Dominant Alleles of the Basic Helix-Loop-Helix Transcription Factor ATR2
Activate Stress-Responsive Genes in Arabidopsis
Gromoslaw A. Smolen, Laura Pawlowski, Sharon E. Wilensky and Judith Bender
1Department of Biochemistry and Molecular Biology, Johns Hopkins University, Bloomberg School of Public Health, Baltimore, Maryland 21205 Manuscript received February 15, 2002
Accepted for publication April 4, 2002
ABSTRACT
Members of the R/B basic helix-loop-helix (bHLH) family of plant transcription factors are involved in a variety of growth and differentiation processes. We isolated a dominant mutation in an R/B-related bHLH transcription factor in the course of studying Arabidopsis tryptophan pathway regulation. This mutant,atr2D, displayed increased expression of several tryptophan genes as well as a subset of other stress-responsive genes. Theatr2Dmutation creates an aspartate to asparagine change at a position that is highly conserved in R/B factors. Substitutions of other residues with uncharged side chains at this position also conferred dominant phenotypes. Moreover, overexpression of mutantatr2D, but not wild-typeATR2, conferred pleiotropic effects, including reduced size, dark pigmentation, and sterility. There-fore,atr2Dis likely to be an altered-function allele that identifies a key regulatory site in the R/B factor coding sequence. Double-mutant analysis withatr1D, an overexpression allele of the ATR1Myb factor previously isolated in tryptophan regulation screens, showed thatatr2Dandatr1Dhave additive effects on tryptophan regulation and are likely to act through distinct mechanisms to activate tryptophan genes. The dominantatrmutations thus provide tools for altering tryptophan metabolism in plants.
P
LANTS are capable of integrating a wide range of bHLH factor acts along with the GLABRA1 (GL1) Myb-tissue, developmental, and environmental signals to related factor, plus a WD-repeat-containing factor achieve a complex pattern of gene expression. Immedi- TRANSPARENT TESTA GLABRA1 (TTG1), to promote ate effectors of this spatial and temporal regulation in- the formation of trichomes (leaf hairs; Oppenheimer clude members of the R/B-related basic helix-loop-helix et al.1991;Walkeret al.1999;Payneet al.2000). Fur-(bHLH) family. The R and B factors are two highly thermore, in vitro studies have implicated the Arabi-related maize proteins that control transcription of pig- dopsis RD22BP1 bHLH factor and the ATMYB2 Myb ment biosynthesis genes. These factors are the founding factor in activating a drought-regulated generd22(Abe members of a large class of plant proteins characterized et al.1997). The emerging view is that various bHLH/ by conserved sequences near the amino terminus in- Myb heterodimer combinations serve as transcriptional cluding an acidic domain plus a bHLH DNA binding/ activators for a number of plant biosynthetic and devel-dimerization domain near the carboxy terminus (Puru- opmental genes, with WD-repeat factors perhaps per-ggananandWessler 1994). R/B bHLH family mem- forming a modifying or stabilizing function (Molet al. bers have been shown to regulate such diverse processes 1998;Payneet al.2000;Speltet al.2000).as cell shape determination, tissue-specific pigment ac- We recovered a dominant mutation,atr2D, in an R/B-cumulation, and adaptation to the environment. related factor in the course of studying tryptophan path-Extensive genetic and biochemical studies have shown way regulation in Arabidopsis. Theatr2Dmutation confers that maize R and B factors require a Myb cotranscription constitutively activated expression of several tryptophan factor, C1 or Pl, to activate target gene expression lead- pathway genes, as well as perturbed levels of other stress-ing to purple anthocyanin pigmentation (reviewed in responsive genes. The mutation changes an aspartic acid Molet al.1998). Genetic studies have revealed a more to an asparagine at a position near the amino terminus complex system in petunia, where the AN1 bHLH factor of the predicted ATR2 protein sequence that is highly plus the AN2 Myb factor and a WD-repeat factor AN11 conserved in related plant transcription factors. Muta-are all required for floral pigmentation (de Vettenet tion of this conserved residue to other amino acids with al.1997; Mol et al. 1998; Quattrocchio et al. 1999; uncharged side chains also resulted in similar dominant Speltet al.2000). In Arabidopsis, the GLABRA3 (GL3) phenotypes. Overexpression of the atr2D mutant, but
not wild-type ATR2, resulted in pleiotropic phenotypes including dark pigmentation and sterility. This result 1Corresponding author:Department of Biochemistry and Molecular
suggests thatatr2Dis an altered-function allele and that
Biology, Johns Hopkins University, Bloomberg School of Public
ATR2is not normally involved in tryptophan and other
Health, 615 N. Wolfe St., Baltimore, MD 21205.
E-mail: jbender@mail.jhmi.edu. stress-responsive gene regulation. The conservation of
atr2D to the MZA15 bacterial artificial chromosome clone. aspartic acid at theatr2Dposition suggests that mutation
MZ2L, at the centromere-proximal end, was a 1088-bp frag-of the residue to asparagine might generate activated
ment PCR amplified with the primers MZ2LF 5⬘-CTCC alleles of other R/B family members. Possible mecha- GATCGCTTCTTAATCC-3⬘and MZ2LR 5⬘-CAATTATGGGC nisms for how this single amino acid change can gener- CTGTAGTAC-3⬘. The Col product cleaves withTaqI into frag-ments of 157, 169, 242, and 520 bp whereas the Lerproduct ate activated stress phenotypes are discussed.
cleaves with TaqI into fragments of 157, 411, and 520 bp. Previously, we characterized another dominant atr
MZ2R, at the centromere-distal end, was a 1190-bp fragment mutation,atr1D, which was found to encode an
overex-PCR amplified with the primers MZ2RF 5⬘-TTGCTTCCATG pression allele of a Myb transcription factor (Benderand GAAGGTCTC-3⬘ and MZ2RR 5⬘-TCCCACCACAGAGGTGT Fink 1998). The atr1D plants upregulated the expres- TCG-3⬘. The Col product cleaves withAluI into 810- and 380-sion of primary tryptophan biosynthetic genes (Bender bp fragments whereas the Lerproduct is uncleaved.
BclI cleaves at sites flanking theATR2gene to yield a 12-andFink1998) as well as secondary tryptophan
biosyn-kb fragment. A library was constructed fromBclI-digestedatr2D thetic genes (Smolen and Bender 2002). The ATR1
mutant genomic DNA by ligating into BamHI-cut DASH Myb transcription factor is likely to be an endogenous (Stratagene, La Jolla, CA) arms and packaging with Gigapack regulator of the tryptophan pathway, because increased II Gold (Stratagene)in vitropackaging extracts. The library ATR1 steady-state message levels correlate with trypto- was probed with an ATR2 cDNA probe to identify clones carrying theatr2Dfragment. A wild-type genomicATR2clone phan pathway activation in a recessiveatrmutant,atr4
was obtained by hybridization screening of a wild-type Col (SmolenandBender2002). Theatr4complementation
genomiclibrary provided by J. Mulligan and R. Davis (Stan-group consists of loss-of-function mutations in the
cyto-ford University). A full-length expressed sequence tag (EST) chrome P450 gene CYP83B1. The elevatedATR1 mes- clone ofATR2was obtained from the Arabidopsis Biological sage levels in cyp83B1 seedlings are thought to result Resource Center at Ohio State University.
Plant transformation: The isogenic genomic ATR2 and from perturbations in multiple signaling pathways
con-atr2Dconstructs consisted of 6.0-kbSacI to EcoRV fragments verging onATR1gene expression.
cloned into theSacI toSmaI sites of the pBIN19 vector (Bevan Given the precedent for Myb and bHLH factors to
1984). To make the35S-ATR2and35S-atr2Dconstructs, site-act in combination, we examined whether the ATR1 directed mutagenesis (Kunkelet al.1987) was performed on Myb and the atr2D-activated mutant bHLH might inter- a genomic clone of each allele (1) to create aBamHI site at act to control tryptophan gene expression. Using a vari- 8–13 bp upstream of the translational start codon, removing the upstream out-of-frame ATG, and (2) to create aBamHI ety of approaches, we found that ATR1 and atr2D act
site at a position corresponding to the 3⬘ end of the EST independently of each other to activate the tryptophan
cDNA sequence, 296–301 bp downstream of the translational pathway. In particular, the atr1D atr2Ddouble mutant stop codon. The resulting 2.09-kbBamHI fragment of each displays additive activation of several tryptophan pri- allele was then cloned into theBamHI site of vector pCaMV35S mary and secondary metabolism genes. Therefore, the (Hullet al. 2000). Clones were transformed into wild-type Col (ATR2) plants by anAgrobacterium tumefaciens-mediatedin dominantatrmutations provide novel tools for the study
plantamethod (CloughandBent1998). Primary T1 trans-and metabolic engineering of tryptophan primary trans-and
formants were scored for transgene copy number by preparing secondary biosynthetic pathways.
genomic DNA from a single leaf and performing Southern blot analysis with digests and probes that could distinguish the endogenousATR2gene from the transgene signal. Lines MATERIALS AND METHODS
that displayed a transgene band intensity one-half that of the endogenous locus were identified as putative single-copy iso-atr2Dmutant isolation:Columbia (Col) M2 seeds (ⵑ50,000)
lates. Copy number was confirmed by 3:1 segregation of the generated as described (Niyogiet al.1993) by EMS
mutagene-kanamycin resistance transgene marker in the next generation sis were screened for resistance to 5-methyl-tryptophan (5MT)
and by additional Southern blot analysis on homozygous prog-by plating surface-sterilized seeds on 150⫻25-mm petri plates
eny lines. containing 100 ml of minimal plant nutrient sucrose (PNS)
Mutagenesis ofATR2codon 94:The 6.0-kbSacI-EcoRVATR2 medium (HaughnandSomerville1986) supplemented with
genomic fragment in a pBlueScript KS II⫹(Stratagene) vector 15m5MT (solubilized in 100 mmNaOH). Plates were sealed
was subjected to site-directed mutagenesis (Kunkelet al.1987) with Parafilm and were incubated at 22⬚ under continuous
to alter the GAT aspartic acid codon 94 to AAT asparagine, illumination with yellow long-pass filters as previously
de-GAA glutamic acid, CAA glutamine, TCG serine, or GCT ala-scribed (BenderandFink1998). Seedlings were monitored
nine. To facilitate screening, each mutagenesis at codon 94 was between 7 and 10 days postgermination for root growth. The
designed to also create a nearby novelAvrII site by alteration of same conditions were used to test T2 and T3 generation
trans-codon 90 CTC leucine to CTA leucine. Mutant variants of genic plants and the panel of dominant tryptophan mutants
ATR2were confirmed by sequencing and then subcloned into for 5MT resistance, except that seeds were plated on 100⫻
pBIN19 and transformed into wild-type Col plants as described 100-mm square petri plates containing 50 ml of medium, and
above. the plates were screened with clear glass rather than with
Yeast two-hybrid assays:TheATR1,ATR2, andatr2Dsegments yellow long-pass filters.
tested in yeast two-hybrid analysis were amplified by PCR and
Positional cloning of theatr2Dlocus:Theatr2Dmutation
subcloned asSalI toNotI fragments into the vectors pDBLeu was mapped to the lower arm of chromosome 5 near the
and pEXP-AD502 from the ProQuest kit (Life Technologies). DFR marker using standard cleaved amplified polymorphic
Full-lengthATR1was amplified using ATR1F (5⬘-GTCGACC sequence (CAPS) analysis (Koniecznyand Ausubel1993).
ATGGTGAGGACACCATGTTGC-3⬘) and ATR1R1 (5⬘-GCGG Analysis with additional standard Colvs.Landsbergerecta(Ler)
CCGCTCAGACAAAGACTCCAACCATATTG-3⬘). Truncated polymorphic markers (http://www.arabidopsis.org) localized
ATR1(1–122) was amplified using ATR1F and ATR1R2 (5⬘-GCG the mutation several centimorgans centromere-distal to DFR.
TABLE 1
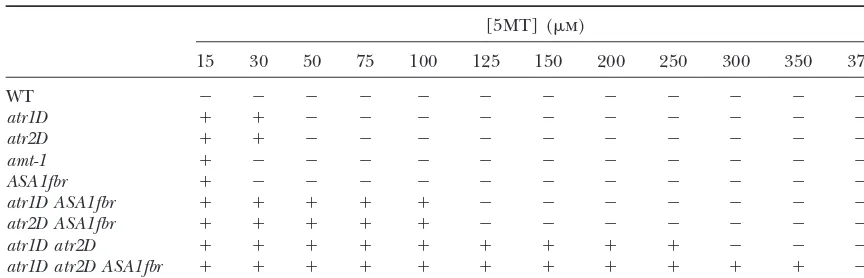
5MT resistance of tryptophan regulation multiple mutants
[5MT] (m)
15 30 50 75 100 125 150 200 250 300 350 375
WT ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺
atr1D ⫹ ⫹ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺
atr2D ⫹ ⫹ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺
amt-1 ⫹ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺
ASA1fbr ⫹ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺
atr1D ASA1fbr ⫹ ⫹ ⫹ ⫹ ⫹ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺
atr2D ASA1fbr ⫹ ⫹ ⫹ ⫹ ⫹ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺
atr1D atr2D ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫺ ⫺ ⫺
atr1D atr2D ASA1fbr ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫺
Seedlings of the indicated genotypes were germinated on agar medium containing the indicated concentra-tions of 5MT in micromolar. A⫹symbol indicates an elongated root phenotype, and a⫺symbol indicates no root elongation.
cDNA isolated from a Lerstrain seedling library (Minetet al. CTATCTTCTGGCAG-3⬘and atr2DR 5⬘-CTCTTTATCTTCCT CTCCTTTGTAGTAACCAG-3⬘, where the underlined base is 1992) was used as template for bothATR1amplifications.
Full-lengthATR2 andatr2D were amplified with ATR2F (5⬘-GTC mismatched with the genomic sequence. This primer set am-plifies a 110-bp fragment. ForATR2template DNA, the frag-GACCATGAACGGCACAACATCATCAATC-3⬘) and ATR2R1
(5⬘-GCGGCCGCTCAATAGTTTTCTCCGACTTTCGTC-3⬘). ment cleaves withAluI into 78- and 32-bp products, whereas foratr2D template DNA the fragment does not cleave with TruncatedATR2andatr2D(1–371) were amplified with ATR2F
and ATR2R2 (5⬘-GCGGCCGCTCATGATCTAACCACAGTAC AluI.
RNA gel blot analysis:Total RNA was prepared from whole TAAACG-3⬘). Since the ATR2locus is encoded by a single
exon, the genomic clones of ATR2and atr2Dwere used as seedlings grown aseptically on PNS medium for 10 days post-germination before RNA extraction or from the indicated amplification templates.
All two-hybrid analyses were done in the yeast strain MaV230 adult plant tissues sources in Figure 2B. RNA gel blot analysis was performed by formaldehyde gel electrophoresis, transfer according to a protocol provided with the ProQuest system.
The interactions were assessed by scoring three independent to nylon membranes, and hybridization with radiolabeled probes as previously described (Melquistet al.1999). Probes reporter genes (HIS3,URA3, andlacZ). All interactions tested
yielded internally consistent results between the three reporter were full-length or nearly full-length cDNA fragments forATR1 (At5g60890),ATR2(At5g46760),ASA1(At5g05730),ASA2 (At2g-genes.
Expression of fusion proteins was monitored by immu- 29690), ASB1 (At1g25220), CYP79B2 (At4g39950), CYP83B1 (At4g31500),CHS(At5g13930),PDF1.2(At5g44420), andTUB4 noblot analysis using antibodies against the Gal4 DNA-binding
domain (SC-510, Santa Cruz) or the Gal4 transcription activa- (At5g44340) and partial cDNA fragments forTSB1(At5g54810), PR1(At2g19990),LOX2(At3g45140), andCYP79B3(At2g22330). tion domain (SC-1663, Santa Cruz).
Dominant tryptophan mutant plant strains:The S115F mu- A-tubulin (TUB) cDNA probe was used as a control to correct for loading differences. Band intensities were quantitated us-tation in the ASA1fbrtransgene was constructed using
site-directed mutagenesis (Kunkelet al.1987) on a genomic clone ing a Fuji Phosphoimager and MACBAS 2.2 software. Results were reproduced in two or three independent experiments. ofASA1extending fromⵑ4.8 kb upstream of the start codon
to 5.7 kb downstream of the stop codon. This mutant genomic Assessment of mutant plant morphologies:The morpholog-ical consequences of dominant tryptophan regulatory muta-clone was then submuta-cloned into theSalI site of the plant
trans-formation vector pBIN19 (Bevan1984). The transgene was tions were scored by germinating seedlings in potting medium (Scott’s Metromix 360) and growing them under continuous introduced into wild-type Columbia plants (CloughandBent
1998), and a transgenic line that displayed 3:1 segregation of illumination atⵑ22⬚. The altered morphologies of theatr1D atr2Ddouble mutant and theatr1D atr2D ASA1fbrtriple mutant the kanamycin resistance transgene marker plus heritable
5MT resistance was selected for further analysis. Southern blot were consistently observed in several independent experi-ments.
assays indicated that this line contains a four-copy array of the
ASA1fbrtransgene. Soluble tryptophan measurements:For soluble tryptophan
measurements, seedlings were grown aseptically on PNS me-The double- and triple-mutant strains combining atr1D,
atr2D, and ASA1fbrwere made by screening F2 progeny of dium for 10 days postgermination. The extraction procedure was performed as previously described (LiandLast1996). crosses for resistance to 50m5MT, a concentration at which
the parental single mutants are sensitive (Table 1). Candidates Tryptophan measurements were performed by the Biore-source Center of Cornell University (Ithaca, NY) using pre-from this screen were subsequently tested for their genotypes
using molecular markers. Theatr1Dgenotype was confirmed viously published HPLC methods (HeinriksonandMeredith 1984). Three independently prepared samples were analyzed by a PCR-based assay for a restriction site polymorphism
cre-ated by theatr1Dmutation (BenderandFink1998). TheASA1fbr in parallel for each strain tested. genotype was confirmed by scoring transgene kanamycin
resis-tance. Theatr2Dmutation was confirmed by a PCR-based assay
that converts the base change into a restriction site change RESULTS by combining it with a nearby mismatch in a PCR primer,
Isolation and characterization of the atr2Dmutant:
which results in PCR amplification of a novel DNA species (Neff
de-Figure1.—Theatr2Dmutation confers dominant 5MT re-sistance. Seedlings of wild-type Col (ATR2/ATR2), a Colatr2D
Figure2.—Gene expression patterns in ATR2 andatr2D homozygote (atr2D/atr2D), a Colatr2D/ATR2F1heterozygote
plants. (A) Replicate gel blots of seedling RNAs were probed made by crossing Colatr2Das the male with wild-type Col as
with cDNA fragments of the indicated genes. TheTUBprobe the female, a representative homozygous T3 transgenic line
was used to normalize for differences in loading. (B) RNA of Col transformed with a single-copy wild-typeATR2genomic
samples prepared from leaves (leaf), flowers and buds (flower), clone,ATR2/ATR2(ATR2/ATR2), and a representative
homo-or green siliques (silique) of adult wild-type Col plants grown zygous T3 transgenic line of Col transformed with a
single-in soil, plus 10-day-postgermsingle-ination aseptically grown whole copy mutantatr2Dgenomic clone,ATR2/ATR2(atr2D/atr2D),
Col seedlings (seedling), were used for gel blot analysis with are shown after being grown on PNS medium containing 15
anATR2probe. The ethidium bromide (EtBr)-stained gel is
m5MT for 7 days postgermination.
shown as a loading control. (C) Wild-type Col seedlings were grown aseptically on PNS medium under glass plates for 10 days postgermination and then transferred to liquid PNS me-scribed screen for altered tryptophan regulation (atr) dium for a 6-hr induction before RNA extraction. The follow-Arabidopsis mutants resistant to the toxic tryptophan ing concentrations of inducers were used: 20mmethyl jasmo-nate (MeJA), 20mabscisic acid (ABA), 500msalicylic acid analog, 5MT (BenderandFink1998). The screen
ex-(SA), 20mIAA, 20m6-benzylaminopurine (BAP), 20m ploits the feedback inhibition of anthranilate synthase
gibberellic acid A3(GA), 20m1-aminocyclopropane 1-car-(AS), the first enzyme of the pathway, by the end prod- boxylic acid (ACC), and 1 mbrassinolide (BR). The RNA uct of the primary pathway, tryptophan. Feedback inhi- samples were analyzed by gel blot with anATR2probe. bition limits the flow through the pathway under
condi-tions of abundant tryptophan. 5MT acts by triggering
feedback inhibition of AS activity without substituting played no obvious morphological alterations at any stage of development.
for the nutritional role of tryptophan. Mutants that are
resistant to 5MT will thus include plants with feedback To determine whether the 5MT resistance phenotype of the atr2D mutant involved increased expression of resistance mutations in AS catalytic subunit genes (Niyogi
1993;Krepset al.1996;LiandLast1996) and mutants tryptophan genes, RNA was prepared from aseptically grown whole seedlings of wild-typeATR2 vs. atr2Dand with increased expression of AS and other tryptophan
metabolism genes (Bender and Fink 1998; Smolen analyzed by gel blot with tryptophan gene probes (Fig-ure 2A). The probes tested included those detecting andBender2002). To isolate such resistant mutants,
EMS-mutagenized M2 seeds of the Col strain were plated on two AS catalytic ␣-subunit-encoding genes ASA1 and ASA2 (Niyogi and Fink 1992); a probe that cross-agar medium containing 15m5MT, and seedlings were
scored at 10–14 days for root growth. Under these condi- hybridizes to three highly related AS glutamine amido-transferase-subunit-encoding genesASB1,ASB2, and tions, wild-type seedlings have strongly inhibited root
development whereasatrmutants have unimpaired root ASB3 (Niyogiet al. 1993); and a probe detecting the tryptophan synthase -subunit-encoding gene TSB1 growth (Figure 1). The 5MT resistance phenotype of the
atr2D mutant isolated from this screen was found to (Berlynet al. 1989). This analysis revealed that steady-state transcript levels of theASA1gene, theASBgenes, be dominant in F1heterozygous plants (Figure 1) and
segregated at 75% (533 resistant out of 709 total seed- and theTSB1gene were elevated inatr2Dplants. These tryptophan genes have been shown to be induced by a lings scored) in an F2population from a backcross to
dis-ogiet al. 1993;ZhaoandLast1996;Zhaoet al. 1998; Standard procedures were used to map the mutation to the lower arm of chromosome 5. Theatr2Dmutant Reymondet al. 2000), although the stress responsiveness
of the individualASB genes has not been determined was then crossed to a polymorphic strain carrying visible marker mutations on the upper and lower ends of (Niyogiet al.1993). In contrast, the steady-state levels
of the ASA2 gene, which was previously found to be chromsome 5 flanking theatr2Dlocus (Landsbergerecta ttg yi). The visible marker mutations were used to iden-unresponsive to stress induction, were not affected by
theatr2Dmutation. Because this pattern suggested that tify 502 F2 progeny that had crossed over between the markers and thus had recombination break points stress-inducible genes might be generally upregulated
in theatr2Dmutant background, we tested several other aroundatr2D. The recombinants with break points clos-est to theatr2D5MT resistance phenotype defined the known stress gene probes in this assay, including the
flavonoid compound synthesis gene chalcone synthase minimal atr2D locus as being contained between the ends of a cloned and sequenced 79,995-bp bacterial (CHS;FeinbaumandAusubel1988), the salicylic
acid-responsive genePR1 (Penninckx et al. 1996), methyl artificial chromosome MZA15 (GenBank accession no. AB016882).
jasmonate-responsive genes PDF1.2 (Penninckx et al.
1996) andLOX2(BellandMullet1993), and theATR1 Inspection of the MZA15 sequence revealed that it contained an open reading frame for a bHLH transcrip-Myb gene, which can be either up- or downregulated
in response to various signaling molecules (Smolenand tion factor. This gene was an attractive candidate for the atr2Dtryptophan gene andCHS-activating locus because Bender2002). This analysis showed thatCHS,PDF1.2,
andLOX2were upregulated in theatr2Dmutant, while the relatedRgene in maize is known to encode a tran-scriptional activator of phenylalanine-derived flavonoid PR1 was downregulated and ATR1 was not affected.
Note that althoughPR1expression is not normally de- compound biosynthetic genes such as CHS (reviewed inMolet al.1998). We confirmed that the bHLH open tectable by RNA gel blot in adult plant tissues (Clarke
et al. 2000), it does have a detectable basal level of reading frame is in fact the ATR2 gene, using three approaches. First, we found that a polymorphic PstI expression in seedlings grown under our aseptic
condi-tions (SmolenandBender2002). Collectively, this sam- restriction enzyme site in the central coding sequence of this gene cosegregated perfectly with the mutant phe-pling of probes revealed that a subset of stress response
genes as well as inducible tryptophan genes are activated notype in the mapping population. Second, we cloned and sequenced the bHLH gene from theatr2Dmutant byatr2D.
The previously isolated atr1D mutation conferred a vs.wild-typeATR2and found a single G to A transition mutation in the gene that creates an aspartic acid to seedling phenotype diagnostic of resistance to high
lev-els of exogenous tryptophan (BenderandFink1998). asparagine change in the predicted amino acid se-quence at codon 94 (D94N). Third, we transformed This phenotype assay uses the blue fluorescenttrp1-100
mutant, which carries a missense mutation in thePAT1 isogenic 6.0-kb genomic regions containing the tran-scription factor gene but no other open reading frames gene encoding the second enzyme of the tryptophan
pathway (Roseet al.1992, 1997). The blue fluorescence from either wild-type ATR2oratr2D into the wild-type Col genome. The inserts in these transgene constructs oftrp1-100results from the accumulation of anthranilate
in a sugar-conjugated form, particularly in the cotyle- were completely sequenced and found to differ only at the single base that creates the D94N mutation. All of dons of seedlings. When thetrp1-100mutant is grown
on agar medium containing high levels of exogenous 36 transformants of the wild-type clone ATR2(ATR2) were sensitive to 5MT, whereas 25 of 27 transformants tryptophan, the cotyledon blue fluorescence is
sup-pressed, presumably due to feedback inhibition of AS of the mutant cloneATR2(atr2D)were resistant to 5MT (Figure 1). The two exceptionalATR2(atr2D)lines that activity and reduced production of anthranilate (Bender
andFink1998). However, in thetrp1-100 atr1Ddouble were sensitive to 5MT were also sensitive to the trans-gene selectable marker kanamycin in the second trans- gener-mutant, cotyledons remain fluorescent when grown on
high tryptophan medium. To determine whetheratr2D ation after transformation and contained multiple cyto-sine methylated copies of the transgene, suggesting that behaves similarly toatr1Din this assay, thetrp1-100 atr2D
double mutant was constructed and tested for fluores- these lines carry silenced transgene arrays. The transfor-mation experiment thus demonstrated that the atr2D cence on high tryptophan medium. Unlike thetrp1-100
atr1Ddouble mutant, thetrp1-100 atr2Ddouble mutant locus lies in the 6.0-kb clone and that the activated tryptophan phenotype is conferred by the D94N muta-displayed suppressed cotyledon fluorescence similarly
to thetrp1-100 parental strain (data not shown). This tion.
Overexpression of atr2D but not ATR2 confers
pleio-result suggests thatatr1Dandatr2Dperturb tryptophan
metabolism in different tissues and/or via different tropic effects:During the construction of theATR2(ATR2) and ATR2(atr2D)transgenic lines we found that all of mechanisms.
Positional cloning of theatr2Dmutation:To under- theATR2(ATR2)lines were morphologically identical to untransformed Columbia plants regardless of transgene stand the molecular basis of the atr2D mutation, we
from a LercDNA library (Minetet al.1992) by hybridiza-tion. In both cases, the transcribed region contained an upstream out-of-frame ATG sequence just 8 bp up-stream of the correct ATR2 translational start codon, which could impair optimal translation of the gene. RNA gel blot analysis of ATR2 expression revealed that the gene is well expressed in seedlings, adult leaves, and flowers, with reduced expression in green siliques (Fig-ure 2B). ATR2 steady-state transcript levels were not altered in theatr2Dmutant (Figure 2A), indicating that the activatedatr2Dallele does not affect its own expres-sion. TheATR2gene displayed only modest changes in steady-state message levels when wild-type seedlings were subjected to exogenous treatment with a variety of plant signaling molecules, in contrast to the more dramatic transcriptional regulation of theATR1 Myb-Figure 3.—ATR2 and atr2D overexpression phenotypes.
Representative 5-week-old single-copy primary transformant encoding gene previously detected under these condi-hemizygous T1 plants ofATR2/ATR2(35S-ATR2/o)(left,35S- tions (SmolenandBender2002).
ATR2) and ATR2/ATR2(35S-atr2D/o) (right, 35S-atr2D) are
Database comparisons showed that theATR2gene is shown.
related to a group of plant bHLH transcription factor genes (Figure 4). These genes are characterized by con-served sequences including an acidic region near the ATR2(atr2D)lines had increased purple pigmentation,
reduced size, and reduced fertility. In the most extreme amino terminus plus a basic helix-loop-helix domain near the carboxy terminus (PuruggananandWessler cases, ATR2(atr2D) multiple-insert plants were
com-pletely sterile. These observations suggested that overex- 1994). The codon 94 aspartic acid residue in theATR2 gene occurs in a highly conserved sequence motif. Of pression ofatr2D, but not wild-typeATR2, perturbs gene
expression in a way that leads to 5MT resistance, in- 11 closely related cDNA and genomic sequences identi-fied in the Arabidopsis nucleic acid sequence database creased pigmentation, and morphological changes.
To explicitly test the effects of overexpression, we using a BLAST search with the full-length ATR2 amino acid sequence as a query, 9 sequences also carried an made additional transgenic lines in which theATR2or
atr2D D94N coding sequence was expressed from the aspartic acid at the analogous position (Figure 4B). Moreover, the majority of related cDNA sequences cloned strong constitutive cauliflower mosaic virus 35S promoter
(35S-ATR2and 35S-atr2D). In the wild-type Columbia from other plant species carried an aspartic acid at the analogous position. One additional Arabidopsis geno-(ATR2) background, three independent35S-ATR2
sin-gle-copy primary transformants were phenotypically nor- mic sequence, a maize cDNA, and twoPerilla frutescens cDNAs carried the conservative amino acid substitution mal, whereas four independent single-copy 35S-atr2D
primary transformants were small, bushy, darkly pig- of a glutamic acid at the analogous position. However, one exceptional Arabidopsis sequence, the TT8 gene mented, and completely sterile (Figure 3).
Second-gen-erationATR2(35S-ATR2) lines were found by RNA gel that controls pigment production in the seed coat, car-ried an asparagine at the analogous position (Nesi et blot analysis to have approximately fivefold elevated
steady-state transcript levels ofATR2, but the plants were al.2000). The TT8 protein has relatively low sequence identity with ATR2 and lacks a large block of amino not resistant to 5MT and did not display upregulation
of ASA1 expression (data not shown). The sterility of acids between the conserved acidic and bHLH domains. Thus, it is difficult to predict whether the activated atr2D the ATR2(35S-atr2D) lines precluded detailed analysis.
However, these results confirm that overexpression of D94N factor might act in part by mimicking the effects of TT8 over a broader expression range.
the wild-type ATR2 factor is not sufficient to alter 5MT
resistance or morphology, whereas overexpression of Several mutations atATR2 codon 94 yieldatr2D-like phenotypes:The GAT aspartic acid to AAT asparagine the atr2D mutant factor has pleiotropic consequences,
presumably due to enhanced activation of target gene atr2D mutation was the only change predicted to be recovered at codon 94 ofATR2using EMS mutagenesis, expression.
Theatr2Dmutation alters a highly conserved amino which primarily generates C to T and G to A transitions. Therefore, an interesting question was whether
acti-acid position in a related group of plant transcription
factors:TheATR2gene consists of a single exon encod- vatedatr2D-like alleles ofATR2could be generated by alteration of codon 94 to other amino acids. To address ing a predicted 592-amino-acid polypeptide. A full-length
Columbia cDNA isolate of this gene was available in this question we explicitly mutagenizedATR2codon 94 to several other amino acids including glutamic acid, the Arabidopsis EST collection (GenBank accession no.
se-For each of these constructs the highest-copy-number lines were bushy, darkly pigmented, and had reduced fertility similarly to theatr2D94N overexpression lines. However, all five D94E lines tested were 5MT sensitive and all of these lines were morphologically normal. This result implies that the D94E mutation has a neutral effect, although it is also possible that the mutation renders the protein inactive. Taken together, the muta-genesis experiments suggest that loss of a negatively charged residue at codon 94 of ATR2 yields an altered-function atr2D protein.
The atr2DbHLH mutant factor and the ATR1 Myb factor do not interact in a yeast two-hybrid assay: Be-cause several characterized plant bHLH factors act in combination with partner Myb proteins (see Introduc-tion), we investigated whether the atr2D bHLH might act together with the ATR1 Myb factor previously identi-fied inatrmutant screens (BenderandFink1998). As one approach to this question, we tested for physical interactions using a yeast two-hybrid system. This ap-proach has previously been used to demonstrate interac-tions between the maize R/B proteins and C1 and the Arabidopsis GL3 and GL1 bHLH/Myb combinations (Goff et al.1992;Grotewoldet al.2000;Payneet al. 2000). These studies showed that the regions critical for interaction lie in the amino-terminal regions of both Figure4.—Theatr2Dmutation affects a conserved
amino-partner proteins. Thus, for our studies, we tested full-terminal motif in members of the R/B bHLH factor family. (A)
length proteins and amino-terminal (NT) constructs of R/B bHLH factor conserved structural domains. A diagram of
ATR1, ATR2, and atr2D as DNA-binding fusion “baits” a generic R/B bHLH factor protein structure is shown. N
indicates the amino terminus, C indicates the carboxy termi- or activation-domain fusion “preys.” We found that full-nus, and the solid box indicates theatr2D-proximal region with length ATR1, and full-length and NT ATR2 or atr2D the asterisk indicating the position of the mutated residue. (B) constructs, were all self-activating when used as baits. Sequence alignment of theatr2Dmutant region, shown as the
However, the ATR1 NT construct was not self-activating solid box in A, with homologs from Arabidopsis and other
as a bait, and the ATR2 and atr2D constructs were not plant species. Arabidopsis bHLH-predicted amino acid
se-quences are shown in the top group and other plant species self-activating as preys. Immunoblot detection of fusion are shown in the bottom group. All aligned bHLH proteins protein expression for these constructs revealed that are indicated by their GenBank protein accession numbers.
protein products of the predicted sizes were expressed Except for protein BAA97217, the Arabidopsis bHLH genes
for the ATR1 NT bait and the ATR2 and atr2D NT either have been assigned a function by mutation (GL3 and
preys. However, the full-length ATR2 and atr2D preys TT8) or have been cloned as cDNAs and/or ESTs. The
bHLH-predicted amino acid sequences from other plant species are were not detectably expressed. Therefore, the ATR1 NT all based on cDNA sequences. An asterisk above the sequences bait construct was used together with ATR2 NT or atr2D indicates the position of the atr2D mutation. Pv indicates
NT prey constructs to assess activation of three different Phaseolus vulgaris(bean), Zm indicates Zea mays(corn), Os
reporter genes—lacZ, HIS3, andURA3. Neither of the indicatesOryza sativa(rice), Pf indicatesPerilla frutescens, Am
combinations tested showed activation of any of the indicatesAntirrhinum majus(snapdragon), Ph indicates
Petu-nia hybrida, and Gh indicatesGerbera hybrida. reporter genes (data not shown), suggesting that nei-ther the ATR1 NT/ATR2 NT pair nor the ATR1 NT/ atr2D NT pair has a significant physical interaction. lected because it is found at the analogous position Theatr2Dandatr1Dmutations have additive effects
Figure5.—5MT resistance in tryptophan regulation multi- Figure6.—Tryptophan gene expression in tryptophan reg-ple mutants. Representative seedlings of the indicated geno- ulation multiple mutants. RNA samples prepared from seed-types are shown after being grown on PNS medium supple- lings of the indicated genotypes were subjected to RNA gel mented with either (A) 15m5MT or (B) 300m5MT for blot analysis with the indicated probes.
7 days postgermination.
5MT resistance in double- and triple-mutant plants, we performed RNA gel blot analysis of steady-state tran-(ASA1fbr) as double or triple mutants. The ASA1fbr
transgene carries a genomic clone of ASA1 with the script levels for various tryptophan metabolism genes. Because theASA1fbrtransgenic strain carried four extra feedback resistance mutation S115F. This mutation was
predicted to confer feedback resistance on the basis of copies of theASA1gene at an ectopic locus (materials and methods), we expected that this strain would dis-the phenotype of an analogous mutation in dis-the
con-served position of the Salmonella AS␣-subunit-encod- play elevated ASA1 transcript levels. ASA1 expression was indeed increased approximately sevenfold in the ing genetrpE(CaligiuriandBauerle1991). TheASA1
S115F mutation was previously engineered into a 35S- ASA1fbrstrain (Figure 6). In double-mutant combina-tion strains,ASA1expression was upregulated to a higher ASA1cDNA transgene and found to confer 5MT
resis-tance in transgenic Arabidopsis (Niyogi1993). Trans- degree than in the parental single mutants. For exam-ple, theatr2D ASA1fbrstrain had strongly elevatedASA1 genic plant extracts contained feedback-resistant AS
activity inin vitroassays and hadⵑ1.5-fold elevated levels levels (Figure 6). This activation is presumably due to the transcriptional activation effect ofatr2Don the four of soluble tryptophan. For comparison, we also assayed
the genetically isolated feedback-resistantASA1mutant ASA1fbrtransgene sequences as well as on the endoge-nous ASA1gene. Surprisingly, theatr1D atr2D ASA1fbr amt-1, which carries a D341N missense mutation (Kreps
et al.1996). triple mutant had reducedASA1transcript levels com-pared to the two atr ASA1fbr double mutants. The re-In root growth assays for 5MT resistance, the
double-and triple-mutant strains displayed root resistance strong- duced expression in the triple mutant might reflect a downregulation mechanism that is activated when ei-er than that of the single mutants (Table 1). At 300m
5MT, only the triple atr1D atr2D ASA1fbr mutant had ther ASA1 transcripts or tryptophan pathway metabo-lites exceed a threshold level (see discussion). The resistant root growth (Figure 5, Table 1). These results
show that the individual mutations confer additive ef- tryptophan synthase geneTSB1 was upregulated in all strains carrying theatr2Dmutation, without significant fects on the 5MT resistance phenotype.
Figure7.—Adult plant morphologies of tryptophan regula-tion multiple mutants. Representative 4-week-old plants of the indicated genotypes are shown. Other strains used in this study were morphologically indistinguishable from the wild-type Col (WT) control.
mutations. Similar results were obtained forASB(data not shown).
Two Arabidopsis cytochrome P450 enzymes,CYP79B2 and CYP79B3, catalyze the conversion of tryptophan into indole-3-acetaldoxime (Hullet al.2000). Because overexpression ofCYP79B2leads to modest 5MT resis-tance, we investigated theCYP79B2andCYP79B3
steady-state message levels in the panel of dominant mutant Figure8.—Soluble tryptophan levels in tryptophan regula-strains. Both genes were upregulated in all the strains tion multiple mutants. Soluble tryptophan levels were mea-sured in triplicate samples for seedlings of the indicated geno-carrying theatr1Dmutation (Figure 6). Both genes were
types. also slightly upregulated in the atr2D mutant
back-ground. In theatr1D atr2Ddouble-mutant and the triple-mutant backgrounds, there was an additive effect of the
by the ASA1fbr-induced increased flow of metabolites twoatrmutations onCYP79B2andCYP79B3expression.
through the tryptophan pathway. The cytochrome P450 enzyme CYP83B1 acts on the
Taken together, these analyses suggest thatatr1Dand indole-3-acetaldoxime product of the
CYP79B2/B3-cat-atr2Dhave distinct effects on tryptophan gene regula-alyzed reactions to convert it into 1-aci
-nitro-2-indolyl-tion, implying independent mechanisms of gene activa-ethane, a precursor for indole glucosinolate synthesis
tion. The tryptophan gene expression profiles suggest (Baket al. 2001; Hansen et al. 2001). As previously
re-that in the triple mutant, the strong upregulation of pri-ported (SmolenandBender2002), theCYP83B1gene
mary pathway genes (ASA1,ASB, andTSB1), strong up-was upregulated in atr1D mutant backgrounds.
How-regulation of secondary pathway genes (CYP79B2 and ever, there was no significant additional activation via
CYP79B3), and a feedback resistance allele ofASA1 com-atr2D or ASA1fbr. Moreover, it is unlikely that the
bine to uniquely deregulate the pathway and confer CYP83B1gene product contributes significantly to 5MT
strong 5MT resistance and altered morphology. resistance, because loss-of-function alleles in the gene
Soluble tryptophan levels are perturbed in dominant
are 5MT resistant (Smolen and Bender 2002). This
tryptophan regulation mutants:Feedback resistance mu-resistance is presumably due to perturbations in
expres-tations in ArabidopsisASA1increase soluble tryptophan sion levels of other tryptophan genes in the cyp83B1
levels (Niyogi1993;Krepset al.1996;LiandLast1996), background.
but theatrmutations had not been previously character-Beyond the altered patterns of 5MT resistance and
ized by this assay. We therefore measured soluble trypto-tryptophan gene expression, the atr1D atr2D
double-phan levels in our complete panel of mutant plants mutant and theatr1D atr2D ASA1fbrtriple-mutant strains
(Figure 8). This analysis showed that only the feedback had distinct adult plant (4-week-old) phenotypes
(Fig-resistanceASA1alleles conferred increased soluble tryp-ure 7). The atr1D atr2D double mutants displayed a
tophan levels relative to wild-type plants: ⵑ3-fold ele-modest reduction in size and flowered a few days earlier
vated foramt-1andⵑ1.5-fold elevated forASA1fbr. These than did wild-type plants. Theatr1D atr2D ASA1fbrtriple
levels are similar to those previously reported for each mutants displayed more severe defects, including
strong-feedback resistance mutation (Niyogi1993;LiandLast ly reduced size and a bushy morphology. None of the
1996). In contrast, theatr1D andatr2Dsingle mutants other single or double mutants in the panel displayed
both displayed reduced soluble tryptophan levels rela-any obvious morphological effects (data not shown).
tive to wild-type plants. This reduction might reflect the These results suggest that the morphological transition
cy-tochrome P450 genes CYP79B2 andCYP79B3 (Figure lies (Figure 4). However, the activatedatr2Dphenotypes could also occur through any of several other mecha-6) and/or activation of other as-yet-unidentified
trypto-phan metabolism genes. Especially in the case ofatr2D, nisms, including alterations in protein stability, nuclear localization, or DNA binding for the mutant atr2D fac-it seems likely that other tryptophan secondary
metabo-lism genes are activated because the slight upregulation torvs.wild-type ATR2. Furthermore, because theatr2D mutation stimulates expression of a number of stress-of CYP79B2 andCYP79B3 does not correlate with the
depletion of soluble tryptophan levels, especially in light inducible genes such asCHSandPDF1.2in addition to inducible tryptophan biosynthetic genes (Figure 2), the of the coordinate upregulation of primary pathway genes
(Figures 2A and 6). When theatrmutations were com- mutant phenotypes could occur indirectly due to a gen-eral stress response caused by the altered form of the bined withASA1fbr, wild-type or higher levels of soluble
tryptophan were observed. This pattern suggests that protein rather than by direct interaction ofatr2D with target genes. A specific possibility is that theatr2D muta-ASA1fbris able to counteract the tryptophan-depleting
effects of theatrmutations. tion creates a dominant negative protein that interferes with the normal function of ATR2 or other bHLH fac-We also assayed the panel of mutant plants for
pertur-bations in the levels of two other tryptophan pathway tors, thus indirectly causing stress-response phenotypes. More extensive genetic and molecular analysis ofatr2D metabolites. First, we performed a thin layer
chromatog-raphy assay on seedlings for the presence of the patho- should allow us to distinguish among these scenarios. For example, an interesting question is whether the anal-gen defense compound camalexin (ZhaoandLast1996;
Zhaoet al. 1998). Neither wild-type nor mutant plants ogous mutation inATR2-related Arabidopsis bHLH genes will yield a similar profile of stress-responsive gene acti-displayed any detectable camalexin by this assay,
indicat-ing that none of the mutant combinations deregulates vation as observed in atr2D. This result would suggest that the effect of the dominant mutation is to make a camalexin synthesis. Second, we inspected the panel of
mutant plants for blue fluorescence under ultraviolet general structural change in bHLH proteins that pro-vokes an activated stress response in the host plant cell. light diagnostic of accumulation of anthranilate, the
first compound in the tryptophan pathway. None of the Our explicit mutagenesis ofATR2codon 94 revealed that changes of the aspartic acid to asparagine, gluta-mutants was fluorescent, indicating that there is not a
significant accumulation of anthranilate in these strains. mine, serine, or alanine result in dominant transgene constructs that confer 5MT resistance to wild-typeATR2 plants. In contrast, conversion of the codon to glutamic DISCUSSION
acid results in a construct that does not confer 5MT re-sistance or any other obvious phenotypic effects. These By screening for mutants with deregulated tryptophan
pathway phenotypes, we have identified a novel domi- results suggest that an uncharged amino acid side chain at codon 94 yields an activated atr2D-like factor whereas nant allele of the Arabidopsis ATR2 bHLH transcription
factor. This factor is a member of a family of plant tran- a negatively charged amino acid side chain at codon 94 (either aspartic acid or glutamic acid) yields a version scriptional activators that share structural homology
with the maize R and B factors. The R/B group is charac- of ATR2 with normal functions. Because overexpression of wild-typeATR2is not sufficient to activate 5MT resis-terized by a conserved amino-terminal region with an
acidic patch and the bHLH domain located near the car- tance or other atr2D phenotypes (Figure 3), it seems unlikely that tryptophan gene regulation is the normal boxy terminus of the protein (Puruggananand
Wes-sler1994). Significantly, the region where the activat- role of this protein. However, we are currently pursuing isolation of an atr2 loss-of-function allele to clarify ing mutation occurs in ATR2 is highly conserved in
other members of the group, with the majority of factors whether wild-type ATR2 has any involvement in trypto-phan and other stress-responsive gene regulation. having an aspartic acid residue and a minority having
a glutamic acid residue at this position (Figure 4). Several lines of evidence argue thatatr1D, a Myb over-expression allele previously isolated in the atr genetic Therefore, similarly to the atr2D allele, altering the
charge at this position could generate activated alleles screen (BenderandFink 1998), and theatr2DbHLH allele activate the tryptophan pathway through distinct of other R/B family members.
Given the precedent for R/B-related factors to work mechanisms. First, atr1D was originally recovered by screening for a cotyledon phenotype diagnostic of resis-together with Myb factors (see Introduction), it is
possi-ble that ATR2 and atr2D have partner Myb proteins. tance to high levels of exogenous tryptophan (Bender andFink 1998), butatr2Ddoes not confer this pheno-Thus, the activated phenotypes in the atr2D mutant
could be conferred through altered atr2D protein/pro- type. Second, ATR1 does not display detectable interac-tion with either wild-type ATR2 or mutant atr2D in a tein interactions with partner Mybs. Consistent with this
possibility, the Myb interaction domains of GL3, R, and yeast two-hybrid assay. Third, the atr1D atr2D double mutant displays additive 5MT resistance (Figure 5, Table B bHLH factors have been mapped to amino-terminal
ure 7), suggesting that each single mutation confers its 79B2, CYP79B3, and CYP83B1 (Hull et al. 2000; Bak et al.2001;Hansenet al.2001). The doubleatr1D atr2D effects independently of the other locus. The ability
of each dominant atr mutation to enhance the 5MT mutant displays significant upregulation of these three genes, as well as upregulation of key genes in the pri-resistance ofASA1fbr suggests that the feedback
resis-tance of this allele is incomplete, as is also suggested by mary tryptophan pathway (Figure 6). These patterns of gene expression suggest that flux into secondary meta-the lower accumulation of soluble tryptophan that this
allele confers relative to the amt-1 allele (Figure 8; bolic pathways might be significantly perturbed in this strain. Consistent with this view, when a tryptophan feed-Niyogi1993;LiandLast1996). It is also possible that
5MT has toxic effects beyond feedback inhibition of AS back-resistant allele ofASA1, which further increases flux through the primary tryptophan pathway, is combined that are ameliorated by theatrmutations. For example,
5MT might be misincorporated into proteins, and the with the atr1D and atr2D mutations in a triple-mutant strain, this strain displays a unique altered morphology stimulation of tryptophan secondary metabolism
en-zymes that directly deplete 5MT levels might limit this (Figure 7). The altered morphology could result from increased production of IAA, increased production of misincorporation. However, studies in both yeast and
plants suggest that this compound is not significantly indole glucosinolates, and/or increased production of other tryptophan pathway metabolites. Genetic suppres-used in protein synthesis (Miozzariet al. 1977; Sasse
et al. 1983). sor analysis of this morphological transition could iden-tify key biosynthetic genes responsible for the effect. When used in combination, the dominant atr
muta-tions allow deregulation of multiple tryptophan primary Furthermore, metabolic labeling studies with the triple-mutant strain could elucidate the major routes of sec-and secondary metabolism genes (Figure 6). These two
dominant mutations thus provide a much more facile ondary metabolism that are deregulated in this back-ground. The triple dominant mutant strain therefore means of manipulating the tryptophan pathway than
does the alternative strategy of overexpressing multiple serves as a novel tool for studies of tryptophan secondary metabolism in plants.
biosynthetic genes from separate transgene constructs.
Another advantage of theatrmutations as tryptophan We thank the Arabidopsis Biological Resource Center for the Ler metabolism tools is that they activate tryptophan gene ttg yimapping strain and theATR2EST cDNA clone, Dr. Chris Town for seeds of theamt-1strain, and Dr. Krishna Niyogi for a genomic
expression from native promoters, so that gene products
clone ofASA1and reagents for generating the S115F mutation. We
are expressed in appropriate tissues. Although theatr
-also thank Lisa Bartee, Juan Quiel, Christine Prater, and Julie Blum
activated expression of tryptophan genes is not as high
for technical assistance. This material is based upon work supported
as the expression levels that could be achieved by expres- by the National Science Foundation under grant IBN 9723172 to J.B. sion from a strong viral promoter such as 35S, this is
not necessarily a disadvantage because extremely high
levels of expression can sometimes cause RNA silencing LITERATURE CITED (reviewed inVanceand Vaucheret2001), defeating
Abe, H., K. Yamaguchi-Shinozaki, T. Urao, T. Iwasaki, D.
Hoso-the purpose of an overexpression construct as a means kawaet al., 1997 Role of Arabidopsis MYB and MYC homologs
in drought- and abscisic acid-regulated gene expression. Plant
of metabolic engineering. In fact, the downregulation
Cell9:1859–1868.
of ASA1 expression we observed in the triple-mutant
Bak, S., F. E. Tax, K. A. Feldmann, D. W. GalbraithandR.
Feyerei-atr1D atr2D ASA1fbrstrain (Figure 6) could be due to sen, 2001 CYP83B1, a cytochrome P450 at the metabolic branch
point in auxin and indole glucosinolate biosynthesis in
Arabi-this strain expressingASA1at high enough levels to
pro-dopsis. Plant Cell13:101–111.
voke weakASA1-directed RNA silencing. Alternatively,
Bell, E., andJ. E. Mullet, 1993 Characterization of anArabidopsis
accumulation of a tryptophan-derived metabolite could lipoxygenase gene responsive to methyl jasmonate and wounding.
Plant Physiol.103:1133–1137.
trigger downregulation ofASA1inatr1D atr2D ASA1fbr
Bender, J., andG. R. Fink, 1998 A Myb homologue, ATR1, activates
plants. This downregulation ofASA1could represent a
tryptophan gene expression inArabidopsis.Proc. Natl. Acad. Sci.
natural threshold for tryptophan pathway activation. USA95:5655–5660.
Berlyn, M. B., R. L. LastandG. R. Fink, 1989 A gene encoding
Tryptophan secondary metabolism in plants provides
the tryptophan synthase beta subunit ofArabidopsis thaliana.Proc.
a number of important agricultural and medicinal
com-Natl. Acad. Sci. USA86:4604–4608.
pounds including the growth regulator indole acetic Bevan, M., 1984 BinaryAgrobacteriumvectors for plant
transforma-tion. Nucleic Acids Res.12:8711–8721.
acid (IAA), the defense compound DIMBOA in maize
Caligiuri, M. G., and R. Bauerle, 1991 Identification of amino
(Frey et al. 1997), indole glucosinolate defense
com-acid residues involved in feedback regulation of the anthranilate
pounds in Brassicas (Chapple et al. 1994), and anti- synthase complex fromSalmonella typhimurium. Evidence for an
amino-terminal regulatory site. J. Biol. Chem.266:8328–8335.
cancer alkaloids vincristine and vinblastine in
Catharan-Chapple, C. C. S., B. W. Shirley, M. Zook, R. Hammerschmidtand
thus roseus(Kutchan1995). There is thus considerable
S. Somervilee, 1994 Secondary metabolism inArabidopsis, pp.
interest in understanding and manipulating the biosyn- 989–1030 in Arabidopsis, edited by E. Meyerowitzand C. R.
Somerville. Cold Spring Harbor Laboratory Press, Cold Spring
thetic enzymes that convert tryptophan into secondary
Harbor, NY.
compounds. Recent genetic studies in Arabidopsis have
Clarke, J. D., S. M. Volko, H. Ledford, F. M. AusubelandX. Dong,
identified some of the components of these secondary 2000 Roles of salicylic acid, jasmonic acid, and ethylene incpr
-induced resistance in Arabidopsis. Plant Cell12:2175–2190.
CYP-Clough, S. J., andA. F. Bent, 1998 Floral dip: a simplified method 2000 TheTT8gene encodes a basic helix-loop-helix domain protein required for expression ofDFRandBANgenes in Arabi-forAgrobacterium-mediated transformation ofArabidopsis thaliana.
dopsis siliques. Plant Cell12:1863–1878. Plant J.16:735–743.
Niyogi, K. K., 1993 Molecular and genetic analysis of anthranilate
de Vetten, N., F. Quattrocchio, J. MolandR. Koes, 1997 The
synthase inArabidopsis thaliana, pp. 97–144, Ph.D. Thesis,
Depart-an11locus controlling flower pigmentation in petunia encodes
ment of Biology, Massachusetts Institute of Technology, Cam-a novel WD-repeCam-at protein conserved in yeCam-ast, plCam-ants, Cam-and Cam-animCam-als.
bridge, MA. Genes Dev.11:1422–1434.
Niyogi, K. K., andG. R. Fink, 1992 Two anthranilate synthase genes
Feinbaum, R. L., andF. M. Ausubel, 1988 Transcriptional
regula-in Arabidopsis: defense-related regulation of the tryptophan path-tion of theArabidopsis thalianachalcone synthase gene. Mol. Cell.
way. Plant Cell4:721–733. Biol.8:1985–1992.
Niyogi, K. K., R. L. Last, G. R. FinkandB. Keith, 1993 Suppressors
Frey, M., P. Chomet, E. Glawischnig, C. Stettner, S. Grunet al.,
oftrp1fluorescence identify a new Arabidopsis gene,TRP4, en-1997 Analysis of a chemical plant defense mechanism in grasses.
coding the anthranilate synthasesubunit. Plant Cell5:1011– Science277:696–699.
1027.
Goff, S. A., K. C. ConeandV. L. Chandler, 1992 Functional analysis
Oppenheimer, D. G., P. L. Herman, S. Sivakumaran, J. Eschand
of the transcriptional activator encoded by the maize B gene:
M. D. Marks, 1991 Amybgene required for leaf trichome
differ-evidence for a direct functional interaction between two classes
entiation in Arabidopsis is expressed in stipules. Cell67:483–493. of regulatory proteins. Genes Dev.6:864–875.
Payne, C. T., F. ZhangandA. M. Lloyd, 2000 GL3encodes a bHLH
Grotewold, E., M. B. Sainz, L. Tagliani, J. M. Hernandez, B.
protein that regulates trichome development in Arabidopsis
Bowen et al., 2000 Identification of the residues in the Myb
through interaction with GL1 and TTG1. Genetics156:1349– domain of maize C1 that specify the interaction with the bHLH
1362. cofactor R. Proc. Natl. Acad. Sci. USA97:13579–13584.
Penninckx, I. A., K. Eggermont, F. R. Terras, B. P. Thomma, G. W.
Hansen, C. H., L. Du, P. Naur, C. E. Olsen, K. B. Axelsenet al.,
De Samblanxet al., 1996 Pathogen-induced systemic activation
2001 CYP83B1 is the oxime-metabolizing enzyme in the
glucosi-of a plant defensin gene in Arabidopsis follows a salicylic acid-nolate pathway inArabidopsis.J. Biol. Chem.276:24790–24796. independent pathway. Plant Cell8:2309–2323.
Haughn, G. W., and C. Somerville, 1986 Sulfonylurea-resistant Purugganan, M. D., andS. R. Wessler, 1994 Molecular evolution
mutants inArabidopsis thaliana.Mol. Gen. Genet.204:430–434. of the plantRregulatory gene family. Genetics138:849–854.
Heinrikson, R. L., andS. C. Meredith, 1984 Amino acid analysis Quattrocchio, F., J. Wing, K. van der Woude, E. Souer, N. de
by reverse-phase high-performance liquid chromatography: pre- Vettenet al., 1999 Molecular analysis of theanthocyanin2gene column derivatization with phenylisothiocyanate. Anal. Biochem. of petunia and its role in the evolution of flower color. Plant
136:65–74. Cell11:1433–1444.
Hull, A. K., R. VijandJ. L. Celenza, 2000 Arabidopsiscytochrome Reymond, P., H. Weber, M. DamondandE. E. Farmer, 2000
Differ-P450s that catalyze the first step of tryptophan-dependent indole- ential gene expression in response to mechanical wounding and 3-acetic acid biosynthesis. Proc. Natl. Acad. Sci. USA97:2379– insect feeding in Arabidopsis. Plant Cell12:707–720.
2384. Rose, A. B., A. L. CasselmanandR. L. Last, 1992 A
phosphoribo-Konieczny, A., andF. M. Ausubel, 1993 A procedure for mapping sylanthranilate transferase gene is defective in blue fluorescent
Arabidopsis mutations using co-dominant ecotype-specific PCR- Arabidopsis thalianatryptophan mutants. Plant Physiol.100:582– based markers. Plant J.4:403–410. 592.
Kreps, J. A., T. Ponappa, W. DongandC. D. Town, 1996 Molecular Rose, A. B., J. LiandR. L. Last, 1997 An allelic series of blue
fluo-basis of alpha-methyltryptophan resistance inamt-1, a mutant of rescenttrp1mutants ofArabidopsis thaliana.Genetics145:197–
Arabidopsis thalianawith altered tryptophan metabolism. Plant 205.
Physiol.110:1159–1165. Sasse, F., M. BuchholzandJ. Berlin, 1983 Site of action of growth inhibitory tryptophan analogues inCatharanthus roseuscell
sus-Kunkel, T. A., J. D. RobertsandR. A. Zakour, 1987 Rapid and
pension cultures. Z. Naturforsch. Sect. C Biosci.38:910–915. efficient site-specific mutagenesis without phenotypic selection.
Smolen, G., and J. Bender, 2002 Arabidopsis cytochrome P450
Methods Enzymol.154:367–382.
cyp83B1mutations activate the tryptophan biosynthetic pathway.
Kutchan, T. M., 1995 Alkaloid biosynthesis—the basis for metabolic
Genetics160:323–332. engineering of medicinal plants. Plant Cell7:1059–1070.
Spelt, C., F.Quattrocchio, J. N. M.Moland R.Koes, 2000
anthocy-Li, J., andR. L. Last, 1996 TheArabidopsis thaliana trp5mutant has
anin1of petunia encodes a basic helix-loop-helix protein that a feedback-resistant anthranilate synthase and elevated soluble
directly activates transcription of structural anthocyanin genes. tryptophan. Plant Physiol.110:51–59.
Plant Cell12:1619–1631.
Melquist, S., B. LuffandJ. Bender, 1999 ArabidopsisPAIgene
Vance, V., and H. Vaucheret, 2001 RNA silencing in plants—
arrangements, cytosine methylation and expression. Genetics153:
defense and counterdefense. Science292:2277–2280. 401–413.
Walker, A. R., P. A. Davison, A. C. Bolognesi-Winfield, C. M.
Minet, M., M.-E. DufourandF. Lacroute, 1992 Complementation
James, N. Srinivasanet al., 1999 TheTRANSPARENT TESTA
ofSaccharomyces cerevisiaeauxotrophic mutants byArabidopsis
thali-GLABRA1locus, which regulates trichome differentiation and
anacDNAs. Plant J.2:417–422.
anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat
Miozzari, G., P. Niederberger andR. Hutter, 1977 Action of
protein. Plant Cell11:1337–1349.
tryptophan analogues inSaccharomyces cerevisiae.Arch. Microbiol. Zhao, J., andR. L. Last, 1996 Coordinate regulation of the
trypto-115:307–316. phan biosynthetic pathway and indolic phytoalexin accumulation
Mol, J., E. GrotewoldandR. Koes, 1998 How genes paint flowers in Arabidopsis. Plant Cell8:2235–2244.
and seeds. Trends Plant Sci.3:212–217. Zhao, J., C. C. WilliamsandR. L. Last, 1998 Induction of
Arabi-Neff, M. M., J. D. Arabi-Neff, J. ChoryandA. E. Pepper, 1998 dCAPS, dopsis tryptophan pathway enzymes and camalexin by amino acid
a simple technique for the genetic analysis of single nucleotide starvation, oxidative stress, and an abiotic elicitor. Plant Cell10: polymorphisms: experimental applications inArabidopsis thaliana 359–370.
genetics. Plant J.14:387–392.