3940
FORMULATION AND EVALUATION OF MUCOADHESIVE DRUG
DELIVERY OF ANTI-DIABETIC DRUG
Nagaveni P*1, Chandra Sekhar KB2, Jayachandra Reddy P3
1S.V.U. College of Pharmaceutical Sciences, S.V. University, Tirupati, 517502, Chittoor District- Andhra Pradesh, India.
2Director, JNTUA-Oil Technological Pharmaceutical Research Institute,
Anantapuramu-515001, Andhra Pradesh, India.
3Professor & Principal, Krishna Teja Pharmacy College, Tirupati-517506, Andhra Pradesh, India.
*Corresponding author E-mail: nagavenipatp@gmail.com
ARTICLE INFO ABSTRACT
Key Words
Hydrophilic polymers, Correlation coefficient,
Diffusion coefficient, Swelling index.
The present investigation was carried out to develop a novel mucoadhesive drug delivery system for the delivery of Nateglinide in a non-invasive dosage form with enhanced bioavailability that bypasses the hepatic first pass metabolism by delivering the drug unidirectionally towards buccal mucosa. Drug-polymer interaction studies through substantial observation, FTIR analysis revealed that there was no considerable interaction among drug and polymers and therefore the selected raw materials greatly suitable for the formulation of buccal films of Nateglinide. The prepared buccal films were evaluated for their flexibility, thickness, moisture content, and tensile strength. The in-vitro permeation study was carried out using a trans-diffusion cell. It was concluded that selective mucoadhesive polymers can be successfully used as a carrier for enhancing permeation and bioavailability of Nateglinide.
INTRODUCTION:
Nateglinide [N-(trans-4-isopropyl cyclo hexyl carbonyl)-D-phenyl alanine] is a novel, highly physiologic, glucose regulator recently approved for the treatment of type-2 diabetes mellitus. Nateglinide has a rapid onset and short duration of insulinotropic action that results in reduction of glucose level1. In recent years several advancements has been made in research and development of oral drug delivery system. Concept of novel drug delivery system arose to overcome certain aspect related to physicochemical properties of drug molecule
and the related formulations. Various gastro retentive approaches that have recently become leading methodologies in the field of site-specific orally administered controlled release drug delivery. GRDDS has become leading methodology in site specific orally administered controlled release drug delivery system. Various drugs like which are unstable in alkaline pH, soluble in acidic pH, having narrow absorption window and site of action specific to stomach can be developed by using this technique2-4.
Journal of Global Trends in Pharmaceutical Sciences
3941
MATERIALS AND METHODS
The following materials were used: Nateglinide (Yarrow Chemical Pvt.Ltd., Mumbai), Chitosan, Carbopol, Polyvinyl Pyrolidine, HPMC and Propylene Glycol (S.D Fine chemicals, India). All the solvents and chemicals were used analytical grade satisfying Pharmacopoeial standards.
The solvent evaporation method was followed in this study for the preparation of buccal films of Nateglinide. This method was most frequently employed for fabrication of buccal films as well as transdermal patches in the research field of pharmacy by the scientists.
PREFORMULATIONS
Preformulation5-6 is the process
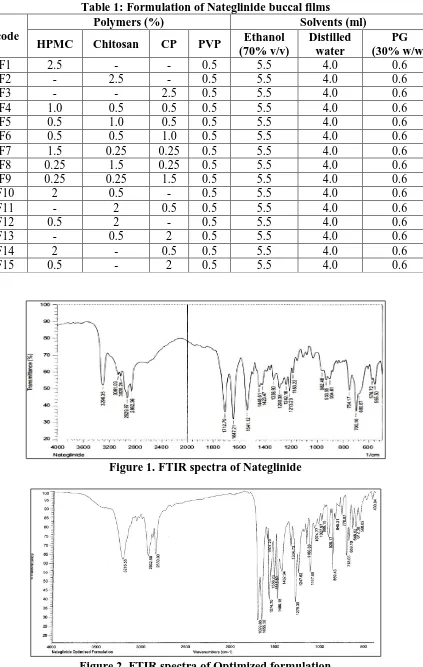
which investigate the physicochemical properties of drug or excipients alone or combined form. In this research, FTIR study (KBR pellet technique) was performed for the drug and optimized formulation and reported in the figure no.1&2.
FORMULATION OF BUCCAL FILMS Formulation of buccal films comprises two steps which includes preparation7 of drug free films and preparation of drug loading buccal films. Drug free buccal films
The weighed quantities of polymers were dissolved in ethanol (70%). Triethanolamine was used to neutralize CP polymeric solution. Propylene Glycol (PG) in the concentration of 30% w/w was used as plasticizer and permeation enhancer, subjected for levigation along with polymeric solutions. The solution was stirred seldom to get paste like consistency. To remove the air bubbles, the solution was subjected to sonication in a bath sonicator. Then this was placed on a surface of glass and with the use of ring having shape of ‘O’ has 4 cm in diameter was enclosed with funnel for reducing the disappearance of solvent and kept to complete dryness at room temperature throughout night. After drying films were collected and protected with aluminium foil8-10. The films were later
placed in desiccators for further use. Drug containing buccal films
The weighed quantities of polymers were dissolved in ethanol (70%).
Triethanolamine was used to neutralize CP polymeric solution. After levigation with 30% w/w PG, accurately weighed respective quantities of drug samples were addd in polymeric solutions. The solution was stirred seldom to get paste like consistency. Then the remaining procedure is same like fabrication of drug free films. Patches were intended to release the drug from one side only, for that reason an impermeable backing membrane was positioned on the other side of the patch. Finally, in vacuum desiccators, the patches were dried for 4 h at room temperature. After clear examination, the dried patches were taken, examined for any imperfections or air bubbles and specific diameter patches produced using a specially fabricated circular stainless steel cutter. The diameter of the patches was measured using vernier callipers. By using aluminum foil samples were packed and stored in a glass container at room temperature11-12. The compositions of of films are given in table 1.
Physicochemical evaluation
The buccal films of Nateglinide was evaluated for various
physico-chemical parameters13-15.
SEM studies
SEM frequently used to establish size distribution of particles, topography of surface, texture and to look at the morphology of cracked or sectioned
surface. Three dimensional surfaces
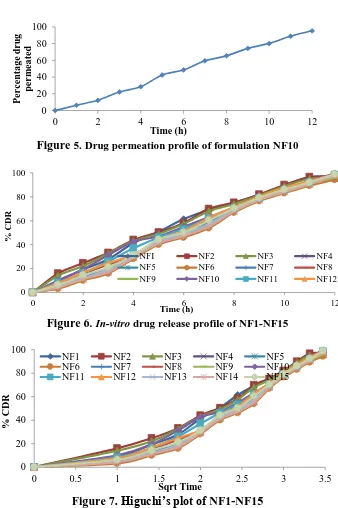
usually generating from SEM for relief images occurred from secondary electrons. The buccal film surface possessing the proportions of drug and polymer observed under microscopic examination to obtain the morphology information and porosity of the film and reported in figure no 2 & 3. Thickness
Each film thickness was measured with digital vernier calipers (Absolute digimate) in six different locations of the film and the standard thickness was calculated and reported in table 2.
Weight of films
3942 weighed and the mean of three films
calculated and reported in table 2. Folding endurance
Folding endurance test performed by taking the individual film and folded constantly up to 300 times manually or unless it broke at the same place. How many times the film could be folded at the same position without breaking given the folding endurance value and the average of three films were noted in table 2.
Surface pH
Surface pH of the buccal films (2 cm diameter), with no backing membrane was measured by a modified method. Buccal films were placed for 2 hr on the surface of agar plate, prepared by dissolving 2% (m/v) agar in warmed IPB (pH 6.75) under stirring and then pouring the solution into a petridish until it gelled at room temperature. The surface pH was determined with help of a combined glass electrode in contact with the surface of the film, kept it to equilibrate for 1 minute. The procedure was repeated thrice and the average was noted in table 2.
Percentage Moisture Absorption (PMA) PMA test of the buccal films carried out for testing the integrity of films physically at high moisture environment. After cutout the mass of 1 cm in diameter 3 films was weighed accurately and then
placed in desiccator contain AlCl3
saturated solution, keeping the RH at 79.5%. The films were taken following 3 days, weighed and PMA was estimated. The average of 3 films was calculated and reported in table 2.
Percentage Moisture Absorption
=Final weight – Initial weight
Initial weight X100
Percentage Moisture Loss (PML)
PML test performed to ensure the stability of films at dry condition. After cutout the mass of 1 cm in diameter 3 films was weighed accurately and then placed in desiccators contain anhydrous
CaCl2 in fusion state. The films were taken
following 3 days, weighed and PML was
estimated. The average of 3 films was determined and reported in table 2.
Percentage Moisture Loss
=Initial weight – Final weight
Initial weight X100
Swelling Percentage (%S)
50 ml of pH 6.8 phosphate buffer added in a thoroughly cleaned petridish in to which buccal films were placed. Increments in the mass of the film were taken with the intervals of 15 min up to 1 hr and the weight was calculated and reported in table 2.
Drug content
Three equal parts of each buccal film formulation was placed in a 100 ml phosphate buffer (pH 6.8). These samples were subjected for stirring up to 24 hr fallowed by filtration. The filtrate was diluted suitably and the absorbance was determined at respective wavelengths by using UV Spectrophotometer. The average of three films was considered as drug content present in the film and reported in table 2.
Buccoadhesive strength
Modified balance method was chosen to measure the buccoadhesive
strengthof the films. Fresh buccal mucosa
3943 This force of detachment indicated the
strength of buccoadhesive nature of the buccal film in grams are reported in figure 3. Adhesion Force (N) = (Bioadhesive strength (g) ×9.8) /1000
Bond strength (N m–2) = Adhesion force / surface area.
Ex-vivo permeation
Franz-diffusion cell was used for the drug permeation study of films with fresh buccal mucosa of sheep at 37 ± 1°C. The tissue preparation was similar to that explained before. Freshly obtained buccal mucosa was placed for connecting the donor and receptor compartments, thus the mucosa of smooth surface faced the donor compartment. After the animal mucosa was attached on one side of an open-ended tube, and it was served as a donor compartment. The film was located in such a way that it must be stuck on surface of mucous membrane. The diffusion cell was
maintained at 37±20C and the receptor
compartment was stimulated at a rate of 100 rpm. At pre-determined time intervals 1ml sample was taken using a butterfly canula and syringe. Sample filtered through 0.45 µm filter and diluted suitably for analyzing drug content using UV spectrophotometer at 216 nm.
In-vitro drug release
The dissolution study was carried out using USP Type-2 rotating paddle dissolution test apparatus. Therefore, to provide sink condition, 100 ml of simulated saliva solution (pH 6.8) was taken as the dissolution medium in a 250
ml glass beaker maintained at 37 ± 0.5 0C
which was stirred at 50 rpm. 2 cm in diameter film was fixed by using acyanoacrylate adhesive on the glass disk. At the bottom of the dissolution vessel the disk was kept so that the film remains on the upper side of the disk. At pre-determined time intervals 5 ml samples withdrawn and replaced with same volume of dissolution medium. These samples have been filtered using 0.45 µm filter and diluted suitably with simulated saliva
solution (pH 6.8) and assayed
spectrophotometrically 216 nm
respectively. The drug release mechanism from the buccal films was analyzed by ruling the best fit of the release data to Higuchi and Korsmeyer - Peppa’s plots.’ For each model the release rate constants ‘k’ and ‘n” were estimated by linear regression analysis using Microsoft Excel 2003 software.
RESULTS AND DISCUSSION
The prepared buccal films were smooth, uniform in thickness, mass, drug content and showed no visible cracks or folds. The formulated buccal films were
evaluated for various physical and
chemical parameters and the obtained results were given in the table 2. In the IR spectral analysis of Nateglinide exhibits characteristic peaks at 1712 (C=O), 3061 (CH Stetching) 3299 (NH), 1448, 1647 (Aromatic CH Str) and physical mixture of Nateglinide and their admixture with polymers the characteristic absorption peaks at 3215 (CH-S), 1699 & 1655 (C=O), 1574 (CH Stretching Aromatic)
were observed. The characteristic
3944 Table 1: Formulation of Nateglinide buccal films
F. code
Polymers (%) Solvents (ml)
HPMC Chitosan CP PVP Ethanol
(70% v/v)
Distilled water
PG (30% w/w)
NF1 2.5 - - 0.5 5.5 4.0 0.6
NF2 - 2.5 - 0.5 5.5 4.0 0.6
NF3 - - 2.5 0.5 5.5 4.0 0.6
NF4 1.0 0.5 0.5 0.5 5.5 4.0 0.6
NF5 0.5 1.0 0.5 0.5 5.5 4.0 0.6
NF6 0.5 0.5 1.0 0.5 5.5 4.0 0.6
NF7 1.5 0.25 0.25 0.5 5.5 4.0 0.6
NF8 0.25 1.5 0.25 0.5 5.5 4.0 0.6
NF9 0.25 0.25 1.5 0.5 5.5 4.0 0.6
NF10 2 0.5 - 0.5 5.5 4.0 0.6
NF11 - 2 0.5 0.5 5.5 4.0 0.6
NF12 0.5 2 - 0.5 5.5 4.0 0.6
NF13 - 0.5 2 0.5 5.5 4.0 0.6
NF14 2 - 0.5 0.5 5.5 4.0 0.6
NF15 0.5 - 2 0.5 5.5 4.0 0.6
Figure 1. FTIR spectra of Nateglinide
3945
Figure 3. SEM picture of drug free film & drug containing film
Table 2: Physico-chemical evaluation of buccal films of Nateglinide
F. Code Thicknes s (mm) Weight (mg) Fold. end Surfac
e pH PMA PML % S Q
Drug content
(mg)
NF1 0.48
±0.02
178.23
±0.91 320±5.0
6.73± 0.005
5.21±
0.07 5.97±0.12
120.9 ±0.9 8.39± 0.35 49.50± 0.22
NF2 0.40
±0.01
171.18
±0.91 300±3.0
6.79± 0.005
7.32±
0.04 5.14±0.72
99.6 ±0.69 5.46± 0.34 48.70± 0.32
NF3 0.47
±0.01
176.53
±0.80 315±1.0
6.71± 0.015
9.24±
0.09 4.74±0.10
118.4± 0.72 5.95± 0.34 49.20± 0.45
NF4 0.39
±0.01
168.31
±0.58 298±6.0
6.64±
0.050 10.32±0.11 4.14±0.20
124.2 ±0.99 4.38± 0.35 49.66± 0.35
NF5 0.35
±0.02
166.37
±0.80 281±4.0
6.60±
0.015 12.13±0.09 4.08±0.03
122.4± 0.6 3.76± 0.08 48.56± 0.25
NF6 0.41
±0.01
172.12
±1.00 318±5.0
6.69±
0.03 14.21±0.06 3.88±0.02
128.0± 0.85 5.18± 0.32 49.63± 0.25
NF7 0.40
±0.21
170.53
±0.80 310±1.0
6.70± 0.03
7.86±
0.27 6.44±0.10
120.4± 0.72 8.67 ±0.35 49.50± 0.03
NF8 0.38
±0.05
169.31
±0.48 296±6.0
6.82± 0.015
6.18±
0.13 7.13±0.08
114.2± 0.99 9.27± 0.52 48.80± 0.20
NF9 0.36
±0.02
166.37
±0.20 320±4.0
6.81± 0.005
5.34±
0.12 9.12±0.07
130.4± 0.6 9.37± 0.43 49.94± 0.12
NF10 0.39
±0.01
168.12
±1.00 320±5.0
6.77± 0.001
4.12±
0.13 10.06±0.06
125± 0.85 9.98± 0.59 48.45± 0.31
NF11 0.34
±0.01
165.17
±1.10 286±2.0
6.67± 0.003
3.85±
0.22 9.05±0.04
128.6± 0.4 9.46± 0.59 48.43± 0.29
NF12 0.39
±0.01
169.27
±1.10 294±1.0
6.74± 0.008
3.93±
0.33 8.04±0.08
123.2± 0.63 9.56± 0.59 48.35± 0.28
NF13 0.38
±0.01
172.37
±0.60 304±3.0
6.67±
0.005 11.26±0.24
5.72± 0.01 77.4± 0.7 5.91± 0.38 18.90± 0.25
NF14 0.36
±0.01
171.07
±0.90 305±2.0
6.63± 0.005 9.89± 0.22 6.13± 0.02 72.51± 0.6 6.32± 0.20 18.90± 0.15
NF15 0.32
±0.01
168.43
±0.50 302±2.0
6.61± 0.017 7.02± 0.06 7.45± 0.52 69.56± 0.65 6.94± 0.31 19.30± 0.21 Mean± SD (n=3)
3946 Figure 4. Buccoadhesive strength of Nateglinide buccal films
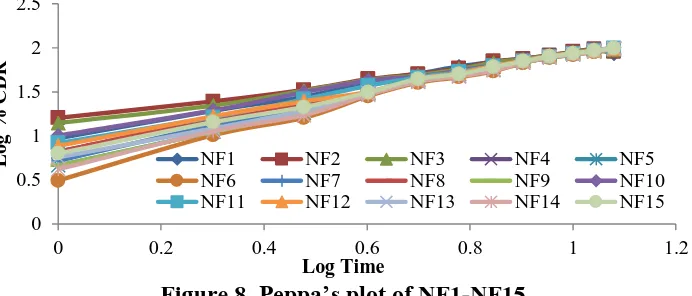
Figure 5. Drug permeation profile of formulation NF10
Figure 6. In-vitro drug release profile of NF1-NF15
Figure 7. Higuchi’s plot of NF1-NF15
0 5 10 15 20 25 30 35 40
B
io
ad
h
e
si
o
n
S
tr
e
n
th
Formulation Code
0 20 40 60 80 100
0 2 4 6 8 10 12
P
e
r
c
e
n
tage
d
r
u
g
p
e
r
m
e
ate
d
Time (h)
0 20 40 60 80 100
0 2 4 6 8 10 12
%
C
D
R
Time (h)
NFI NF2 NF3 NF4
NF5 NF6 NF7 NF8
NF9 NF10 NF11 NF12
0 20 40 60 80 100
0 0.5 1 1.5 2 2.5 3 3.5
%
C
D
R
Sqrt Time
NF1 NF2 NF3 NF4 NF5
NF6 NF7 NF8 NF9 NF10
3947 Figure 8. Peppa’s plot of NF1-NF15
The formulation NF10 has shown maximum folding endurance. After determination of surface pH for films the results showed that all the formulations in the range of pH 6 to 6.82. Therefore formulations did not cause irritation while administration and hence they attain patient compliance. SEM photographs showed that the buccal films were uniform in pores on the surface which has smooth surface and completely covered with the polymer and drug also distributed throughout the films. Moisture Absorption & Moisture Loss tests were conducted for the films for ensuring the physical stability while they exposed to greater humid environment and also to check reliability & integrity of the film during dry environment. In case of Nateglinide buccal films, the moisture absorbed in terms of percentage in the formulation NF6 has shown the highest value of moisture absorption which is 14.21±0.06%. The formulation NF11exhibited significantly high value of loss of moisture which is 10.06±0.06 this might be occurred due to presence of PVP and absence of carbopol. The films started to swell within 10 min due to presence of swellable polymers like HPMC and carbopol and chitosan, and maximum degree of swelling was observed after 60-120 min. The formulation NF10 shows higher percentage of swelling (130.4±0.6) than the rest. At most transmission of water vapor attained in the formulation NF11 has shown 9.98±0.59 among all the films. The formulation NF6 was attained least transmission of water vapor of 3.76±0.08 among entire films. All the films are showing significant drug content shown 48.35±0.28 to 49.94±0.12 for
both the selected candidates. Recovery was possible in the range of 18.9 to 19.95 mg for Nateglinide formulations. The actual drug content was high by increase in the concentration of polymer due to formation of viscous films, which leads to better retention of the drug in the films. No correlation was found between the bioadhesion force and the residence time of the polymers. Maximum bioadhesive force was seen in the carbopol containing films may be due to its anionic nature. The bioadhesive strength shown by Nateglinide buccal films was agreeable for keeping them in buccal cavity. The mixture of HPMC and carbopol exhibited satisfactory adhesion were shown in the figure 4. The maximum buccoadhesive strength is obtained in NF10 formulations. In-vitro permeation profile of formulation NF10 has shown 95.35% of diffusion phenomena was observed in figure 5. The decrease in drug diffusion observed from Ex-vivo study compared to In-vitro, may be due to the lesser permeability of buccal mucosa over egg membrane and also the presence of backing membrane in the ex-vivo study, which make the release of the drug unidirectional. The drug permeation study of optimized formulations NF10 through sheep buccal mucosa was shown in the figures 4. The formulation NF10 showed 99.6 ± 0.58 on 12 hr. In formulations NF1 to NF9 drug release decreased with increasing concentrations of polymers and maximum release was observed in NF10 formulation due to optimum concentrations of combination HPMC, carbopol and chitosan polymers (Figure 6 - Figure 8). Since carbopol is insoluble in simulated saliva and swelling behavior of carbopol is attributed to
0 0.5 1 1.5 2 2.5
0 0.2 0.4 0.6 0.8 1 1.2
Lo
g
%
C
D
R
Log Time
NF1 NF2 NF3 NF4 NF5
NF6 NF7 NF8 NF9 NF10
3948
unchanged COOH group that get hydrated by forming hydrogen bonds on imbibing with water and therefore extending polymer chain. The release was, thus controlled by the viscoelastic relaxation of the matrix during solvent penetration as well as the diffusivity of the drug in the gel layer formed as the patch swelled. From the release kinetic models, optimized
formulation NF10 follows Non-fickian
diffusion release mechanism from all the films.
CONCLUSION
From the all prepared formulations, the NF10 buccal film was found to be considered as optimized formulation. The formulation NF10 comprising HPMC, chitosan and PVP polymers and which has provides as good buccal film. It showed highest swelling index, bioadhesive strength and in-vitro drug release profile. Hence the present investigation concluded that, Nateglinide buccoadhesive drug delivery system with HPMC, chitosan and PVP meet the ideal requirement for buccal devices which can be good way to bypass the extensive hepatic first pass metabolism and increase bioavailability.
REFERENCES
1. Chowdary, KPR, Mucoadhesive microspheres for controlled drug delivery. Biol. Pharm. Bull. 2004; 27(11): 1717-1724
2. Cleary, Adhesion of polyether-modified poly (acrylic acid) to mucin. Langmuir 2004; 20(22): 9755-9762.
3. Chavanpatil, Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for olfloxacin. International Journal
of Pharmaceutics 2006; 316 (1-2):
86 – 92.
4. Säkkinen, Evaluation of microcrystalline chitosan for gastro-retentive drug delivery. Eur J. of
Pharma. Sciences 2003; 19(5): 345 –
353.
5. Illum, Adhesive drug delivery composition. US Patent 6 387 408, April 13, 1998.
6. Tur KM., Ch’ng, HS. Evaluation of possible mechanism(s) of bioadhesion. International Journal
of Pharmaceutics 1998; 160(1):
61-74.
7. Thongborisute, Evaluation of mucoadhesiveness of polymers by Biacore method and mucin-particle method. International Journal of
Pharmaceutics 2008; 354(1-2):
204-209.
8. Caliceti, Development and in vivo evaluation of an oral insulin-PEG delivery system. European Eur J. of
Pharma. Sciences 2004; 22 (4): 315
– 323.
9. Thira, Mucoadhesive properties of various pectins on gastrointestinal mucosa: an in-vitro evaluation using texture analyzer. Eur. J. Pharm.
Biopharm. 2007; 67: 132–140.
10.Takeuchi. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Advanced Drug Delivery
Reviews 2005; 57(11): 1583-1594.
11.El-Samaligy MS. Formulation and evaluation of diclofenac sodium buccoadhesive discs. Int. J. of
Pharm. 2004; 286(1-2): 27-39.
12.Jayvadan. Formulation and evaluation of mucoadhesive glipizide microspheres. AAPS Pharm. Sci Tech. 2005; 6(1):51-55. 13.Myung. Mucoadhesive microspheres
prepared by interpolymer complexation and solvent diffusion method. Int. J. Pharma. 2005; 288(2): 295-303.
14.Eaimtrakarn. Retention and transit of intestinal mucoadhesive films in rat small intestine. Int. J. of Pharm. 2001; 224: 61-67.
15.Hagesaether. Mucoadhesion and drug permeability of free mix films of pectin and chitosan: In-vitro and
ex-vivo study. Eur. J. of Pharm. and