organic papers
o2632
Yinet al. C28H32N6O6 doi:10.1107/S1600536805022725 Acta Cryst.(2005). E61, o2632–o2634
Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
N,N
000-Bis(N,N-dimethyl-p-toluidine)bis(ethoxy-carbonyl)glycoluril
Guo-Dong Yin, Bao-Han Zhou, Yun-Feng Chen and An-Xin Wu*
Key Laboratory of Pesticide and Chemical Biology of the Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, People’s Republic of China
Correspondence e-mail: chwuax@mail.ccnu.edu.cn
Key indicators
Single-crystal X-ray study
T= 292 K
Mean(C–C) = 0.003 A˚
Rfactor = 0.048
wRfactor = 0.132
Data-to-parameter ratio = 15.7
For details of how these key indicators were automatically derived from the article, see http://journals.iucr.org/e.
#2005 International Union of Crystallography Printed in Great Britain – all rights reserved
In the title compound (systematic name: diethyl 4,8-dioxo-2,6-di-p -tolyl-1,3,5,7-tetrahydro-2,3a,4a,6,7a,8a-hexaazacyclo-penta[def]fluorene-8b,8c-dicarboxylate), C28H32N6O6, the
dihedral angles between the two fused five-membered rings in the glycoluril unit and between the two terminal benzene rings are 71.8 (2) and 88.5 (1), respectively.
Comment
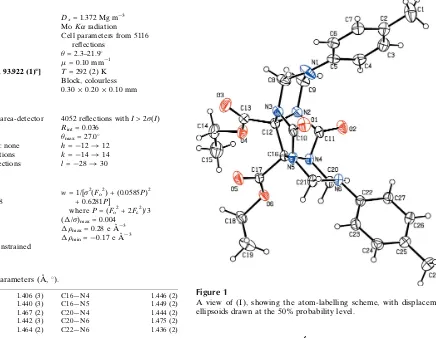
Since Mock and co-workers first characterized the chemical nature and structure of cucurbit[6]uril (CB[6]; Freemanet al., 1981), many receptors based on glycoluril have been reported, including Nolte’s molecular clips and molecular baskets (Rowanet al., 1999), Rebek’s molecular capsules (Hofet al., 2002), CB[n] homologues (n= 5,7 and 8; Leeet al., 2003) and CB[n] (n= 5–7) derivatives (Lee et al., 2003; Lagona et al., 2003). In this paper, we report the crystal structure of the title compound, (I), a new type of receptor based on glycoluril (Fig. 1).
Selected bond lengths and angles are listed in Table 1. The crystal packing is mainly governed by intermolecular C— H O interactions (Table 2 and Fig. 2). The dihedral angle between the two fused five-membered rings in the glycoluril unit is 71.8 (2) and that between the two terminal benzene
rings is 88.5 (1).
Experimental
p-Tolylamine (2.14 g, 20 mmol) and formaldehyde (4.8 g, 80 mmol) were added to a solution of diethoxycarbonyl glycoluril (2.86 g, 10 mmol) in dimethyformamide (50 ml) under a nitrogen atmo-sphere. The mixture was stirred overnight. The solvent was then evaporated to dryness and the residue purified by column chroma-tography (hexane–ethyl acetate, 4:1), to obtain the title compound as a white solid (yield 60%, 3.27 g). Crystals of (I) suitable for X-ray diffraction were grown by slow evaporation of a dichloromethane– methanol (2:1) solution of the title compound under ambient conditions.
Crystal data
C28H32N6O6
Mr= 548.60
Monoclinic, P21=n
a= 9.7963 (7) A˚ b= 11.3007 (8) A˚ c= 24.0399 (17) A˚
= 93.9220 (10)
[OR 93.922 (1)
]
V= 2655.1 (3) A˚3 Z= 4
Dx= 1.372 Mg m
3
MoKradiation Cell parameters from 5116
reflections
= 2.3–21.9
= 0.10 mm1
T= 292 (2) K Block, colourless 0.300.200.10 mm
Data collection
Bruker SMART CCD area-detector diffractometer
’and!scans
Absorption correction: none 21931 measured reflections 5746 independent reflections
4052 reflections withI> 2(I) Rint= 0.036
max= 27.0
h=12!12 k=14!14 l=28!30
Refinement
Refinement onF2
R[F2> 2(F2)] = 0.048
wR(F2) = 0.132 S= 1.02 5746 reflections 365 parameters
H-atom parameters constrained
w= 1/[2(F
o2) + (0.0585P)2
+ 0.6281P] whereP= (Fo
2
+ 2Fc 2
)/3 (/)max= 0.004
max= 0.28 e A˚
3
min=0.17 e A˚
[image:2.610.118.554.75.413.2]3
Table 1
Selected geometric parameters (A˚ ,).
C5—N1 1.406 (3)
C8—N1 1.440 (3)
C8—N3 1.467 (2)
C9—N1 1.442 (3)
C9—N2 1.464 (2)
C16—N4 1.446 (2)
C16—N5 1.449 (2)
C20—N4 1.444 (2)
C20—N6 1.475 (2)
C22—N6 1.436 (2)
N1—C8—N3 110.60 (16)
N1—C9—N2 111.20 (16)
N4—C20—N6 110.61 (14) N5—C21—N6 109.79 (14)
N3—C8—N1—C5 120.5 (2) N2—C9—N1—C5 120.8 (2)
N5—C21—N6—C22 176.08 (14) N4—C20—N6—C22 175.80 (14)
Table 2
Hydrogen-bond geometry (A˚ ,).
D—H A D—H H A D A D—H A
C18—H18B O5i
0.97 2.53 3.336 (2) 141
Symmetry code: (i)xþ1;yþ1;z.
All H atoms were positioned geometrically (C—H = 0.93–0.97 A˚ ) and refined as riding, allowing for free rotation of the methyl groups. The constraintUiso(H) = 1.2Ueq(C) or 1.5Ueq(methyl C) was applied.
Data collection:SMART(Bruker, 1997); cell refinement:SAINT (Bruker, 1999); data reduction: SAINT; program(s) used to solve structure: SHELXS97(Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics: SHELXTL (Bruker, 2001); software used to prepare material for publication:SHELXTL.
The authors are grateful to the Central China Normal University, the National Natural Science Foundation of China (grant No. 20472022) and the Hubei Province Natural Science Fund (grant Nos. 2004ABA085 and 2004ABC002) for finan-cial support.
References
Bruker (1997).SMART. Version 5.054. Bruker AXS Inc., Madison, Wisconsin, USA.
Bruker (1999).SAINT. Version 6.01. Bruker AXS Inc., Madison, Wisconsin, USA.
Bruker (2001). SHELXTL. Version 6.12. Bruker AXS Inc., Madison, Wisconsin, USA.
Freeman, W. A., Mock, W. L. & Shih, N. Y. (1981).J. Am. Chem. Soc.103, 7367–7368.
Hof, F., Craig, S. L., Nuckolls, C. & Rebek, J. Jr. (2002).Angew. Chem. Int. Ed.
41, 1488–1508.
organic papers
Acta Cryst.(2005). E61, o2632–o2634 Yinet al. C
28H32N6O6
o2633
Figure 1
[image:2.610.318.565.412.581.2]A view of (I), showing the atom-labelling scheme, with displacement ellipsoids drawn at the 50% probability level.
Figure 2
Lagona, J., Fettinger, J. C. & Isaacs, L. (2003). Org. Lett. 5, 3745– 3747.
Lee, J. W., Samal, S., Selvapalam, N., Kim, H. J. & Kim, K. (2003).Acc. Chem. Res.36, 621–630.
Rowan, A. E., Elemans, J. A. A. W. & Nolte, R. J. M. (1999).Acc. Chem. Res.
32, 955–1006.
Sheldrick, G. M. (1997). SHELXS97 and SHELXL97. University of Go¨ttingen, Germany.
organic papers
o2634
Yinet al. Csupporting information
sup-1 Acta Cryst. (2005). E61, o2632–o2634
supporting information
Acta Cryst. (2005). E61, o2632–o2634 [https://doi.org/10.1107/S1600536805022725]
N
,
N
′
-Bis(
N
,
N
-dimethyl-
p
-toluidine)bis(ethoxycarbonyl)glycoluril
Guo-Dong Yin, Bao-Han Zhou, Yun-Feng Chen and An-Xin Wu
Diethyl 4,8-dioxo-2,6-di-p-tolyl-1,3,5,7-tetrahydro-2,3a,4a,6,7a,8a- hexaazacyclopenta[def]fluorene-8
b,8c-dicarboxylate
Crystal data
C28H32N6O6 Mr = 548.60 Monoclinic, P21/n Hall symbol: -P2yn a = 9.7963 (7) Å b = 11.3007 (8) Å c = 24.0399 (17) Å β = 93.922 (1)° V = 2655.1 (3) Å3 Z = 4
F(000) = 1160 Dx = 1.372 Mg m−3
Mo Kα radiation, λ = 0.71073 Å Cell parameters from 5116 reflections θ = 2.3–21.9°
µ = 0.10 mm−1 T = 292 K Block, colourless 0.30 × 0.20 × 0.10 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Radiation source: fine-focus sealed tube Graphite monochromator
φ and ω scans
21931 measured reflections 5746 independent reflections
4052 reflections with I > 2σ(I) Rint = 0.036
θmax = 27.0°, θmin = 1.7° h = −12→12
k = −14→14 l = −28→30
Refinement
Refinement on F2 Least-squares matrix: full R[F2 > 2σ(F2)] = 0.048 wR(F2) = 0.132 S = 1.03 5746 reflections 365 parameters 0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[σ2(F
o2) + (0.0585P)2 + 0.6281P] where P = (Fo2 + 2Fc2)/3
(Δ/σ)max = 0.004 Δρmax = 0.28 e Å−3 Δρmin = −0.17 e Å−3
Special details
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full
supporting information
sup-2 Acta Cryst. (2005). E61, o2632–o2634
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2,
conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
x y z Uiso*/Ueq
supporting information
sup-3 Acta Cryst. (2005). E61, o2632–o2634
C20 0.06913 (17) 0.18153 (14) 0.06289 (8) 0.0386 (4) H20A 0.0461 0.1036 0.0764 0.046* H20B 0.1171 0.1716 0.0292 0.046* C21 −0.02406 (18) 0.36961 (14) 0.03209 (8) 0.0408 (4) H21A 0.0228 0.3658 −0.0021 0.049* H21B −0.1076 0.4148 0.0248 0.049* C22 −0.15066 (17) 0.18803 (15) 0.01167 (8) 0.0370 (4) C23 −0.1840 (2) 0.22592 (17) −0.04231 (8) 0.0481 (5) H23 −0.1463 0.2956 −0.0550 0.058* C24 −0.2730 (2) 0.16081 (19) −0.07762 (8) 0.0511 (5) H24 −0.2939 0.1881 −0.1137 0.061* C25 −0.33176 (18) 0.05680 (16) −0.06091 (8) 0.0444 (5) C26 −0.3002 (2) 0.02148 (16) −0.00636 (9) 0.0467 (5) H26 −0.3393 −0.0475 0.0064 0.056* C27 −0.21240 (18) 0.08536 (16) 0.02966 (8) 0.0431 (4) H27 −0.1945 0.0596 0.0662 0.052* C28 −0.4260 (2) −0.01417 (19) −0.10009 (10) 0.0618 (6) H28A −0.5114 0.0269 −0.1065 0.093* H28B −0.4419 −0.0902 −0.0839 0.093* H28C −0.3847 −0.0245 −0.1349 0.093* N1 0.0511 (2) 0.42966 (16) 0.24513 (8) 0.0695 (6) N2 0.19975 (14) 0.31725 (12) 0.19006 (6) 0.0380 (3) N3 0.11522 (16) 0.50804 (12) 0.15830 (6) 0.0406 (4) N4 0.15708 (14) 0.24132 (11) 0.10461 (6) 0.0346 (3) N5 0.06230 (14) 0.42731 (12) 0.07490 (6) 0.0364 (3) N6 −0.05734 (14) 0.25002 (12) 0.05008 (6) 0.0375 (3) O1 −0.09907 (14) 0.54211 (12) 0.11467 (7) 0.0579 (4) O2 0.09622 (15) 0.13432 (11) 0.18109 (6) 0.0551 (4) O3 0.38907 (16) 0.56791 (13) 0.17714 (7) 0.0713 (5) O4 0.45356 (13) 0.38474 (12) 0.15453 (6) 0.0512 (4) O5 0.32248 (15) 0.48640 (11) 0.03966 (6) 0.0574 (4) O6 0.35394 (12) 0.28994 (10) 0.03618 (5) 0.0422 (3)
Atomic displacement parameters (Å2)
U11 U22 U33 U12 U13 U23
supporting information
sup-4 Acta Cryst. (2005). E61, o2632–o2634
C13 0.0443 (11) 0.0483 (11) 0.0401 (11) −0.0093 (9) 0.0052 (8) −0.0071 (9) C14 0.0358 (11) 0.0806 (16) 0.0815 (17) −0.0162 (11) −0.0005 (11) −0.0084 (13) C15 0.0404 (12) 0.0850 (18) 0.109 (2) 0.0002 (12) 0.0058 (13) 0.0004 (16) C16 0.0309 (9) 0.0290 (8) 0.0412 (10) −0.0018 (7) 0.0058 (7) 0.0021 (7) C17 0.0337 (9) 0.0358 (9) 0.0398 (10) −0.0029 (7) 0.0051 (7) 0.0006 (8) C18 0.0414 (11) 0.0533 (11) 0.0560 (13) 0.0023 (9) 0.0214 (9) 0.0029 (9) C19 0.0747 (16) 0.0973 (19) 0.0502 (14) 0.0191 (14) 0.0177 (12) 0.0141 (13) C20 0.0353 (9) 0.0309 (8) 0.0497 (11) −0.0016 (7) 0.0036 (8) 0.0011 (8) C21 0.0374 (10) 0.0337 (9) 0.0508 (11) −0.0008 (7) 0.0000 (8) 0.0058 (8) C22 0.0306 (9) 0.0373 (9) 0.0433 (11) 0.0011 (7) 0.0050 (7) −0.0003 (8) C23 0.0472 (11) 0.0479 (11) 0.0496 (12) −0.0093 (9) 0.0052 (9) 0.0062 (9) C24 0.0501 (12) 0.0613 (12) 0.0416 (11) −0.0001 (10) 0.0010 (9) 0.0044 (10) C25 0.0385 (10) 0.0441 (10) 0.0500 (12) 0.0054 (8) −0.0009 (8) −0.0058 (9) C26 0.0454 (11) 0.0364 (9) 0.0576 (13) −0.0061 (8) −0.0022 (9) 0.0015 (9) C27 0.0430 (10) 0.0402 (10) 0.0457 (11) −0.0051 (8) −0.0012 (8) 0.0048 (8) C28 0.0612 (14) 0.0615 (13) 0.0603 (14) −0.0027 (11) −0.0135 (11) −0.0086 (11) N1 0.0861 (14) 0.0548 (11) 0.0735 (13) 0.0293 (10) 0.0479 (11) 0.0238 (10) N2 0.0386 (8) 0.0368 (8) 0.0393 (9) 0.0020 (6) 0.0076 (6) 0.0032 (6) N3 0.0453 (9) 0.0334 (7) 0.0444 (9) 0.0024 (6) 0.0128 (7) 0.0002 (7) N4 0.0319 (7) 0.0289 (7) 0.0433 (9) −0.0021 (6) 0.0050 (6) 0.0029 (6) N5 0.0318 (7) 0.0291 (7) 0.0486 (9) −0.0007 (6) 0.0049 (6) 0.0014 (6) N6 0.0315 (8) 0.0322 (7) 0.0486 (9) −0.0020 (6) 0.0012 (6) 0.0035 (6) O1 0.0451 (8) 0.0470 (8) 0.0827 (11) 0.0154 (6) 0.0127 (7) 0.0018 (7) O2 0.0631 (9) 0.0419 (7) 0.0616 (9) −0.0086 (6) 0.0141 (7) 0.0151 (7) O3 0.0635 (10) 0.0575 (9) 0.0937 (12) −0.0198 (8) 0.0108 (9) −0.0297 (9) O4 0.0327 (7) 0.0521 (8) 0.0686 (9) −0.0071 (6) 0.0011 (6) −0.0061 (7) O5 0.0616 (9) 0.0366 (7) 0.0778 (10) −0.0061 (6) 0.0325 (8) 0.0071 (7) O6 0.0403 (7) 0.0400 (7) 0.0482 (8) −0.0001 (5) 0.0160 (6) 0.0007 (6)
Geometric parameters (Å, º)
supporting information
sup-5 Acta Cryst. (2005). E61, o2632–o2634
C8—H8A 0.9700 C20—H20A 0.9700 C8—H8B 0.9700 C20—H20B 0.9700 C9—N1 1.442 (3) C21—N5 1.442 (2) C9—N2 1.464 (2) C21—N6 1.463 (2) C9—H9A 0.9700 C21—H21A 0.9700 C9—H9B 0.9700 C21—H21B 0.9700 C10—O1 1.209 (2) C22—C23 1.384 (3) C10—N5 1.377 (2) C22—C27 1.391 (2) C10—N3 1.384 (2) C22—N6 1.436 (2) C11—O2 1.207 (2) C23—C24 1.386 (3) C11—N2 1.380 (2) C23—H23 0.9300 C11—N4 1.388 (2) C24—C25 1.380 (3) C12—N3 1.450 (2) C24—H24 0.9300 C12—N2 1.458 (2) C25—C26 1.385 (3) C12—C13 1.539 (2) C25—C28 1.504 (3) C12—C16 1.560 (2) C26—C27 1.381 (3) C13—O3 1.190 (2) C26—H26 0.9300 C13—O4 1.315 (2) C27—H27 0.9300 C14—C15 1.455 (3) C28—H28A 0.9600 C14—O4 1.456 (2) C28—H28B 0.9600 C14—H14A 0.9700 C28—H28C 0.9600 C14—H14B 0.9700
supporting information
sup-6 Acta Cryst. (2005). E61, o2632–o2634
N1—C8—H8A 109.5 H21A—C21—H21B 108.2 N3—C8—H8A 109.5 C23—C22—C27 118.03 (17) N1—C8—H8B 109.5 C23—C22—N6 123.40 (16) N3—C8—H8B 109.5 C27—C22—N6 118.56 (16) H8A—C8—H8B 108.1 C22—C23—C24 120.53 (18) N1—C9—N2 111.20 (16) C22—C23—H23 119.7 N1—C9—H9A 109.4 C24—C23—H23 119.7 N2—C9—H9A 109.4 C25—C24—C23 122.08 (19) N1—C9—H9B 109.4 C25—C24—H24 119.0 N2—C9—H9B 109.4 C23—C24—H24 119.0 H9A—C9—H9B 108.0 C24—C25—C26 116.76 (17) O1—C10—N5 125.74 (19) C24—C25—C28 121.45 (19) O1—C10—N3 126.27 (18) C26—C25—C28 121.79 (18) N5—C10—N3 107.82 (15) C27—C26—C25 122.14 (18) O2—C11—N2 125.98 (18) C27—C26—H26 118.9 O2—C11—N4 125.96 (17) C25—C26—H26 118.9 N2—C11—N4 107.96 (14) C26—C27—C22 120.41 (18) N3—C12—N2 112.28 (13) C26—C27—H27 119.8 N3—C12—C13 111.92 (14) C22—C27—H27 119.8 N2—C12—C13 110.50 (15) C25—C28—H28A 109.5 N3—C12—C16 103.86 (14) C25—C28—H28B 109.5 N2—C12—C16 102.83 (13) H28A—C28—H28B 109.5 C13—C12—C16 115.03 (14) C25—C28—H28C 109.5 O3—C13—O4 126.92 (18) H28A—C28—H28C 109.5 O3—C13—C12 124.17 (18) H28B—C28—H28C 109.5 O4—C13—C12 108.90 (15) C5—N1—C8 123.48 (17) C15—C14—O4 108.59 (18) C5—N1—C9 124.11 (17) C15—C14—H14A 110.0 C8—N1—C9 112.33 (17) O4—C14—H14A 110.0 C11—N2—C12 111.53 (15) C15—C14—H14B 110.0 C11—N2—C9 121.92 (15) O4—C14—H14B 110.0 C12—N2—C9 115.71 (14) H14A—C14—H14B 108.4 C10—N3—C12 110.52 (14) C14—C15—H15A 109.5 C10—N3—C8 119.70 (16) C14—C15—H15B 109.5 C12—N3—C8 115.88 (15) H15A—C15—H15B 109.5 C11—N4—C20 120.64 (14) C14—C15—H15C 109.5 C11—N4—C16 110.73 (14) H15A—C15—H15C 109.5 C20—N4—C16 116.35 (13) H15B—C15—H15C 109.5 C10—N5—C21 123.29 (15) N4—C16—N5 111.11 (13) C10—N5—C16 111.84 (15) N4—C16—C17 115.21 (13) C21—N5—C16 116.28 (13) N5—C16—C17 108.74 (13) C22—N6—C21 113.93 (14) N4—C16—C12 104.19 (13) C22—N6—C20 111.51 (13) N5—C16—C12 102.85 (13) C21—N6—C20 110.21 (13) C17—C16—C12 114.05 (13) C13—O4—C14 118.40 (16) O5—C17—O6 126.60 (16) C17—O6—C18 116.47 (14)
supporting information
sup-7 Acta Cryst. (2005). E61, o2632–o2634
supporting information
sup-8 Acta Cryst. (2005). E61, o2632–o2634
N4—C11—N2—C12 15.94 (18) C12—C13—O4—C14 177.77 (17) O2—C11—N2—C9 −24.9 (3) C15—C14—O4—C13 −173.3 (2) N4—C11—N2—C9 158.63 (15) O5—C17—O6—C18 −0.5 (3) N3—C12—N2—C11 102.77 (16) C16—C17—O6—C18 179.37 (15) C13—C12—N2—C11 −131.51 (15) C19—C18—O6—C17 −89.2 (2) C16—C12—N2—C11 −8.25 (17)
Hydrogen-bond geometry (Å, º)
D—H···A D—H H···A D···A D—H···A
C18—H18B···O5i 0.97 2.53 3.336 (2) 141