Copyright2000 by the Genetics Society of America
Chromosome Nondisjunction and Instabilities in Tapetal Cells Are Affected by
B Chromosomes in Maize
A. Mauricio Chiavarino, Marcela Rosato, Silvia Manzanero, Guillermo Jime´nez,
Mo´nica Gonza´lez-Sa´nchez and Marı´a J. Puertas
Departamento de Gene´tica, Facultad de Biologı´a, Universidad Complutense, 28040 Madrid, Spain Manuscript received December 10, 1999
Accepted for publication February 21, 2000
ABSTRACT
Abnormal mitosis occurs in maize tapetum, producing binucleate cells that later disintegrate, following a pattern of programmed cell death. FISH allowed us to observe chromosome nondisjunction and micronu-cleus formation in binucleate cells, using DNA probes specific to B chromosomes (B’s), knobbed chromo-somes, and the chromosome 6 (NOR) of maize. All chromosome types seem to be involved in micronucleus formation, but the B’s form more micronuclei than do knobbed chromosomes and knobbed chromosomes form more than do chromosomes without knobs. Micronuclei were more frequent in 1B plants and in a genotype selected for low B transmission rate. Nondisjunction was observed in all types of FISH-labeled chromosomes. In addition, unlabeled bridges and delayed chromatids were observed in the last telophase before binucleate cell formation, suggesting that nondisjunction might occur in all chromosomes of the maize complement. B nondisjunction is known to occur in the second pollen mitosis and in the endosperm, but it was not previously reported in other tissues. This is also a new report of nondisjunction of chromo-somes of the normal set (A’s) in tapetal cells. Our results support the conclusion that nondisjunction and micronucleus formation are regular events in the process of the tapetal cell death program, but B’s strongly increase A chromosome instability.
T
HE tapetum is the innermost cell layer that lines Recent investigations have correlated the expression each anther locule. It is in immediate contact with of certain unique classes of mRNAs with the differenti-the sporogenous tissue to differenti-the inside and to differenti-the middle ated state of the tapetum.Yokoiet al. (1997) first dem-layer to the outside of the anther. Tapetal tissue has a onstrated that an anther-specific promoter directed ta-secretory role, providing nutrients required for micro- petum-specific expression in rice.Huanget al. (1997) spore and pollen grain development (reviewed inPac- characterized a tapetum-specific transcript in Liliumlon-ini1997;Raghavan1997). Particularly in Poaceae, all giflorum temporarily expressed during microspore de-pollen mother cells and de-pollen grains remain in physical velopment. In Brassica oleracea, pollen grains are covered contact with the tapetum at every stage of development, with a lipophilic pollen coat containing several forms because a single cylinder of pollen, one cell thick, is of oleosin. Different transcripts that originate from a arranged in a locule such that each grain is in contact single gene whose expression is restricted to the
tape-with the tapetum (Kirpeset al. 1996). tum encode the forms (Ruiteret al. 1997). In Zea mays,
The tapetum is in a dynamic state during its short the enzyme xylanase is present in the pollen coat and life period, lasting from primary parietal cell formation the Xyl gene was found to be specifically expressed in until the dehiscent pollen stage when anther walls break the tapetum after the tetrads had become individual down to allow pollination. Tapetal cells begin to synthe- microspores (Bih et al. 1999).Alche´ et al. (1999) re-size DNA after the microsporocytes enter meiosis (Hes- ported the localization of the protein Ole e I within the lop-HarrisonandMackenzie 1967). During meiosis pollen wall and in the tapetum. The transcripts are they undergo aberrant divisions producing bi- or
multi-present in both the microspores and the tapetum and nucleate cells. Marked RNA synthesis occurs in the
tape-absent in other tissues. tal cells at all stages of meiosis with a peak at diplotene
In early articles, abnormal divisions of tapetal nuclei (WilliamsandHeslop-Harrison1979;Raghavanet
have been reported in a number of species (Maheswari al. 1992), indicating that long before the tapetum begins
1950), and tapetal ontogeny and its role in pollen matu-to disintegrate it acquires a complex set of specifications
ration at the ultrastructural level has been reviewed by in the form of mRNAs.
Echlin (1973). These investigations suggest that nu-clear aberrations are an intrinsic feature of tapetal cytol-ogy. As a matter of fact, there are a large number of
Corresponding author: Marı´a J. Puertas, Departamento de Gene´tica,
articles reporting that male sterility is associated with Facultad de Biologı´a, Universidad Complutense, 28040 Madrid, Spain.
E-mail: majetas@eucmax.sim.ucm.es disturbances in the regular pathway to tapetal
tions of macerated anthers for in situ hybridization were made tion (Lee et al. 1979; reviewed in Raghavan 1997);
according toZhonget al. (1996).
and certain male sterile mutants show abnormal tapetal
The following repetitive DNA sequences were used in FISH development (Aarts et al. 1997; Jin et al. 1997). Re- as probes:
cently, it has been pointed out that the tapetal
degenera-1. pZmBs, a clone containing the maize B chromosome-spe-tion is not an uncontrolled event, butPapiniet al. (1999)
cific sequence (AlfenitoandBirchler1993), kindly pro-andWanget al. (1999) proposed that it is a process of vided by J. A. Birchler (Columbia, MO). This probe labels
programmed cell death (PCD). the B centromeric regions and, in especially good slides,
In maize, tapetal cells undergo the last mitosis when a small B telomeric region is also labeled.
2. pZm4-21, a clone containing the maize 180-bp knob repeat the microspores are at leptotene-zygotene stage, but cell
(Peacocket al. 1981), kindly provided by J. A. Birchler.
division is not complete since cytokinesis does not take
3. pTa71, a clone containing the rDNA gene unit, the 5.8S, place and the cell becomes binucleate at pachytene. At 18S, and 28S genes and the intergenic spacer from Triticum about the dyad stage, restitution 4n nuclei are common. aestivum (GerlachandBedbrook1979).
The main tapetal RNA synthesis occurs during meiotic
The probes were labeled by nick translation with biotin-prophase, with a further period of accumulation in the
16-dUTP (Boehringer Mannheim, Mannheim, Germany; interval tetrad to young spores. Protein accumulation 1093070000) or digoxigenin-11-dUTP (Boehringer Mann-occurs up to midmeiotic prophase; after this there is a heim 1093088000), using a nick translation kit (Boehringer
Mannheim 976776). pause, followed by further synthesis from meiotic
meta-Slides were incubated with RNase A (1g/ml, Sigma) in phase I to the final dissolution of the tissue (Carniel
2⫻SSC for 1 hr at 37⬚; 1⫻SSC is 0.15mNaCl, 0.015msodium 1961;MossandHeslop-Harrison1967).
citrate. Subsequently the slides were rinsed three times for 5 The present work uses fluorescence in situ hybridiza- min in 2⫻SSC, fixed in 4% paraformaldehyde (Sigma) in tion (FISH) with probes specific to B chromosomes 1⫻SSC for 10 min at room temperature, washed in 2⫻SSC, and then sequentially dehydrated in an ethanol series of 70, (B’s), knobbed chromosomes, and the nucleolar
orga-95, and 100%, 3 min each, and air-dried. Prior to hybridiza-nizer region (NOR), located in chromosome 6, to study
tion, the chromosome preparations were denatured in 70% chromosome nondisjunction and micronucleus forma- (v/v) formamide in 2⫻ SSC at 62⬚
for 1 min, dehydrated tion during the last tapetal mitosis, which gives rise to through an ice-cold ethanol series, and air-dried.
the binucleate tapetal tissue. Both nondisjunction and The hybridization mixture, containing 2 ng/ml of a specific repetitive probe, was denatured by boiling for 10 min, micronuclei are produced by abnormal segregation of
quenched on ice for 7 min, and added to the denatured slides. chromatids at anaphase. Micronuclei are produced by
Hybridization was performed overnight at 37⬚. Posthybridiza-lagging chromatids not included in the poles and non- tion washes of the slides were done with 2⫻
SSC at room disjunction is produced when the chromatids fail to temperature and 1⫻ SSC at 37⬚, both for 30 min. Biotin-divide and both are included in the same pole. Mi- labeled probe detection was performed with avidin conjugated to Cy3 (Amersham PA 43000). Digoxigenlabeled probe in-cronuclei might also be related with nondisjunction
direct detection was performed with mouse anti-digoxigenin when the centromeres do not separate and the
chromo-(Boehringer Mannheim 1333062). The secondary antibody some lags at the plate.
was anti-mouse conjugated to fluorescein isothiocyanate We use maize anthers from plants with and without (Boehringer Mannheim 124616). After detection, the slides B chromosomes belonging to lines selected for high or were washed in detection buffer (4⫻SSC, 0.2% Tween 20) and counterstained with 4⬘,6-diamidino-2-phenylindole (DAPI; low B transmission rate in female 1B⫻male 0B crosses
Boehringer Mannheim 236276). Slides were mounted in Vec-(Rosatoet al. 1996).
tashield (Vector, Burlingame, CA; H 1000).
Hybridization signals were photographed, using an epiflu-orescence Olympus microscope, on Fujicolor Professional 400 MATERIALS AND METHODS
NPH film and the negatives were scanned at 1350 dpi with a Nikon film scanner. The images were optimized for best con-Plants (0B and 1B) of the female high (H) and low (L) B
trast and brightness by means of commercial image-processing transmission rate lines (Rosato et al. 1996) of Pisingallo, a
software. native race of Zea mays from northwest Argentina (Rosatoet
A sample of root tips was studied for control. The roots al. 1998), were used in the present study.
were not subjected to any pretreatment so that we could ob-Root tips were scored for B number, and then the plants
serve normal mitotic anaphases and telophases. FISH in the were grown in an experimental field. Male inflorescences at
root tips was done using the same probes and procedure as meiosis were fixed in 3:1 ethanol:acetic acid and refrigerated
described above for the anthers. at 4⬚ until analyzed. Anther squashes for determining the
convenient meiotic stage were made in 1% acetocarmine. Binucleate tapetal cells were scored in anthers from
diplotene-metaphase I using Feulgen staining. The last tapetal mitosis RESULTS was found in anthers at zygotene.
Binucleate tapetal cells were scored with standard The spreading procedure was chosen to prepare nuclei for
in situ hybridization because no mechanical pressure is applied Feulgen staining for the presence of micronuclei in 0B to distribute the cells on the slides, thus largely preserving and 1B plants of the L and H lines, using at least six the three-dimensional information. For cell wall digestion, anthers per individual. Table 1 shows that the frequency anthers were incubated in 0.3% (w/v) cytohelicase (Sepracor,
of micronuclei in 1B plants was 12.63% in the L line, France), 0.3% (w/v) pectolyase (Sigma, St. Louis; P-3026) and
whereas only 3.92% of the tapetal cells showed mi-0.3% (w/v) cellulase Onozuka RS (Yakult Honsa, Tokyo) in
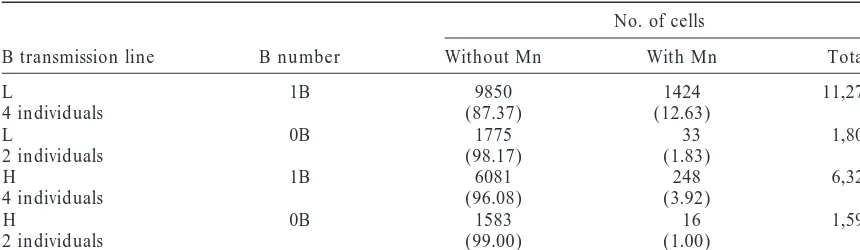
TABLE 1
Binucleate tapetal cells with and without micronucleus in 1B and 0B plants of the L and H lines
No. of cells
B transmission line B number Without Mn With Mn Total
L 1B 9850 1424 11,274
4 individuals (87.37) (12.63)
L 0B 1775 33 1,808
2 individuals (98.17) (1.83)
H 1B 6081 248 6,329
4 individuals (96.08) (3.92)
H 0B 1583 16 1,599
2 individuals (99.00) (1.00)
Percentages are shown in parentheses. Mn, micronucleus.
there are nonsignificant differences between individuals the L line with micronuclei showed the B label in the micronucleus (Figure 1C), indicating that most mi-within lines, but there are significant differences
be-tween lines (F⫽28.85; P⬍ 0.000001) and between 0B cronuclei do not correspond to the B and that A chro-mosomes (A’s) are also unstable in tapetal mitosis (Fig-and 1B plants (F⫽20.08; P⬍0.000001). The interaction
is also significant (F⫽ 24.14; P⬍ 0.000001), indicating ure 1, D and E). Nevertheless, the B’s seem to be more unstable, because if all chromosomes were equally un-that the B dose differently affects the H or L genotype.
Table 1 shows that micronuclei are mainly formed in stable the expected frequency of B micronuclei would be 1/21⫽4.76%, because 21 chromosomes are present; the L line, but not necessarily corresponding to the B’s,
because there are micronuclei in 0B plants. but a frequency of 16.47% is found (Table 2).
A contingency2test was made to test if B
nondisjunc-To test the frequency of micronuclei corresponding
to the B’s, we carried out FISH with the pZmBs probe, tion was related to the formation of A chromosome micronuclei, resulting in significant differences (2 ⫽
specifically labeling 1B plants of both lines. The results
are summarized in Table 2. In this table, cells with and 5.05, 1 d.f., P⬍ 0.025). The deviation is such that the number of cells with B nondisjunction plus A mi-without micronuclei have to be considered separately,
because the cells with micronuclei were preferentially cronuclei is higher than expected and, conversely, the number of cells with B normal disjunction plus A mi-scored to study the distribution of the B label. In most
cells, B labels were normally distributed (Figure 1A), cronuclei is lower than expected. In the H line the number of cells with micronuclei is very low, and no but in a number of cases, two B labels were found in
the same nucleus of the binucleate cell, indicating that calculations can be made.
To determine the nature of the A chromosomes form-B nondisjunction had occurred in the preceding mitosis
(Figure 1B). A contingency 2 test showed that there ing micronuclei we used FISH with the pZmBs and the
pZm4-21 probes, specific to the maize B’s and to the are significant differences between lines (2 ⫽ 13.08,
1 d.f., P⫽ 0.0003), indicating that the frequency of B heterochromatic knobs, respectively.
At root tip mitotic metaphase, it was determined that nondisjunction in the tapetal cells is higher in the L
line. this maize race is polymorphic for the heterochromatic
knobs, with five large and at least three small knobs. In Surprisingly, only 16.47% of the binucleate cells of
TABLE 2
Types of binucleate tapetal cells observed with the pZmBs probe in 1B plants
Types of cells
B Without Mn With Mn
transmission
line Normal B nondisjunction Unlabeled Mn B nondisjunction and Mn Labeled Mn
L 517 297 79 68 29
8 individuals (63.51) (36.49) (44.89) (38.64) (16.47)
H 179 56 1 1
3 individuals (76.17) (23.83) (50.00) (50.00)
Figure1.—(A–E) Localization of the B-specific probe (red) and the knob-specific probe (green) in binucleate tapetal cells. (A) Normal disjunction of the B’s and unequal knob distribution. (B) B nondisjunction and unequal knob distribution. The labels corresponding to the centromere and telomere of the two B chromatids are visible side by side. (C) B in the micronucleus. Equal knob distribution. (D) Normal disjunction of the B’s. Knob in the micronucleus. (E) B nondisjunction. Equal knob distribution. Unlabeled micronucleus. (F and G) Localization of the B-specific probe (red) and the specific probe for chromosome 6 (green) in binucleate tapetal cells. (F) Normal distribution of the B and nondisjunction of chromosome 6. (G) Nondisjunction of both the B and chromosome 6. (H and I) Localization of the B-specific probe (red) and the chromosome 6-specific probe (green) in the last tapetal telophase before binucleate cell formation. (H) B nondisjunction, chromosome 6 label on the bridge. (I) B nondisjunction, normal disjunction of chromosome 6, unlabeled delayed chromatid and bridge. ( J–L) Localization of the knob-specific probe (green) in the last tapetal telophase. ( J) Knob in a delayed chromatid. (K) Unlabeled delayed chromatid. (L) Knob on the bridge.
particular, chromosome 6 shows one small knob on the tion between the two nuclei of the binucleate cell was evident in a number of cells (Figure 1, A and B). This short arm and a DAPI⫹ interstitial band on the long
arm. The NOR is located on the short arm. The large indicates that not only the B, but also the knob carrying chromosomes undergo nondisjunction in the tapetal number of knobs makes it difficult to count the number
TABLE 3
Types of binucleate tapetal cells without micronucleus observed with the pZmBs and the pZm4-21 probes in 1B plants
Types of cells
Normal for the B’s, B nondisjunction, B nondisjunction,
unequal knob equal knob unequal knob
B transmission line Normal distribution distribution distribution
L 87 63 54 45
4 individuals (34.94) (25.30) (21.69) (18.07)
H 71 33 15 19
2 individuals (51.45) (23.91) (10.87) (13.77)
Percentages are shown in parentheses.
Also in this case we scored cells with and without tion. The small number of cells in some classes did not allow determining if the eight classes appeared with micronuclei separately. Table 3 shows the types of cells
without micronuclei observed with both probes in 1B random assortment.
In the H line only five cells with a micronucleus were plants of both lines. All four possible combinations were
found: normal disjunction of B’s and knobs, B disjunc- found, two with B label and three with knob label, indi-cating again that all chromosomes form fewer mi-tion and unequal distribumi-tion of knobs, B
nondisjunc-tion and equal distribunondisjunc-tion of knobs, and B nondis- cronuclei in this line.
Table 4 shows the types of binucleate tapetal cells junction and unequal distribution of knobs. A
contin-gency2test was made between the observed and the observed in 0B and 1B plants using the pTa71 probe,
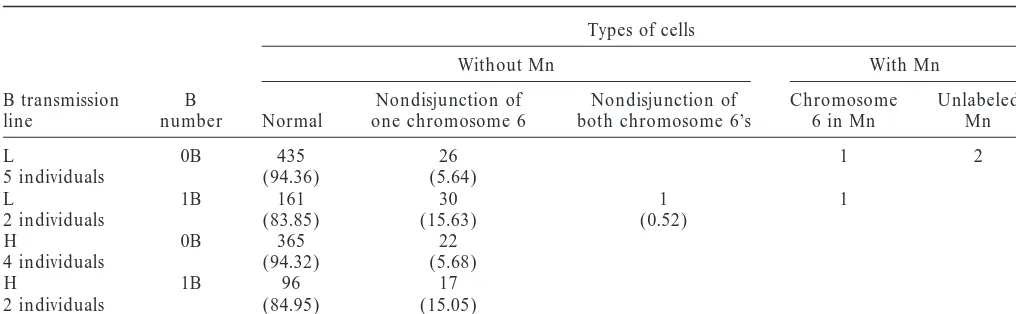
specific to chromosome 6, which is the only maize chro-expected distributions assuming independent
assort-ment, resulting in nonsignificant differences for the L mosome carrying the nucleolar organizing region, lo-cated on the short arm. Cells with nondisjunction of one line (2⫽0.29, 1 d.f., P⫽0.59), whereas in the H line
the distributions significantly differ (2 ⫽ 6.36, 1 d.f., or both chromosome 6’s were observed. Nonsignificant
differences were found between the number of cells P⫽0.01).
B nondisjunction frequency is 0.3976, and the fre- undergoing nondisjunction of chromosome 6 in the L and H lines, irrespective of the presence of B’s (2 ⫽
quency of knob unequal distribution is 0.4337 in the L
line. In the H line B nondisjunction frequency is 0.2464 0.23, 1 d.f., P⫽0.63), but nondisjunction of chromo-some 6 was more frequent in 1B than in 0B plants (2⫽
and the frequency of knob unequal distribution is
0.3768 (Table 3). Since there is only one B and there 28.4, 1 d.f., P⫽0.00001). Cells with micronuclei were observed only in the L line indicating again that this are eight chromosomes with knobs, it seems that the B
tends to undergo nondisjunction with higher frequency line is prone to micronucleus formation.
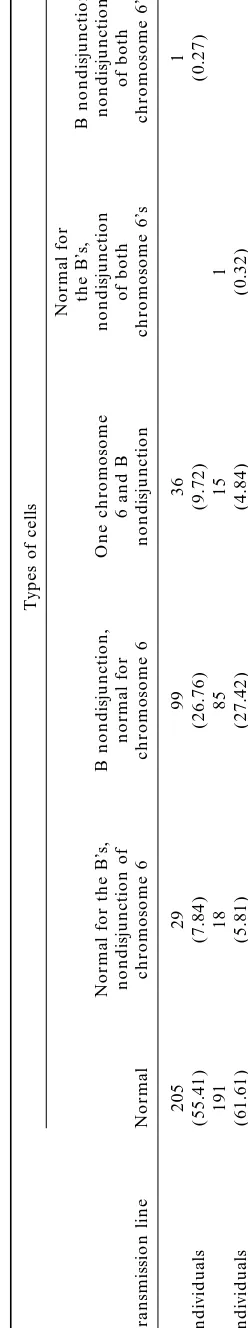
The behavior of the B chromosome and chromosome than knobbed A chromosomes. B nondisjunction
fre-quency is significantly higher in the L line (2 ⫽ 9.0, 6 was studied carrying out FISH with the pZmBs and
the pTa71 probes. The types of binucleate cells without 1 d.f., P⬍0.003), but the frequency of unequal
distribu-tion of knobs is similar in both lines (2⫽1.19, 1 d.f., micronuclei are shown in Table 5 and Figure 1, F and
G. A contingency2test showed that nondisjunction of
P⫽0.28).
The double FISH allowed us to determine that three the B chromosome and nondisjunction of chromosome 6 do not occur as independent events in the L line types of micronuclei can be formed: B micronuclei,
knob micronuclei, and micronuclei without label corre- (2 ⫽12.02, 1 d.f., P⫽ 0.0005). The deviation is such
that more cells are observed than expected when both sponding to A chromosomes or chromosome fragments
lacking knobs (Figure 1, C, D, and E, respectively). In chromosomes undergo normal disjunction or both chromosomes undergo nondisjunction. Conversely, the L line we found 42 binucleate cells with one
micro-nucleus, 6 showing B label (14.28%), 25 showing knob fewer cells are observed than expected when only one chromosome undergoes nondisjunction. However, the label (59.52%), and 11 without label (26.19%). As there
is 1 B chromosome and there are 8 knobbed chromo- contingency 2 test showed that nondisjunction of the
B chromosome and nondisjunction of chromosome 6 somes and 12 chromosomes without knobs, the
proba-bility of forming micronuclei decreases in the order B⬎ occur as independent events in the H line (2⫽ 2.26,
1 d.f., P⫽ 0.09). The frequency of nondisjunction of
knobbed chromosome ⬎ chromosome without knob.
Cells showing the three types of micronuclei were found chromosome 6 is 0.1784 in the L line and 0.1172 in the H line.
in all eight possible combinations between B
TABLE 4
Types of binucleate tapetal cells observed with the pTa71 probe, which labels the NOR in chromosome 6, in 0B and 1B plants
Types of cells
Without Mn With Mn
B transmission B Nondisjunction of Nondisjunction of Chromosome Unlabeled
line number Normal one chromosome 6 both chromosome 6’s 6 in Mn Mn
L 0B 435 26 1 2
5 individuals (94.36) (5.64)
L 1B 161 30 1 1
2 individuals (83.85) (15.63) (0.52)
H 0B 365 22
4 individuals (94.32) (5.68)
H 1B 96 17
2 individuals (84.95) (15.05)
Percentages are shown in parentheses. Mn, micronucleus.
B label (13.64%), 5 showed the chromosome 6 label rate line. This indicates that both the B’s and the geno-type influence A chromosome instability.
(22.73%), and 14 showed no label (63.63%). In the H
Micronucleus formation seems to be more a con-line only 3 cells with a micronucleus were found.
trolled than a random event. First, not all chromosomes The same probes already described were used for
were found forming micronuclei with the same fre-FISH in four anthers of 1B plants of the L line, where
quency, but the B’s form more micronuclei than do the pollen mother cells were at the zygotene stage, to
ob-knobbed A’s, and the ob-knobbed A’s form more micronuclei serve the last tapetal mitosis, just before the binucleate
than do the A’s lacking heterochromatic knobs. Second, cell formation. Bridges and delayed chromatids were
binucleate cells with two micronuclei were not found, commonly observed either labeled or unlabeled (Figure
and they should have been observed according to the 1, H–L). The delayed unlabeled chromatids indicate
frequency of cells with one micronucleus. For example, that A chromosomes without heterochromatic knobs
in 1B plants of the L line the frequency of binucleate may also suffer an abnormal mitosis.
cells with one micronucleus is 0.1263 (Table 1). The To compare the tapetal mitosis with cell division in
random probability of forming two micronuclei is the root meristem, 100 telophases of root tip cells were
(0.1263)2 ⫽ 0.0159; therefore (0.0159 ⫻ 11,274) ⫽
studied as controls in two 1B plants of the L and two
179.84 cells with two micronuclei are expected, but of the H line. All labels studied were normally
distrib-none were observed. uted in 100% of the cases, indicating that
nondisjunc-Alternatively, it is possible that two micronuclei were tion or any type of chromosome instability does not
not observed because both fused in a single restitution occur either for the B or for the A chromosomes in any
micronucleus; however, the probability that this oc-of the lines.
curred in all cases seems to be negligible.
Nondisjunction of B chromosomes is known to occur typically at the second pollen mitosis (Randolph1941; DISCUSSION
Roman 1947;Carlson1986). It has been recently re-The use of FISH with DNA probes specific to B chro- ported to occur also at the first pollen mitosis (Rusche
mosomes, knobbed chromosomes, and chromosome 6 et al. 1997) and in the endosperm (Alfenito and
(NOR) has allowed us to detect that these particular Birchler 1990), but it was not previously reported in chromosomes, and probably all maize chromosomes, other tissues. The B-specific probe allowed us to de-undergo a peculiar behavior in binucleate tapetal cells. scribe this phenomenon in tapetal cells. Similarly, the Nondisjunction of A chromosomes and micronuclei other specific probes detected nondisjunction of the occur in tapetal cells of plants with the normal 0B chro- knobbed A chromosomes including chromosome 6 mosome complement, indicating that these aberrations (NOR). This is also a new report of A chromosome are regular events in the process of anther maturation, nondisjunction in tapetal cells.
But the most remarkable result is that nondisjunction of A chromosomes is significantly increased from 5 to 15% by the presence of B chromosomes (Table 4), al-though it is not influenced by the genotype H or L. Our hypothesis is that there is a basal level of a trans-acting substance producing nondisjunction in tapetal cells, whose concentration and/or effect is increased threefold by B chromosomes. The results shown in Ta-bles 3 and 5 support this hypothesis because B and knobbed chromosome nondisjunction or B’s and chro-mosome 6 nondisjunction are not independent events. The observed number of binucleate cells where both chromosomes behave normally or both undergo nondis-junction is higher than expected. In addition, we have observed more cells with B nondisjunction and A micro-nucleus than expected if these were independent events (Table 2), indicating again that B chromosomes induce a general instability in the A’s.
Rhoades et al. (1967) and Rhoades and Dempsey
(1973) carried out elegant genetic experiments where they followed the pattern of loss for specific marker genes affecting aleurone color or endosperm develop-ment. They demonstrated that B chromosomes caused elimination of chromatin from knob-bearing members of the A set in aleurone cells. They hypothesized that the B’s suppressed the replication of the heterochromatic knobs at the second microspore mitosis thus producing dissimilar sperm cells.
The results reported in the present article indicate that A chromosome instability induced by B chromo-somes in the tapetum is, in all probability, a phenome-non related to knob elimination in the aleurone. Inter-estingly, both the tapetum and the aleurone are tissues playing nutritive roles essential to the pollen and em-bryo, respectively. Both tissues degenerate after their nutritive role is accomplished. Aleurone cells form a secretory tissue that releases hydrolases to digest the endosperm and nourish the embryo; they are unneces-sary for postembryonic development and die as soon as germination is complete following PCD (Kuoet al. 1996; Wanget al. 1996;PennellandLamb1997).
There are a large number of articles demonstrating that tapetal aberrant mitosis, ploidy changes, and degen-eracy are essential events for the normal maturation of pollen grains. However, only recently it has been pointed out that the process of tapetal degeneracy is actually a process of PCD (Papini et al. 1999;Wanget al. 1999) with the cellular remnants necessary for pollen development acting as secretion products.
Our results support the view that chromosome insta-bility is a regular event occurring during tapetal degen-eracy, which might be one of the first steps in the PCD process, first, because the aberrations occur as con-trolled events in normal 0B plants and are influenced by the genotype and, second, because of the similarity between our observations and those of Rhoadeset al.
TA BL E 5 Types o f b inucleate tapetal cells without micronucleus o bserved with the pZmBs and the pTa71 probes in 1B plants Types o f cells Normal for the B ’s, B nondisjunction, Normal for the B ’s, B nondisjunction, One chromosome nondisjunction nondisjunction nondisjunction o f normal for 6 a nd B o f both o f both B transmission line Normal chromosome 6 chromosome 6 nondisjunction chromosome 6’s chromosome 6’s L 2 05 29 99 36 1 4 individuals (55.41) (7.84) (26.76) (9.72) (0.27) H 1 91 18 85 15 1 4 individuals (61.61) (5.81) (27.42) (4.84) (0.32) Percentages are shown in parentheses.
(1967) in the aleurone.
Alfen-itoandBirchler1990), but in the archesporial mitosis
589–597.
they become unstable, increasing A chromosome insta- Bih, F. Y., S. S. H. Wu, C. Ratnayake, L. L. Walling, E. A. Nothnagel
et al., 1999 The predominant protein on the surface of maize bilities. It is possible that the onset of PCD produces B
pollen is an endoxylanase synthesized by a tapetum mRNA with nondisjunction, which, irrespective of its influence on A
a long 5⬘leader. J. Biol. Chem. 274: 22884–22894.
chromosomes, is an essential feature of B chromosomes Carlson, W. R.,1986 The B chromosome of maize. Crit. Rev. Plant Sci. 3: 201–226.
when it occurs at pollen grain mitosis, because it is
Carniel, K.,1961 Das Antherentapetum von Zea mays. Oesterr. Bot. necessary for their own transmission and persistence in
Z. 108: 89–96.
populations. Echlin, P.,1973 The role of the tapetum during microsporogenesis
of Angiosperms, pp. 41–61 in Pollen, Development and Physiology, B nondisjunction, and chromatin elimination from
edited byJ. Heslop-Harrison.Butterworths, London.
knobbed A chromosomes induced by the B’s, has been
Gerlach, W. L.,andJ. R. Bedbrook,1979 Cloning and characteriza-related to the suppression of heterochromatin replica- tion of ribosomal RNA genes from wheat and barley. Nucleic
Acids Res. 7: 1869–1885. tion at second pollen division (RhoadesandDempsey
Heslop-Harrison, J.,andA. Mackenzie,1967 Autoradiography of 1972, 1973), although direct evidence of nondisjunction soluble [2-14C] thymidine derivatives during meiosis and mi-induced by lack of replication has never been obtained. crosporogenesis in Lilium anthers. J. Cell Sci. 2: 387–400.
Huang, J. C., F. C. ChangandC. S. Wang,1997 Characterization On the contrary,AlfenitoandBirchler(1990), using
of a lily tapetal transcript that shares sequence similarity with a markers on B-A translocations, reported replicative non- class of intranuclear pathogenesis-related (IPR) proteins. Plant disjunction of B chromosomes in the endosperm. Mol. Biol. 34: 681–686.
Jin, W., H. T. HornerandR. G. Palmer,1997 Genetics and cytology Our results support the view that nondisjunction does
of a new genic male-sterile soybean [Glycine max (L) Merr]. Sex. not result from faulty replication of chromosomes or Plant Reprod. 10: 13–21.
chromosome segments, because the number of fluores- Kirpes, C. C., L. G. Clark andN. R. Lersten,1996 Systematic significance of pollen arrangement in microsporangia of Poaceae cent labels in tapetal telophase or binucleate cells always
and Cyperaceae: review and observations on representative taxa. correspond to that expected if all chromosomes were Am. J. Bot. 83: 1609–1622.
fully replicated (Figure 1). Particularly, in the case of B Kuo, A., S. Capelluti, M. Cervantes-Cervantes, M. Rodrı´guezand
D. S. Bush,1996 Okadaic acid, a protein phosphatase inhibitor, chromosomes, replicative nondisjunction seems evident
blocks calcium changes, gene expression, and cell death induced since the B-specific probe labels both chromosome by gibberellin in wheat aleurone cells. Plant Cell 8: 259–269. ends. Micronuclei carrying fluorescent labels also seem Lee, S. L. J., V. E. GracenandE. D. Earle,1979 The cytology of
pollen abortion in C-cytoplasmic male-sterility corn anthers. Am. to correspond to full chromatids and not to
chromo-J. Bot. 66: 656–667.
some fragments, because unlabeled chromatin is always Maheswari, P.,1950 An Introduction to the Embryology of Angiosperms. present in addition to the label in the micronucleus. McGraw-Hill, New York.
Moss, G. I.,andJ. Heslop-Harrison,1967 A cytochemical study Similarly, abnormal segregation at tapetal telophase
of DNA, RNA and protein in the developing maize anther. Ann. produced delayed chromatids and not small fragments. Bot. 31: 555–574.
Pacini, E.,1997 Tapetum character states: analytical keys for tape-Tapetal tissue has an important role in developing
tum types and activities. Can. J. Bot. 75: 1448–1459. anthers of angiosperms. In a further work we will analyze
Papini, A., S. Mosti and L. Brighigna, 1999 Programmed-cell-whether the abnormalities in the tapetum due to the death events during tapetum development of angiosperms.
Proto-plasma 207: 213–221. B’s have any influence on pollen viability. It is
interest-Peacock, W. J., E. S. Dennis, M. M. RhoadesandA. Pryor,1981 ing that the L line, which tends to lose the B’s, is that
Highly repeated DNA sequence limited to knob heterochromatin producing more abnormalities. This may influence the in maize. Proc. Natl. Acad. Sci. USA 78: 4490–4494.
Pennell, R. I.,andC. Lamb,1997 Programmed cell death in plants. B polymorphism at the population level since those
Plant Cell 9: 1157–1168. plants transmitting more B’s seem to induce less
instabil-Raghavan, V.,1997 Molecular Embryology of Flowering Plants.
Cam-ity in the normal A chromosomes. bridge University Press, Cambridge.
Raghavan, V., C. JiangandR. Bimal,1992 Cell- and tissue-specific This work was supported by grant PB 98-0678 of the DGICYT of
expression of rice histone gene transcripts during anther and
Spain. Mauricio Chiavarino is a postdoctoral grant holder of the pollen development in henbane (Hyoscyamus niger). Am. J. Bot.
Fundacio´n Antorchas (Argentina) and Marcela Rosato is a postdoc- 77:778–783.
toral grant holder of the CONICET (Argentina). Randolph, L. F.,1941 Genetic characteristics of the B chromosomes
in maize. Genetics 26: 608–631.
Rhoades, M. M.,andE. Dempsey,1972 On the mechanism of chro-matin loss induced by the B chromosome of maize. Genetics 71: 73–96.
LITERATURE CITED
Rhoades, M. M., andE. Dempsey, 1973 Chromatin elimination
Aarts, M. G. M., R. Hodge, K. Kalantidis, D. Florack, Z. A. Wilson induced by the B chromosome of maize. I. Mechanism of loss
et al., 1997 The Arabidopsis MALE STERILITY 2 protein shares and the pattern of endosperm variegation. J. Hered. 64: 13–18.
similarity with reductases in elongation/condensation com- Rhoades, M. M., E. DempseyandA. Ghidoni,1967 Chromosome
plexes. Plant J. 12: 615–623. elimination in maize induced by supernumerary B chromosomes.
Alche´, J. D. D., A. J. Castro, A. Olmedilla, M. D. C. Ferna´ndez, Proc. Natl. Acad. Sci. USA 57: 1626–1632.
R. Rodrı´guezet al., 1999 The major olive pollen allergen (Ole Roman, H.,1947 Mitotic nondisjunction in the case of interchanges
e I) shows both gametophytic and sporophytic expression during involving the B-type chromosome in maize. Genetics 32: 391–409.
anther development, and its synthesis and storage takes place in Rosato, M., A. M. Chiavarino, C. A. Naranjo, M. J. Puertasand
the RER. J. Cell Sci. 112: 2501–2509. L. Poggio,1996 Genetic control of B chromosome transmission
Alfenito, M. R.,andJ. A. Birchler,1990 Studies on B chromosome rate in Zea mays ssp. mays (Poaceae). Am. J. Bot. 83: 1107–1112.
stability during development. Maydica 35: 359–366. Rosato, M., A. M. Chiavarino, C. A. Naranjo, J. Ca´mara
Herna´n-dezandL. Poggio,1998 Genome size and numerical
characteriza-phism for the B chromosome in races of maize (Zea mays ssp. Williams, E. G.,andJ. Heslop-Harrison,1979 A comparison of
mays, Poaceae). Am. J. Bot. 85: 168–174. RNA synthetic activity in the plasmodial and secretory types of
Ruiter, R. K., G. J. VanEldik, R. M. A. VanHerpen, J. A. M. Schrau- tapetum during the meiotic interval. Phytomorphology 29: 370–
wenandG. J. Wullems,1997 Characterization of oleosins in 381.
the pollen coat of Brassica oleracea. Plant Cell 9: 1621–1631. Yokoi, S., T. Tsuchiya, K. ToriyamaandK. Hinata,1997
Tapetum-Rusche, M. L., H. L. Mogensen, L. Shi, P. Keim, M. Rougieret al., specific expression of the Osg6B promoter-beta-glucuronidase
1997 B chromosome behavior in maize pollen as determined gene in transgenic rice. Plant Cell Rep. 16: 363–367.
by a molecular probe. Genetics 147: 1915–1921. Zhong, X., P. F. Fransz, J. Wennekes Van Eden, P. Zabel, A. Van
Wang, H., J. Li, R. M. BostockandD. G. Gilchrist,1996 Apopto- Kammen et al., 1996 High-resolution mapping on pachytene
sis: a functional paradigm for programmed plant cell death in- chromosomes and extended DNA fibres by fluorescence in-situ
duced by a host-selective phytotoxin and invoked during develop- hybridisation. Plant Mol. Biol. Rep. 14: 232–242.
ment. Plant Cell 8: 375–391.
Wang, M., S. Hoekstra, S. Van Bergen, G. E. M. Lamers, B. J. Communicating editor:J. A. Birchler Oppedijket al., 1999 Apoptosis in developing anthers and the
role of ABA in this process during androgenesis in Hordeum