg/day) for 7 consecutive days, caused significant decrease in Hgb (%) (p<0.01), RBC
(x106/mm3) (p<0.001) and MCV (micron3) (p<0.001) concentration as compared to control
rats. The MCHC (g%) and Platelets(x103/mm3) number did not differ to that of controls, but,
increase in number of TLC(x103/mm3) was observed in PHZ-treated rats. When PHZ-treated
groups of rats administered SI at 250 or 500mg/kg, showed a significant decline in Hgb, RBC
and MCV concentration comparable to in controls (Table 3). The TLC values increased by
PHZ treatment were normalized by SI extract treatment comparable to controls. The Hct,
MCHC and platelets did not show any significant change in PHZ or SI treatment alone or in
[image:12.595.120.479.67.285.2]Table 3. Haematological parameters in control, PHZ-treated and PHZ+SI-treated male rats on day 0, 7 and 14 (n=5 number of animals, Mean ± S.D.).
Parameters Days Group I Group II Group III Group IV Group V
Studied Control SI (250mg/kg) PHZ (10mg/kg) PHZ+SI (250mg/kg) PHZ+SI (500mg/kg)
Hgb(g%) Day 0 12.42±0.22 12.42±0.36 12.52± 0.70 12.62±1.13 12.78±0.19
Day 7 12.17±0.12 12.80±0.15 8.12± 0.49** 9.21±0.91** 9.22±0.43**
Day 14 12.40±0.50 12.58±0.41 9.22± 0.48*** 12.56±0.78 12.52±0.41
T-RBC (x106
/mm3
) Day 0 7.04±0.43 6.85±0.60 7.15± 0.52 7.21±0.75 7.42±0.35
Day 7 7.27±0.59 6.94±0.68 3.02± 0.42*** 3.21±0.42*** 3.20±0.61***
Day 14 7.16±0.16 7.13±0.89 3.15± 0.25*** 5.95±0.50 6.55±0.20
Hct (%) Day 0 41.34±1.73 42.92±2.63 42.46± 5.57 41.64±4.97 43.14±1.16
Day 7 45.64±3.27 43.82±3.15 35.28± 2.17 41.64±4.97 43.14±1.16 `
Day 14 45.76±5.57 44.22±4.90 39.42± 2.04 45.50±4.33 45.50±4.71
MCV (micron3
) Day 0 60.64±3.53 62.66±2.43 58.90± 3.83 57.74±2.12 58.14±1.67
Day 7 59.60±1.75 62.24±2.71 108.80± 4.82*** 108.52±5.40*** 107.95±3.77***
Day 14 60.46±2.30 62.14±2.66 104.40± 4.62*** 75.50±3. 80* 68.00±3.71*
MCHC (g%) Day 0 28.58±1.81 29.04±1.91 29.82± 2.42 30.56±2.86 28.54±0.75
Day 7 25.85±1.40 27.60±2.05 25.33± 2.29 25.85±3.05 24.26±4.02
Day 14 27.21±2.53 28.00±1.10 31.28± 2.35 26.86±0.83 27.52±3.54
PC (x103
/mm3
) Day 0 433.20±22.26 426.20±28.53 406.40±22.59 421.20±23.19 430.80±18.09
Day 7 480.80±31.20 475.20±34.52 399.40±40.97 442.40±27.79 465.80±21.16
Day 14 434.00±28.45 408.00±10.04 353.80±35.62 439.00±30.04 489.20±19.28
TLC(x103/mm3) Day 0 15.31±1.94 14.55±3.13 13.45± 2.63 14.28±3.63 12.02±1.51
Day 7 13.45±2.20 13.06±2.32 22.15±2.63*** 24.53±2.31*** 24.83±2.50***
Day 14 14.35±1.30 14.59±3.38 25.51±2.63** 16.77±1.21 13.51±3.80
Significance level, *P < 0.05, **P < 0.01, ***P < 0.001. Hgb- hemoglobin, T-RBC- total red blood cells, Hct- hematocrit, MCV- mean corpuscular volume, MCHC- mean corpuscular hemoglobin concentration, TLC- total leucocytes count, PC- platelet count.
Biochemical analysis
Oral administration of methanol extract of SI stem bark at 200mg/kg body weight did not
show any significant changes in the general metabolic function, liver function or kidney
function as evident by analysis of marker enzymes viz. GLU, CHOL, TG, TP, ALB, ALT,
AST, ALP, T-BIL, BUN, CRTN, Ca and P as compared to control rats. Treatment of PHZ
(10mg/kg body weight) alone caused significant increase in blood GLU concentration and in
marker enzymes of Liver and kidney function e.g. ALT, AST, ALP, T-BIL, and in CRTN
and P as compared to control or SI (500mg/kg) alone treatment. Administration of SI stem
bark extract at 250 or 500 mg/kg to PHZ-induced anemic rats caused decline in GLU, AST,
ALP, T-BIL, CRTN and P levels as compared to PHZ alone treatment, which were
Table 4 : Terminal biochemistry in control, PHZ-treated and PHZ+ SI treated adult male rats for 14 days (Mean ± S.D., n=5 number of animals).
Treatment groups General metabolic function Liver function Kidney function
GLU CHOL TG TP ALB ALT AST ALP T-BIL BUN CRTN Ca P
(Mg/dl) (Mg/dl) (Mg/dl) (Mg/dl) (Mg/dl) (U/L) (U/L) (U/L) (Mg/dl) (Mg/dl) (Mg/dl) (Mg/dl) (Mg/dl)
Gr. I (Control) 106.16 68.20 58.68 7.02 3.30 49.61 128.3 588.12 0.21 28.77 0.57 10.26 9.20
± ± ± ± ± ± ± ± ± ± ± ± ±
14.77 9.81 9.20 0.52 0.17 7.32 19.8 25.39 0.03 7.6 0.05 0.91 0.02
Gr. II (SI-250mg/kg) 101.5 62.2 59.3 7.12 3.1 54.3 132.6 558.7 0.22 23.7 0.62 10.1 9.00
± ± ± ± ± ± ± ± ± ± ± ± ±
7.85 4.5 7.8 0.73 0.21 6.5 12.6 35.6 0.04 4.7 0.06 0.82 0.40
Gr. III (PHZ-10mg/kg) 187.08d 62.70 57.50 7.16 3.24 76.90a 243.38b 964.86c 0.54d 20.53 0.82a 11.63 17.50d
± ± ± ± ± ± ± ± ± ± ± ± ±
14.13 10.60 13.24 0.27 0.27 10.56 40.93 107.98 0.04 1.68 0.11 0.78 1.70
Gr. IV (PHZ+SI-250mg/kg) 158.14b 63.42 53.02 7.33 3.73 74.72b 215.44b 588.99 0.14 25.91 0.73 11.44 9.70
± ± ± ± ± ± ± ± ± ± ± ± ±
16.30 10.62 11.42 0.39 0.22 5.96 26.58 103.72 0.03 2.84 0.07 1.23 1.40
Gr. V (PHZ+SI-500mg/kg) 127.04* 55.36 57.92 7.16 2.93 62.34 155.68 582.24 0.18 18.34 0.68 9.98 8.30
± ± ± ± ± ± ± ± ± ± ± ± ±
11.42 9.18 6.86 0.53 0.13 13.93 44.01 26.37 0.05 1.98 0.03 0.40 1.20
Significance level, a – p<0.05, b – p <0.02, c – p<0.01, d – p<0.001 (Control vs. SI, PHZ and PHZ+ SI); PHZ vs. PHZ+SI,* p<0.01.GLU -Glucose, CHO -Cholesterol, TG -Triglycerides, TP -Total proteins, ALB –Albumin, ALT -Alanine trasparase, AST-Aspartate transferase, ALP-Alkaline phosphatase, T-BiL-Total billirubin, BUN-Blood urea and nitrogen, CRTN-Creatinin, Ca-Calcium and P-Phosphate.
DISCUSSION
In present study, PHZ-induced anemic rats showed significant increase in MDA activity and
decrease in SOD and CAT enzyme activity in blood serum. Oral administration of the
methanol extract of SI stem bark to PHZ-induced anemic rats caused significant decrease in
MDA activity, but increased SOD and CAT enzyme activity equivalent to in SI- treated or
normal rats. Previous studies have been shown the antioxidant properties of SI stem bark
extract using 1,1-diphenyl-2-picrylhydrazyl (DPPH) and SOD assay system.[58, 60-61] Further,
the antioxidant, antiglycation and inhibitory potential of flavonoid fraction of the flower
extract of this plant (SAF) against alpha-glucosidase and alpha-amylase (the enzymes linked
to type 2 diabetes) and LDL oxidation have been reported.[62] Pre-treatment of C2C12 cells
with SAF prevented the increased formation of MDA, ROS and depletion of GSH induced by
H2O2.[62] The ethyl acetate fraction of SAF have also shown the free radical scavenging
activity against the DPPH and superoxide radical, along with hydroxyl radical scavenging
activity and lipid peroxidation inhibitory potential indicating significant antioxidant and
xanthin oxidase (XO; key enzyme linked to inflammation) inhibitory potential.[48] SI leaves
been reported to show significant (P <0.05) antioxidant activity at the dose of 500 μg/ml.[12]
In concurrence, previously reported studies with herbal extracts e.g. A. pterocarpoides, M.
arboreus and H. madagascariensis[63,64] have been demonstrated to show an increase in the
rate of inhibition of DPPH, as a function of time and the extract‟s concentration which reflect
on the kind of scavenging radical kinetic: the fast, intermediate and slow kinetics. The
ameliorative effects of SI leaves ethanolic extract on hyperglycemia and lipid profile have
been demonstrated to be due its contents including phytosterols, phenols, tannin and
flavonoids.[65-67] In plant tissues many phenolic compounds (in addition to tocopherols) are
potential antioxidants, flavonoids, tannins and lignin precursors may work as
ROS-scavenging compounds. Antioxidants act as a cooperative network, employing a series of
redox reactions. Interactions between ascorbic acid and glutathione, and ascorbic acid and
phenolic compounds are well known.[68] Therefore, the radical scavenging activity of SI
extract may to be due to its polyphenolic constituents that play an important role in
anti-oxidative effects, act as reducing agents/antioxidants via several mechanisms including the
scavenging of free radicals, chelation of transition metals, as well as the mediation and
inhibition of enzymes.[46, 69]
The body and organ weights (viz. adrenal, brain, testes, heart and kidney) did not show any
significant changes in SI, PHZ and PHZ+SI extract treated rats as compared to controls. But,
there was a significant increase in weights of liver, lung and spleen in PHZ-treated anemic
rats as compared to control. Oral administration of SI extract (250 or 500mg/kg) caused a
significant decrease in these organ weights gain to normalcy. Histopathological studies
showed higher erythroblastic islands in spleen with a higher excessive erythrocytic
congestion, abundant macrophages than in liver and kidney in PHZ-treated anemic rats.
Previous study has been demonstrated that PHZ treatment induces an increase in spleen,
kidney and liver weights at 60mg/kg dose and showed severe splenomegaly and marked
splenic erythroid hyperplasia on day 6 of post injection.[58, 70] Erythroblastic islands reported
in the spleen, liver and kidney, this being indicative of compensatory erythropoietic activity.
In ultrastructural study, sequential transformation of PHZ-induced hemolytic anemia as well
as transformation of typical erythrocytes into erythrocytes full of protuberances and
transformation of Heinz bodies into damaged erythrocytes by splenic lytic activity have been
reported.[70] The treatments with SI stem bark extract show recovery of these changes similar
to controls. Previous study on SI leaves extract also shown favorable histopathological
The terminal serum biochemistry on day 15 of autopsy, showed significant increase in GLU,
ALT, AST, ALP, T-BIL, CRTN and P levels by PHZ-treatment as compared to control or
SI(250mg/kg) alone treated rats. This increase in by SI treatment was decreased to normal
level by oral administration of SI extract (500 mg/kg) in PHZ-treated male rats. Previously,
Kumar et al.[12] has been shown a significant (P<0.01) reduction in blood GLU levels,
improved body weight and altered biochemical parameters associated with diabetes in
diabetic mice treated with SI leaves extracts (e.g. petroleum ether, chloroform and methanol)
for 21 days. Estimation of blood GLU level has been used as marker of hyperglycemia.
Hyperglycemia induced-oxidative stress caused by free radical generation and decrease in
antioxidant defense system.[71-72] Dyslipidaemia is associated with elevated total cholesterol,
triglycerides and low level of high density lipoprotein (HDL).[73] Treatment with ethanolic
extract of SI leaves has been reported to normalize the altered lipid profile, reduce the
elevated glucose level and attenuates the diabetes-induced renal oxidative stress in dose
dependent manner.[12]
Administration of SI extract (250mg/kg) alone for 14 days to normal rats did not alter Hgb,
RBC, MCV, Platelets(x103/mm3) and TLC(x103/mm3) concentration as compared to controls.
However, PHZ-treatment (10mg/kg/day) for 7 consecutive days, caused significant decrease
in Hgb(%)(p<0.01 ), RBC(x106/mm3) (p<0.001 ) and MCV(micron3)(p<0.001 ) concentration
and increased TLC(x103/mm3) infiltration. Treatment of SI extract at 250 or 500mg/kg body
weight to PHZ-induced anaemic rats, showed significant increase in Hgb, RBC and MCV
concentration which was comparable to in control rats. The Hct, MCHC and platelets did not
show any significant change in PHZ or SI treated either alone or in combination with PHZ
when compared with controls. Similarly, previously reported studies have been demonstrated
that PHZ decreases the Hgb, RBC and MCV and the rate of haematocrit (HCT) below
controls and impairs erythrocyte deformability. It also induces reticulocytosis, increases
osmotic resistance, free plasma Hgb, MCH, MCHC and erythropoietin levels, and
extramedular haematopoiesis in the spleen and liver.[59, 74-79] The numbers of
erythrocyte-committed progenitors and colony-forming units increases during PHZ-induced acute
anaemia.[80] PHZ induce vascular dysfunction and haemodynamic disturbance, but, decreases
in mean arterial pressure and hindlimb vascular resistance.[81] Uncompensated respiratory
alkalosis, increased arterial CO2 tensions and acidosis were also reported following PHZ
administration.[49, 82-83] The plant extracts of Hibiscus cannabinus[52], hydroethanolic extract
shown anti-anaemic activity evidenced by an increase in the contents of Hb, RBC, HCT and
reticulocytes rate in PHZ-induced anaemia. This action could be the result from the highest
antioxidant potential and the presence of iron as reported earlier.[63] The aqueous extract of
Sorghum bicolor (L.) Moench stem bark at the doses of 200, 400 and 800 mg/kg body weight
on iron sufficient and iron deficient weaning rats had also produced a significant increase in
Hgb, packed cell volume and RBCs in iron sufficient and iron deficient groups. There was
also a significant increase in the catalase activity of rat liver and kidney indicating restoration
of anaemic condition in the iron deficient group.[85] Results of the study with SI extract
administration to PHZ-treated rats also explain the recovery of the decreased haematological
parameters (RBCs, Hgb, and MCV) and increased TLC level to normalcy, indicating the
action of the plant extract vis-a-vis haemolysis. Further, the toxic effects of PHZ have been
shown to increase in ROS, Lipid peroxidation, and decrease in GSH which to be reversed by
N-acetyl cysteine, a known ROS scavenger.[86,87] The sub chronic intoxication of rats with
PHZ, results in marked anemia, reticulocytosis, methemoglobinemia and increases
hemocatheresis, total iron content, hepatic ferritin and DNA fragmentation, increases levels
of 8-oxo-7,8-dihydro-2‟-deoxyguanosine (8-oxodGuo), a specific marker of oxidative DNA
damage, and hepatocyte y-glutamyl transpeptidase (y-GT, EC 2.3.2.2) activity.[88] Acute
haemolysis induced by PHZ, exhibited the extramedullary haemopoiesis and increased
erythrophagocytosis in the spleen. In consequence, morphological changes include
splenomegaly and congestion of haemosiderin deposits.[89] Catabolism of haemolysed RBCs
is associated with increased expression and activity of haem oxygenase 1 which suggests
increased capacity for degradation of Hb products, iron level and DMT1 and TFR1 mRNA
expressions in the spleen by PHZ treatment.[79,88,90] Increased splenic erythrophagocytosis
results in a net flux of iron into the circulation from haemolysed RBCs. While the mechanism
of iron exchange between macrophages and transferrin is still undefined, ferroportin mRNA
increased in the spleen of PHZ-treated mice[79], consistent with efflux of iron being mediated
by this protein. Hepatic non-haem iron levels increases significantly after PHZ treatment and
sustained for 7 days after haemolysis[91] which is due to increased absorption driven by
haematopoeitic activity in PHZ-treated animals. Liver iron accumulates initially due to
haemolysis, later on absorbed iron may be diverted to the liver hepatocytes via serum
transferrin.[92] Iron deposition in the liver could in addition to the TFR/DMT1 routes, be due
to influx of non-transferrin bound iron (NTBI). Furthermore, as PHZ-induced haemolysis is
stressful and produces haemderived toxic reactants, the expressions of acute phase proteins
These organs mop up the haem-derived products from circulation.[49] A flavonoid, silybin
dihemisuccinate, an anti-hepatotoxic agent caused protective effects on the hepatic
glutathione depletion and lipid peroxidation induced by PHZ.[95] The protective effects of
quercetin in a model of PHZ-induced oxidant stress, vascular dysfunction and hemodynamic
disturbance, shown to protect the blood glutathione, suppressed plasma malondialdehyde
levels and nitric oxide metabolites and superoxide anion production in rats.[81] A significant
increase in thiobarbituric acid (TBA)-reactivity in the circulating RBC of PHZ-treated rats
increases 3-fold in RBC obtained from the spleen. Since lipid peroxidation accompanies
formation of TBA-reactive malonyldialdehyde, phenylhydrazine induces anemia as a
consequence of peroxidation of RBC membrane lipids and this effect may be a result of the
autoxidation of the drug and the interaction of oxygen radicals with membrane lipids.[96]
CONCLUSION
In summary, results of the present study provide the evidence that the methanolic estract of SI
stem bark could reduce oxidative stress evidenced by its antioxidant activity. Further, SI
extract also showed protective effects on decreased hematological parameters viz. Hgb, RBC
and MCV concentration and increased TLC by PHZ- treated anemic rats. It also normalizes
the increased biochemical parameters such as blood GLU level and marker enzymes for Liver
function (viz. ALT, AST, ALP, T-BIL) and kidney function (CRTN and P) by PHZ
treatment. The increase in organ weights of spleen, liver and kidney and histopathological
alterations induced by PHZ-treatment were also restored to normalcy by oral administration
of SI extract. The prevention of PHZ-induced anemia and related changes in vascular
dysfunction and dynamics by SI extract, explore the traditional use of this plant as an
antioxidant, antianaemic and haematoprotectant.
ACKNOWLEDGEMENT
CDRI communication number: 10000.
Authors’ declaration: Authors have no declaration of interest.
REFERENCES
1. Walaszek Z. Metabolism, uptake and excretion of a D-glucaric acid salt and its potential
use in cancer prevention. Cancer Detect Prev, 1997; 21: 178-190.
2. Lai LS, Chou ST, Chao WW. Studies on the antioxidative activities of Hsian-tsao
3. Jiménez-Estrada1 M, Velázquez-Contreras C, Garibay-Escobar A, Sierras-Canchola D,
Lapizco-Vázquez R, Ortiz-Sandoval C, Burgos-Hernández A, Enrique Robles-Zepeda R.
In vitro antioxidant and antiproliferative activities of plants of the ethnopharmacopeia
from northwest of Mexico. BMC Complementary and Alternative Medicine, 2013, 13: 12
http: //www.biomedcentral.com/1472-6882/13/12.
4. Dhawan BN, Patnaik GK, Rastogi RP, Singh KK, Tandon JS. Screening of Indian plants
for biological activity: part VI. Indian J Exp Biol, 1977; 15: 208-219.
5. Satyavati GV, Prasad DN, Sen SP, Das PK. Investigations into the uterine activity of
Saraca indica Linn. (Ashoka). Ind J Med Res, 1969; 4: 37-45.
6. Mishra V, Agrawal M, Onasanwo SA, Madhur G, Rastogi P, Pandey HP, Palit G,
Narender T. Anti-secretory and cyto-protective effects of chebulinic acid isolated from
the fruits of Terminalia chebula on gastric ulcers. Phytomedicine, 2013; 20: 506-511.
7. Chakkvarthy BK, Gupta S, Gambir SS, Gode KD. Pancreatic Beta cell Regeneration. A
novel antidiabetic mechanism of Pterocarpus marsupium Roxb. Ind J Pharmacy, 1980;
12: 123-127.
8. Pradhan P, Joseph L, Gupta V, Chulet R, Arya H, Verma R, Bajpai A. Saraca asoca
(Ashoka): A Review. J Chemical and Pharmaceutical Res, 2009; 1: 62-71.
9. Das AV, Padayatti PS, Paulose CS. Effect of leaf extract of Aegle marmelose (L.) Correa
ex Roxb. on histological and ultrastructural changes in tissues of streptozotocin induced
diabetic rats. Ind J Exp Biol, 1996; 34: 341-345.
10.Defronzo RA, Bonadonna RC, Ferrannini I. Pathogenesis of type 2 (non-insulin
dependent) diabetes mellitus: A balanced overview. Diabetologia, 1992; 35: 389-397.
11.Ebrahimzadeh MA, Nabavi SF, Nabavi SM. Antioxidant activities of methanol extract of
Sambucus ebulus L. flower. Pak J Biol Sci, 2009; 12: 447-450.
12.Kumar S, Narwal S, Kumar D, Singh G, Narwal S, Arya R. Evaluation of
antihyperglycemic and antioxidant activities of Saraca asoca (Roxb.) De Wild leaves in
streptozotocin induced diabetic mice. Asian Pacific Journal of Tropical Disease, 2012; 2:
170-176.
13.Ebrahimzadeh MA, Nabavi1 SM, Nabavi SF and Eslami B. Antioxidant Activity of the
Bulb and Aerial Parts of Ornithogalum sintenisii L (Liliaceae) at Flowering Stage. Trop J
Pharm Res, 2010; 9: 141-148.
14.Ghosh D, Bera TK, Chatterjee K, Ali KM, De D. Antidiabetic and antioxidative effects of
foenum-graecum L. (methi) in separate and composite manner in streptozotociin-induced diabetic
male albino rat. Trop J Pharm Res, 2009; 1: 1-10.
15.Middelkoop TB, Labadie RP. The action of Saraca asoca Roxb. de Wilde bark on the
PGH2 synthetase enzyme complex of the sheep vesicular gland. Zeitschrift fur
Naturforschung, 1985; C 40: 523-526.
16.Shahid M, Shahza DA, Malik A, Anis M. Antibacterial activity of aerial parts as well as
in vitro raised calli of the medicinal plant Saraca asoca (Roxb.) de Wilde. Cancer J
Microbiol, 2007; 53: 75-81.
17.Chatterjee PC, Barat B, Mandal A. Studies on the effect of plant seed extracts on different
isolates of Pseudomonas aeruginosa. The Japanese journal of experimental medicine,
1980; 50: 263.
18.Annapurna J, Bhalerao UT, Iyengar DS. Antimicrobial activity of Saraca asoca leaves.
Fitoterapia, 1999; 70: 80-82.
19.Shinde A, Chikhali U, Patil S, Naikwade N. Antitumor, Antioxidant and Cytotoxic
Activity of Saraca Indica Linn. Leaves Extract. Int J Drug formulation and Res, 2011; 2:
312-324.
20.Verma A, Jana GK, Chakraborty R, Sen S, Sachan S, Mishra A. Analgesic Activity of
Various Leaf Extracts of Saraca indica Linn. Der Pharmacia Lettre, 2010; 2: 352-357.
21.Sharma A, Gupta S, Sachan S, Mishra, Banarji AA. Anthelmintic activity of the leaf of
Saraca indica Linn. Asian Journal of Pharmacy and Life Science, 2011; 1: 391-395.
22.Pal SC, Maiti AP, Chatterjee BP, Nandy A. Antibacterial activity of flower and flower
buds of Saraca indica. Ind J Med Res, 1985; 82: 188-189.
23.Maruthappan V, Shree KS. Antiulcer activity of aqueous suspension of Saraca indica
flower against gastric ulcers in albino rats. Journal of Pharmacy Research, 2010; 3: 17-20.
24.Ghosh S, Majumder M, Majumder S, Ganguly NK, Chatterjee BP. Saracin: A Lectin
from Saraca indica Seed Integument Induces Apoptosis in Human T- Lymphocytes.
Archives of Biochemistry and Biophysics. 1999; 371: 163-168.
25.Mathew N, Anitha MG, Bala TSL, Sivakumar SM, Narmadha R, Kalyanasundaram M.
Larvicidal activity of Saraca indica, Nyctanthes arbor-tristis, and Clitoria ternatea extracts
against three mosquito vector species. Parasitology Research, 2008; 104: 1017-1025.
26.Shetty P, Krishnamoorthy M, Vijayanarayana K, Subramanyam EVS, Satyanarayana DS.
Antidepressant Activity of Bark of Saraca indica Linn. Asian Journal of Chemistry,
27.Cibin TR, Gayathri DD, Abraham A. Chemoprevention of skin cancer by the flavonoid
fraction of Saraca asoka. Phytotherapy Research, 2010; 24: 666-672.
28.Gupta VK, Kumria R, Garg M, Gupta M. Recent updates on free radicals scavenging
flavonoids: An overview. Asian J Plant Sci, 2010; 9: 108-117.
29.Kaur T, Hussain K, Koul S, Vishwakarma R, Vyas D. Evaluation of Nutritional and
Antioxidant Status of Lepidium latifolium Linn.: A Novel Phytofood from Ladakh. PLoS
ONE, 2013; 8: e69112. doi: 10.1371/journal.pone.0069112.
30.Ilahi I, Samar S, Khan I, Ahmad I. In vitro antioxidant activities of four medicinal plants
on the basis of DPPH free radical scavenging. Pak J Pharm Sci, 2013; 26: 949-952.
31.Osawa T. Novel Natural Antioxidants for Utilization in Food and Biological Systems. In:
Post Harvest Biochemistry of Plant Food Materials in Tropics, Uritani, L., V.V. Garcia
and E.M. Mendoza (Eds.). Japan Scientific Societies Press, Tokyo, Japan, 1994: 241-251.
32.Valko M, Rhodes b CJ, Moncola J, Izakovic M, Mazura M. Free radicals, metals and
antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions, 2006;
160: 1-40.
33.Gil MI, Ferreres F, Thomas-Barberan FA. Effect of postharvest storage and processing on
the antioxidant constituents (Flavonoids and Vitamin C) of fresh cut spinach. J Agric
Food Chem, 1999; 47: 2213-2217.
34.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol,
1995; 38: 357-366.
35.Maxwell SR. Prospects for the use of antioxidant therapies. Drugs. 1995; 49: 345-361.
36.Poulsen HE, Loft S, Prieme H, Vistisen K, Lykkesfeldt J, Nyyssonen K, Salonen JT.
Oxidative DNA damage in vivo: relationship to age, plasma antioxidants, drug
metabolism, glutathione-S-transferase activity and urinary creatinine excretion. Free
Radic Res, 1998; 29: 565-571.
37.Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals
and antioxidants in human health: current status and future prospects. J Assoc Physicians
India, 2004; 52: 794-804.
38.Kim M, Park J, Lim S. Antioxidant activity and cell toxicity of pressurised liquid extracts
from 20 selected plant species in Jeju, Korea. Food Chem, 2010; 122: 546-552.
39.Badmus JA, Odunola OA, Yekeen TA, Gbadegesin AM, Fatoki JO, Godo MO, Oyebanjo
KS, Hiss DC. Evaluation of antioxidant, antimutagenic, and lipid peroxidation inhibitory
activities of selected fractions of Holarrhena floribunda (G. Don) leaves. Biochimica
40.Devasagayam TP, Sainis KB. Immune system and antioxidants, especially those derived
from Indian medicinal plants. Indian J Exp Biol, 2002, 40: 639-655.
41.Govindarajan R, Vijayakumar M, Pushpangadan P. Antioxidant approach to disease
management and the role of 'Rasayana' herbs of Ayurveda. J Ethnopharmacol, 2005; 99:
165-178.
42.Park EJ, Pezzutto JM. Botanicals in cancer chemoprotection. Canc Metast Rev, 2002; 21:
231-255.
43.Duggal JK, Misra K. Leucoanthocyanidins from Saraca asoca stem bark. J Ind Chem
Soc, 1980; 57: 1243.
44.Indrani N, Balasubramanian K. Isolation of condensed tannins from Saraca ashoka -part
I. Leather Science, 1985a; 31: 349-350.
45.Indrani N, Balasubramanian K. Isolation of condensed tannins from Saraca ashoka-–part
II. Leather Science, 1985b; 32: 12-13.
46.Suja M, Rajan S, Thirunalasundari T, Jana B, Thenmozhi S. Pharmacognostical and
phytochemical studies of An Ayurvedic drug Saraca asoca stem bark. Pharmacy Res,
2012; 5: 1119-1121.
47.Nampoothiri SV, Prathapan A, Lijo Cheriana O, Raghu KG, Venugopalan VV,
Sundaresan A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and
Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2
diabetes. Food Chem Toxicol, 2011; 49: 125-131.
48.Prathapana A, Lijo Cheriana O, Nampoothiria SV, Minib S, Raghu KG. In vitro
antiperoxidative, free radical scavenging and xanthine oxidase inhibitory potentials of
ethyl acetate fraction of Saraca ashoka flowers. Nat Product Res, 2011; 2593: 298-309.
49.Latunde-Dada GO, McKie AT, Simpson RJ. Animal models with enhanced
erythropoiesis and iron absorption. Biochimica et Biophysica Acta, 2006; 1762: 414-423.
50.McMillan DC, Powell CL, Bowman ZS, Morrow JD, Jollow DJ. Lipids versus proteins as
major targets of prooxidant, direct-acting hemolytic agents. Toxicol Sci, 2005; 88:
274-283.
51.Berger J. Phenylhydrazine haematotoxicity review. Journal of Applied Biomedicine,
2007; 5: 125-130.
52.Agbor AG, Oben JE, Brahim OB, Ngogang YJ. Haematinic activity of Hibiscus
cannabinus. African J Biotechnol, 2005; 4: 833-837.
53.Biswas S, Bhattacharyya J, Dutta AG. Oxidant induced injury of erythrocyte – role of
54.Golab J, Olszewska D, Mroz P, Kozar K, Kaminski R, Jalili A, Jakobisiak M.
Erythropoietin restores the antitumor effectiveness of photodynamic therapy in mice with
chemotherapy-induced anemia. Clin Cancer Res, 2002; 8: 1265-1270.
55.Xie LD, Liu DH, Sun DG, Yao WJ, Gu L, Yan ZY, Wen ZY. Study on the membrane
shear elastic modul and viscosity of reticulocytes. Progress in Biochemistry and
Biophysics, 2002; 29: 776-780.
56.Spivak JL, Toretti D, Dickerman HW. Effect of Phenylhydrazine-induced Hemolytic
Anemia on Nuclear RNA Polymerase Activity of the Mouse Spleen. Lood, 1973; 42:
257-266.
57.Shukla P, Yadav NK, Singh P, Bansode FW, Singh RK. Phenylhydrazine induced
toxicity: A Review on its haematotoxicity. Int J Basic and Applied Med Sci, 2012; 2:
86-91.
58.Dubey CK, Meena AK, Mishra V, Singh P, Bansode FW, Singh RK. Antioxidant and
haematoprotective activity of the Saraca indica stem bark. IJPSR, 2015; 6: 1430-1437.
59.Berger J. Screening of toxic-haemolytic anaemia in laboratory rats: a model of
phenylhydrazine-induced haemolysis. Haematologia, 1985; 18: 193-200.
60.Yadav NK, Saini KS, Hossain Z, Omer A, Sharma C, Gayen JR, Singh P, Arya KR,
Singh RK. Saraca indica bark extract shows in vitro antioxidant, antibreast cancer
activity and does not exhibit toxicological effects. Oxid Med Cell Longev, 2015; 2015:
205360. doi:10.1155/2015/205360.
61.Pandey K, Ojha V, Yadav S, Sahu SK. Phytochemical Evaluation and Radical
Scavenging Activity of Bauhinia variegata, Saraca asoka and Terminalia arjuna Barks.
Research Journal of Phytochemistry, 2011; 5: 89-97.
62.Prathapan A, Nampoothiri SV, Mini S, Raghu KG. Antioxidant, antiglycation and
inhibitory potential of Saraca ashoka flowers against the enzymes linked to type 2
diabetes and LDL oxidation. Eur Rev Med Pharmacol Sci, 2012; 16: 57-65.
63.Biapa NP, AgborGA, Oben JE, Ngogang JY. Phytochemical study and antioxidant
properties 0f four antianaemic medicinal plants used in Cameroon. African Journal of
Traditional, Complimentary and Alternative Medicines, 2007; 4: 495-500.
64.Biapa NPC, Oben JE, Ngogang JY. Scavenging radical kinetic and Antianaemic
Screening Properties of some Medicinal Plants used in Cameroon. Int J Applied Res Nat
65.Ullah MB, Urmi KF, Howlader MD, Hossain K, Ahmed MT, Hamid K. Hypoglycemic,
Hypolipidemic and Antioxidant effects of leaves methanolic extract of Baccaurea
Ramiflora. IJPPS, 2012; 4: 266-269.
66.Shinde S, Chivate N, Kulkarni P, Naikwade N. Hypolipidemic activity of Psidium
guajava linn leaves extracts in hyperlipidemic rats. IJPPS, 2013; 5: 70-72.
67.Jain A, Jasmine, Sharma S, Saini V. Hypolipidemic, hypoglycemic and antioxidant
potential of Saraca asoca ethanolic leaves extract in streptozotocin induced –
expwerimental diabetes. Int J Pharmacy and Pharmaceutical Sci, 2013; 5(Suppl 1):
302-305.
68.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen
deprivation stress: a review. Ann Bot, 2003; 91: 179-194.
69.Chang TN, Huang SS, Chang YS, Chang CI, Yang HL, Deng JS, Kou YH, Huang GJ.
Analgesic effects and the mechanisms of anti-inflammation of taraxeren-3-one from
Diospyros maritima in mice. Journal of Agricultural and Food Chemistry, 2011; 59:
9112-9119.
70.Roque R, Pina A, Soares C, Martinho A, Messias H. Splenic metastasis in ovary serous
adenocarcinoma. Acta Med Port, 2007; 20: 581-586.
71.Ha H, Lee HB. Reactive oxygen species as glucose signalling molecules in mesangial
cells cultured under high glucose. Kidney Int, 2000; 77: S19–S25.
72.Haidara MA, Mikhailidisc DP, Rateba MA, Ahmed ZA, Yassin HZ, Ibrahim IM, Rashed
LA. Evaluation of the effect of oxidative stress and vitamin E supplementation on renal
function in rats with streptozotocin-induced Type 1 Diabetes. J. Diabetes Complicat,
2008; 23: 130-136.
73.Vaziri ND, Sato T, Liang, K. Molecular mechanism of altered cholesterol metabolism in
focal glomerulosclerosis. Kidney Int. 2003; 63: 1756-1763.
74.Hara H, Ogawa M. Erythropoietic precursors in mice with phenylhydrazine-induced
anemia. American Journal of Hematology, 1975; 1: 453-458.
75.Stern A. Drug-induced oxidative denaturation in red blood cells. Seminars in
Hematology, 1989; 26: 301-306.
76.Finch CA. Erythropoiesis, erythropoietin and iron, Blood, 1982; 60: 1241-1246.
77.Pootrakul P, Kitcharoen K, Yansukon P, Wasi P, Fucharoen S, Charoenlarp P, Brittenham
G, Pippard MJ, Finch CA. The effect of erythroid hyperplasia on iron balance. Blood,
78.Jansson LT, Perkkio MV, Clemons G, Refino CJ,. Dallman PR. Erythropoietin
concentration during the development and recovery from iron deficiency in the rat. Blood,
1985; 65: 959-963.
79.Latunde-Dada GO, Vulpe CD, Anderson GJ, Simpson RJ, Mckie AT. Tissue-specific
changes in iron metabolism genes in mice following phenylhydrazine-induced
haemolysis. Biochim Biophys Acta, 2004; 1690: 169-176.
80.Terszowski G, Waskow C, Conradt P, Lenze D, Koenigsmann J, Carstanjen D, Horak I,
Rodewald HR. Prospective isolation and global gene expression analysis of the
erythrocyte colony-forming unit (CFU-E). Blood, 2005; 105: 1937-1945.
81.Luangaram S, Kukongviriyapan U, Pakdeechote P, Kukongviriyapan V, Pannangpetch P.
Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and
oxidative stress in rats. Food ChemToxicol, 2007; 45: 448-455.
82.Gilmour KM, Perry SF. The effects of experimental anaemia on CO2 excretion in vitro in
rainbow trout, Oncorhynchus mykiss. Fish Physiol Bioch, 1996; 15: 83-94.
83.Pesquero J, Alfaro V, Palacios L. Acid-base analysis during experimental anemia in rats.
Can J Physiol Pharmacol, 2000; 78: 774-780.
84.Turaskar A, More S, Sheikh R, Gadhpayle J, Bhongade SL, Shende V. 2013.
Antianaemic Potential of Swertia chirata on Phenylhydrazine Induced Reticulocytosis in
Rats. Am J Phytomedicine & Clinical Therapeutics AJPCT, 2013; 1(1): 037-041.
85.Oladiji AT,. Jacob TO, Yakubu MT. Anti-anaemic potentials of aqueous extract of
Sorghum bicolor (L.) moench stem bark in rats. J Ethnopharmacol, 2007; 111: 651-656.
86.Clemens MR, Remmer H, Waller HD. Phenylhydrazine-induced lipid-peroxidation of
red-blood-cells invitro and invivo –monitoring by the production of volatile
hydrocarbons. Biochem Pharmacol, 1984; 33: 1715-1718.
87.Amer J, Goldfarb A, Fibach E. Flow cytometric analysis of the oxidative status of normal
and thalassemic red blood cells. Cytometry, 2004; 60A: 73-80.
88.Ferrali M, Signorini C, Sugherini L, Pompella A, Lodovici M, Caciotti B, Ciccoli L,
Comporti M. Release of free, redox-active iron in the liver and DNA oxidative damage
following phenylhydrazine intoxication. Biochem Pharmacol, 1997; 53: 1743-1751.
89.Burkhard MJ, Brown DE, McGrath JP, Meador VP, Mayle DA, Keaton MJ, Hoffman
WP, Zimmermann JL, Abbott DL, Sun SC. Evaluation of the eryhroid regenerative
response in two different models of experimentally induced iron deficiency anemia. Vet
90.CanonneHergaux F, Zhang AS, Ponka P, Gros P. Characterization of the iron transporter
DMT1 (NRAMP2/DCT1) in red blood cells of normal and anemic mk/mk mice. Blood,
2001; 98: 3823-3830.
91.Frazer DM, Inglis HR, Wilkins SJ, Millard KN, Steele TM, McLaren GD, Mckie AT,
Vulpe CD, Anderson GJ. Delayed hepcidin response explains the lag period in iron
absorption following a stimulus to increase erythropoiesis. Gut, 2004; 53: 1509-1515.
92.Kaplan J, Craven C, Alexander J, Kushner J, Lamb J, Bernstein S. Regulation of the
distribution of tissue iron. Lessons learned from the hypotransferrinemic mouse. Ann NY
Acad Sci, 1988; 526: 124-135.
93.Tolosano E, Hirsch E, Patrucco E, Camaschella C, Navone R, Silengo L, Altruda F.
Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient
mice. Blood, 1999; 94: 3906-3914.
94.Lim SK, Kim H, Lim SK, Bin AA, Lim YK, Wang Y, Chong SM, Costantini F,
Baumman H. Increased susceptibility in Hp knockout mice during acute hemolysis.
Blood, 1998; 92: 1870-1877.
95.Valenzuela A, Guerra R. Protective effect of the flavonoid silybin dihemisuccinate on the
toxicity of phenylhydrazine on rat liver. FEBS, 1985; 2281: 291-294.
96.Jain SK, Subrahmanyam D. On the mechanism of phenylhydrazine-induced hemolytic