UDK 536.46:541.124:546.11:519.87
Poenostavljeni kinetični model turbulentnega zgorevanja vodika
A Simplified Kinetic Model of Hydrogen Turbulent Combustion
NIKO SAMEC - ŽELIMIR DOBOVIŠEK - LEOPOLD ŠKERGET - ANTON ČERNEJ
V članku je predstavljen načelni alternativni postopek numeričnega simuliranja zgorevanja vnaprej pomešanega vodika z zrakom v malem modelnem gorilniku. Potek reševanja je razdeljen na dva dela. V prvem delu rešujemo turbulentni reaktivni tok s programskim paketom TASCflow, na podlagi turbulentnega modela k -e in vrtinčnega disipacijskega modela zgorevanja (EDCM-Eddy Dissipation Combustion Model). V drugem delu pa z lastnim modelom delnega neposrednega simuliranja zgorevanja (MDNS) Izboljšamo rezultate, dobljene z programom
TASCflow. Ujemanje modela je delno potrjeno z eksperimentalnimi rezultati.
An alternative approach to numerical combustion simulation of premixed hydrogen and air in a small experimental burner is presented. The solution is divided into two parts. In the first part, the turbulent reacting flow Is described by means of theTASCflow software package, based on k -s turbulent model and te Eddy Dissipation Combustion Model (EDCM). In the second part, using our own model of Partial Direct Combustion Simulation (MDDS), the results of TASCflow computation have been further improved. The new model has been experimentally validated by comparison of some measured data.
0 UVOD
Iz nekaterih literaturnih virov 111], 1121, 1131, 1141 je moč sklepati, da obstajajo v prihodnosti re alne možnosti za uporabo vodika kot goriva. Seveda pa to že sedaj terja ogromno proučevanj in raziskav glede primernosti njegove uporabe z vidika energet ske kompaktnosti, morebitnih zalog, gospodarnosti pridobivanja, varnosti in distribucije ter primer nosti procesa zgorevanja glede na:
— vpliv produktov zgorevanja (H20, NOx) na človeka in okolje,
— morebitne možnosti uporabe v razpoložljivih zgorevalnih napravah.
Pri tem je treba upoštevati, da imajo v večini primerov proučevanja procesov zgorevanja, ekspe rimentalne raziskave še vedno vodilno vlogo. Glede na velike stroške za moderno eksperimentalno opremo in potreben čas za izvajanje preizkusov se vse pogosteje pojavljajo zahteve po njihovi raču nalniški podpori, kar pa omogočajo ustrezni nu merični modeli.
Uvajanje takšnih modelov je koristno še po sebno pri proučevanju zgorevanja novih, alterna tivnih vrst goriva, kakor v našem primeru, ko gre za zgorevanje vnaprej pomešanega vodika z zrakom. Ker je lahko, zaradi fizikalnih lastnosti vodika (preglednica 1), ki imajo pomemben vpliv na potek zgorevanja (problem stabilnosti 111), eksperimen talno proučevanje njegovega kinetskega zgorevanja zelo zahtevno, analitični modeli omogočajo razu mevanje kemijske kinetike.
0 INTRODUCTION
It is generally believed, based ona review li terature 1111, 1121, 1131, 1141, that hydrogen will be used as a fuel in the future. However, this will require comprehensive study and experimen tal investigations, bearnig in mind H2 energy den sity, resources, the economics of production and distribution, safety and suitability of combustion process in terms of:
— the effect of combustion products (H20, NOx) on human health and the environment,
— the possibility of its utilisation in existing gas-burning equipments.
Moreover, it must be taken into account that experimental investigation still plays a dominant role generally in combustion process study. In view of the high price of modern sophisticated experimental equipment and long-term research it is wise to incorporate a computer aid in many cases, based on numerical combustion simulation models.
Preglednica 1: Pregled poglavitnih lastnosti vodika 121, 131 Table 1: Summary of the main properties of hydrogen 121, 131
Glavne fizikalne lastnosti Main physical properties Temperatura tališča
Melting point 13,96 K
Temperatura vrelišča
Boiling point 20,39 K
Gostota
Density 0,08987 kg/m3
Toplotna prevodnost
Thermal conductivity 0,156 W/mK
Molekularna difuzija
Molecular diffusivity 6,1 • 10 5 m /s Kinematična viskoznost
Kinematic viscosity 9,71 • 10"5 m /s Lastnosti zgorevanja
Combustion properties
zrak/a ir
Oksidant Oxidizer
o 2 Največja hitrost zgorevanja _
Maximum burning velocity
Največja temperatura plamena p = 1,013 bar Maximum flame temperature
3,5 m /s 2318 K
14 m /s 3083 K
Posplošeni modeli turbulentnega zgorevanja omogočajo sicer dobro napoved temperaturnega po lja plamena in lokalno porazdelitev reprezentativne komponente produktov zgorevanja, ne omogočajo pa bolj podrobnega proučevanja procesov zgorevanja z vidika mehanizma kemijskih reakcij. Zato smo iz delali poenostavljeni kinetični model turbulentnega zgorevanja homogene zmesi vodika in zraka, ki te melji na kinetiki kemičnih reakcij, Arrheniusovem zakonu določitve konstant njihovih hitrosti in del nem ravnotežnem modelu določitve vmesnih pro duktov.
1 MATEMATIČNI MODEL
Modeliranje procesov zgorevanja, pri katerem želimo poleg kemijske kinetike upoštevati še vplive konvekcije in difuzije, je vedno odvisno od uspeš nosti simultanega reševanja velikega števila, med sebojno odvisnih, parcialnih diferencialnih enačb. Upoštevajoč kompleksnost problema, poteka reše vanje v našem primeru v dveh delih. V prvem delu rešujemo turbulentni reaktivni tok s turbulentnim modelom k -e in zajema sistem desetih enačb:
— splošno enačbo o ohranitvi mase, — tri gibalne Navier-Stokesove enačbe, — energijsko enačbo,
General analytical models of turbulent bur ning flows enable the fairly accurate prediction of the flame temperature field and the local distri bution of representative species of combustion products, but they do not throw enough light to study combustion processes in more detail. Our simplified analytical model for turbulent combus tion of homogenous hydrogen-air mixture is based on chemical kinetics, Arrhenius law of chemical reaction rate and a partial equilibrium model to determine intermediate products.
1 MATHEMATICAL MODEL
The modelling of a combustion process by including, in addition to chemical kinetics, also the influence of convection and diffusion, depends to a great extend on successfully solving simul taneously a set of interrelated partial differential equations. This complex physical system was solved in our case in two steps. In the first step, the reacting turbulent flow is solved, using a k-s turbulent model, and it consists of a system of ten equations:
— a continuity equation,
— dve transportni enačbi (zadoločitev k in s), — tri enačbe ohranitve koncentracij (reaktantl (H2, 0 2) in produkti (H20)), kateremu je treba dodati še enačbo stanja, ki povezuje tlak, tempera turo in gostoto:
— two transport eqs (to determine k and f), — three equations of species conservation (reactants (H2, 0 2) and product H20)) and, additio nally, the equation of state interrelating pressure, temperature and fluid density:
(1) , P= pT 2
i
in kontrolno enačbo masnih deležev: and a control mass fraction equation:
S f , (2) .
Navedeni sistem enačb smo reševali s pro gramskim paketom TASCflow, ki za določitev iz vornega člena konvektivno difuzivne enačbe, upo rablja vrtinčni disipacijski model zgorevanja (EDCM) 1101:
This system of equations was solved by the TASCflow software package, using the EDCM 1101 to determine the source term of the ad- vection-diffusion equation:
-’go riv o ^edc P mit) i fgorlvo’ j > ^edc j +y ^ (3),
kjer sta Aedc = 4, Bedc = 0,5 konstanti modela. Rešitev celotnega sistema enačb je vektor spremenljivk:
x= {v^x, y, z), p ( x, y, z), ki vsebuje poleg spremenljivk sestave reaktantov (H20, 0 2, M2, N2) tudi spremenljivke turbulentne ga reaktivnega toka.
Napoved sestave produktov zaradi poenostav ljenega EDCM, ki temelji na enokoračni blmole- kularni kemični reakciji:
where Aedc = 4, Bsdc= 0.5 are model constants. The solution of the system of equations is a vector of variables:
T(x, y, z), £j (x, y, z)},
containing, in addition to the variables of reactant composition (H20, 0 2, H2, N2) also variables defi ning the properties of the reacting turbulent flow. The predicted product composition is not re liable enough, because of the simplified EDCM, based on a one step bimolecular chemical reaction: 2H2 + 0 2 - 2H20 ,
ni najbolj zanesljiva. Poleg tega ta model ne omo goča napovedi koncentracije dušikovih oksidov, toksičnih komponent v produktih zgorevanja vo dika z zrakom. Zato smo v drugem delu reševa nja z lastnim modelom (model delnega neposredne ga simuliranja — MDNS), zgrajenim na podlagi predpostavk (poenostavitev):
— da je kontrolna prostornina dovolj majhna (kar je praktično moč doseči z zadostno gostoto diskretne mreže),
— da lahko zanemarimo vpliv radikalov in prostih atomov pri izračunu temperaturnega polja, — da imamo nastanek prostih atomov in ra dikalov za časovno neodvisen (kar opravičujejo zelo hitre kemične reakcije njihovega nastanka),
— da v mehanizmu kemičnih reakcij H2/ 0 2 ne upoštevamo reakcij nastajanja vodikovega pe roksida in molekul H02,
— da tok obravnavamo kot dvodimenzionalen, izboljšali rezultate, določene s programom TASCflow, ki se nanašajo na lokalno sestavo pro duktov zgorevanja in določili tudi lokalne koncen tracije NO.
Moreover, this model does not enable us to predict the concentration of NOx , pollutants in the products of combustion of hydrogen in air. So in the next step of computation, our mathematical model was incorporated (Partial Direct Simulation Model - MDDS), based on the following assum ptions (simplifications):
— the control volume is small enough (practice this can be achieved by the use of sufficiently dense discretization grid),
— the influence of radicals and free atoms is neglected during the temperature field calculation,
— formation of free atoms and radicals is considered to be time independent (this is justified by very high rates of their chemical reactions of formation),
— in the mechanism of H2/ 0 2 chemical reactions, the formation of hydrogen peroxide and H02 molecules are neglected,
Z upoštevanjem teh poenostavitev smo, z uporabo modela zgorevanja za laminami tok, po pravili izračunane vrednosti deležev posameznih komponent v produktih zgorevanja. Omenjeni po stopek temelji na predpostavki, da so spremenljiv ke, ki karakterizirajo lastnosti toka, v dovolj majhni kontrolni prostornini konstantne. To omo goča prenos vrednosti spremenljivk iz mrežnih vozlišč programa TASCflow v isto ležeča vozlišča mreže modela delnega neposrednega simuliranja (MDNS). Tako je bilo treba v drugem delu rešiti le sistem petih konvektivno difuzivnih enačb (H20, H,, 0 2, N,, NO), 141, 151:
With these assumptions and by applying the combustion model for laminar flow, the computed values of several product species fractions were corrected. This approach is based on the assump tion that variables describing flow properties remain constant if the control volume is small enough. This enables, the values of variables at grid points predicted with TASCflow to be trans ferred to the same grid points of the Partial Di rect Simulation Model (MDDS). Exploring this simplification, a system of only five advection- -diffusion equations (H20, H2, 0 2, N2, NO), [41, 151:
pvj
9x-,
< S > - U
dxf (4),katerih izvorne člene Si smo računali na podlagi must be solved, where by the computation of the kinetike mehanizma kemičnih reakcij: source terms S, is based on the kinetic mechanism
of chemical reactions:
vil V21 •• • vii
vh V22 ■ Vi2
Vis V2s ■ Vis
VÌM V2M - VM
VN1
VN2
VNs
VNM X j
X 2
X , ->
■
A
Vfl V21
V12 V22
VFs VFs
Vm V2M
V,11 y12
vls
VIM
V,N1 N 2
V,Ns
VNM
' x {
x
2X ,
X N
(5)
z uporabo zakona o delovanju mas: and applying the law of mass action:
m F , N , , , N ,, 1 ( i
S! = M ,£ I ( vis - v \s ) k {s IT <Pi v ‘s - ( vjs - v |; ) A'bs IT is ( —
s = l L 1 - 1 1 = 1 v r
(6) ,
(m — red kemične reakcije) (where m - is the order of chemical reaction) in Arrheni uso vega zakona določitve koeficientov and, further, Arrhenius law is used to determine hitrosti kemičnih reakcij: the chemical reaction rate coefficient:
A'f, b = A T n e x p \- g a (7).
Glede na to, da smo obravnavali navidezno ustaljeni plamen, pri katerem procesi vžiga in ga šenja niso upoštevani, je predpostavka neupošte vanja kemičnih reakcij mehanizma nastanka H20 2 in H02 komponent dokaj upravičena. S tem se je število kemičnih reakcij bistveno zmanjšalo, kar samo reševanje zelo poenostavi brez večjega vpliva na končni rezultat. Tako smo uporabili nasled nji skrajšani mehanizem kemičnih reakcij H2/ 0 2, 191:
*f,
H + O, OH + O (8),
k b, T • Iv
k f 2
O + H2 Z = * OH + H (9), ^b2
k f3
O H + H 2 Z = * H20 + H (10), k bi
k f 4
O H+ OHZ T T T H20 + 0 (11), k b<
k ti
H + H + M T = T H, + M (12), k bi
H + CH + N,
H + OH + H2 O ‘fe 1 b6
‘f7
‘b7
H2 o + N2
h2o + H20
(13),
(14).
Nastanek NO pa smo opisali z Zeldovičevim and NO formation was described by using the
mehanizmom (61: Zeldovich mechanism (61:
O + N, NO + N
N + 0 9 NO + 0
N + OH NO + H
(15) ,
(16) ,
(17).
Nadalje: splošno je znano, da kemični reakciji (16) in (17) potekata zelo hitro in predpostavili smo navidez ustaljeno koncentracijo N-atomov ter tako modelirali nastanek NO z izrazom:
d i m i
dt c
kjer je c = ( p / R m T).
Ker vodik kot gorivo ne vsebuje vezanega ogljika in dušika, mehanizma nastanka zgodnjega in konverznega NO nista pomembna.
Moreover, it is generally recognised, that chemical reactions (16) and (17) are very fast and, assuming a quasi steady-state N-atom con centration, the NO formation may be modelled by the expression:
2A'fs <p0 ^ N2 ) (18),
where c = ( p / R m T).
Vrednosti posameznih členov v enačbi (7) so podane za vsako reakcijo posebej. Za naš primer smo konstante za reakcije mehanizma H2/ 0 2 (v obeh smereh) povzeli po Baulchu 171, Jensenu [81 in za mehanizem nastanka NOx po Warnatzu 161 in so zbrane v preglednici 2.
MDNS zajema tako sistem petih parcialnih diferencialnih enačb, za vsako izmed komponent produktov zgorevanja (H20, 0 2, H2, N2, NO). Po aproksimaciji parcialnih odvodov s koračno me todo in z določitvijo izvornega člena na podlagi vektorja rešitev iz prejšnjega iterativnega kora ka dobimo pet sistemov linearnih enačb. Njihovo število je odvisno od števila vozlišč diskretizirane- ga območja. Vsakega izmed sistemov enačb rešuje mo z Gaussovo eliminacijsko metodo v vsakem iterativnem koraku. Iteracijo usmerja ustrezen dušilno relaksacijski postopek, ki sloni na neizpol njevanju določenih omejitev, ki se nanašajo na za četno sestavo reaktantov. Posebnost MDNS je kon stanten izvorni člen v posameznem iterativnem koraku.
The values of second terms of rate con stants (7) are given for each reaction. In our ca se, these constants of the H2/ 0 2 reaction mecha nism (for both directions) have been taken from Baulch 171, Jensen (81 and from Warnatz 161 for NO formation, and they are given in table 2.
MDDS Incorporates a system of five partial differential equations for each species of combus tion product (H20, 0 2, H,, N2, NO). Applying the method of finite differences to approximate partial derivatives, and after determining the source term based on the solution vector of the previous iterative step, five systems of linear equations are obtained. The number of systems depends on the number of grid points of the discretized do main. Each of the systems is solved by means of the Gaussian elimination method applied to each iterative step. Iteration is directed by a corres ponding dumped relaxation procedure based on restrictions related to the initial mixture com position. Furthermore, the constant value of the source term in each step of the iteration is a special feature of MDDS.
Preglednica 2: Konstantne hitrosti kemičnih reakcij (crn3m m mol’ m s 11 Table 2: Values of chemical reaction — rate constants
Reakcija f
Reaction A n Ea/ R m
(8) 2,2 10U 0 8450
(9) 1,8 IO10 1 4480
(10) 2,2 1013 0 2590
(11) 6,3 1012 0 550
(12) 2,6 1018 -1 0
(13) 8,6 1015 0 0
(14) 6,6 1016 0 0
(15) 1,8 1012 0 38369
(16) 6,4 109 0 3127
(17) 3,0 1013
k b
m A n Ea/ R m m
2 2,24 ig14 0 2021 2
2 8,3 10 1 3500 2
2 9,3 1013 0 10250 2
2 6,8 1013 0 9240 2
3 2,0 1013 1 51570 2
3 7,8 1017 0 59030 2
3 2 2
3,9 10'7 0 59225 2
Lokalne koncentracije vmesnih produktov (prostih atomov O, H in radikala OH) smo do ločili z upoštevanjem parcialnega ravnotežnega modela, zasnovanega na predpostavki o časovni neodvisnosti nastanka vmesnih produktov:
Local concetrations of intermediate products (free atoms O, H and radical OH) were computed by applying the partial equilibrium model, assu ming a steady state relationship of intermediate species formation:
rf[OH]= rf[H] = d [0 ]
dt dt dt
Sistem treh nelinearnih enačb, ki smo ga dobili z uporabo zapisa (19) za prej navedeni me hanizem kemičnih reakcij, smo reševali z dušeno iterativno Newton-Raphsonovo metodo (v vsakem vozlišču diskretne sheme), ki omogoča le izolirano rešitev. V ta namen je moral biti vektor začetnih približkov dovolj pazljivo izbran, vrednosti drugih spremenljivk pa so bile določene s programom TASCflow.
2 PREIZKUS 2 EXPERIMENT Model smo preverili na preprostem primeru
zgorevanja vnaprej pomešanega vodika z zrakom v atmosferskih razmerah v majhnem modelnem go rilniku.
Konstrukcija gorilnika je morala zagotavljati intenzivno mešanje vodika z zrakom in zadostno stabilnost zgorevanja, da bi dosegli stabilen plamen homogene zmesi reaktantov. Homogenost zmesi smo brez večjih težav dosegli z ustrezno namestit vijo mrež različnih gostot v cev gorilnika. Dosti več težav smo imeli s stabilnostjo zgorevanja, kajti pri zgorevanju vodika zaradi njegovih širokih meja vžiga in velike hitrosti zgorevanja prihaja zelo po gosto do vračanja plamena v cev gorilnika. Do vra čanja plamena pride najprej v mejni plasti, kjer se pojavljajo veliki gradienti padanja hitrosti doteka jočih reaktantov. Da bi preprečili tovrsten pojav, smo ejektorsko odsesovali mejno plast, kar je zah tevalo posebno konstrukcijo izstopnega dela goril nika, pri čemer smo povečali hitrost reaktantov ob steni gorilnika, kakor je to shematsko prikazano na sliki 1.
The model was validated, simulating the combustion of premixed hydrogen with air under atmospheric ambient conditions in a small scale experimental burner.
The burner design has to insure intensive mixing of hydrogen with air and sufficient com bustion stability to achieve a stable flame of pre mixed reactants. An homogenous mixture was ob tained very easily by inserting grids with different mesh densities in the burner tube. Flame stability caused more trouble, because of very broad H2 ig nition limits and high burning velocity and a flash- -back into the burner tube happened very often. The flash-back is initiated in the boundary layer, where very steep gradients of inflow reactant ve locity are present. To prevent this, the boundary layer was ejected and special attention was devo ted to the design of the exit part of the burner. Thus, the flow velocity of the reactant stream near the burner walls was increased, and this is schematically shown in Fig 1.
Sl. 1. Shematični prikaz konstrukcije Izstopnega dela modelnega gorilnika z ejektorskim odsesovanjem reaktantov v mejni plasti lil
Fig. 1. Schematic view of experimental burner exit with ejection of reactant boundary layer 111 Zaradi intenziviranja prenosa toplote na hladne
stene, kar dodatno zmanjšuje možnost vračanja plamena v cev, je bil izstopni del gorilnika vodno hlajen. Da bi zagotovili še večjo stabilnost zgore vanja, smo v izstopno odprtino vstavili vodno hlaje ne cevke, ki so opravljale funkcijo stabilizatorja plamena (sl. 1). Kljub omenjenim ukrepom rezulta ti z relativnim razmernikom zraka, manjšim od dva, niso bili dovolj ugodni.
V laboratoriju za zgorevalne komore letalske ga oddelka na FH Aachen smo z ustreznimi plin skimi analizatorji merili vrednosti koncentracij osnovnih komponent produktov zgorevanja (H2, 0 2, NO), ki smo jih uporabili za analizo veljavnosti numeričnega modela. Koncentracijo vodika smo merili z analizatorjem na podlagi detekcije različ nih toplotnih prevodnosti plinskih komponent, koncentracijo kisika z magnetno mehanskim ana lizatorjem in koncentracijo NO s kemično lumi- niscentnim analizatorjem. Koncentracijo vode smo po izmerjenih koncentracijah računali, v vsaki točki merjenja, z naslednjo bilančno enačbo:
kjer je A fR korekcijski faktor, ki upošteva kon centracijo radikalov.
Meritve trenutnih koncentracij radikalov H, OH in 0 niso bile mogoče, kar je onemogočalo popolno testiranje predpostavljenega mehanizma kemičnih reakcij z eksperimentalno analizo pro duktov zgorevanja.
3 REZULTATI
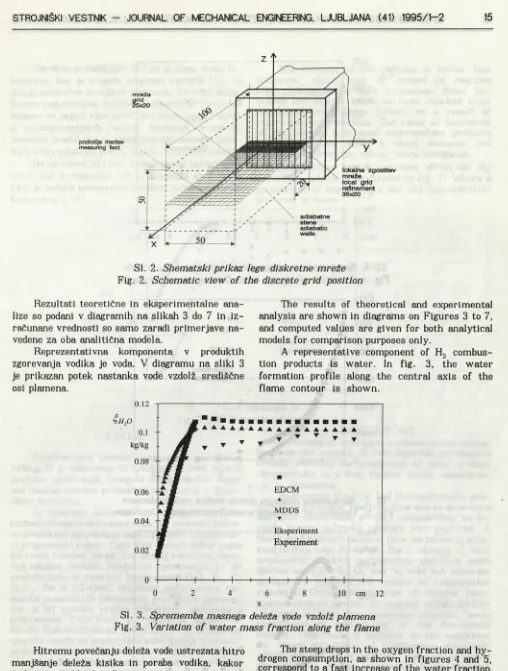
Z vidika ocene ujemanja numeričnega modela smo se želeli s preizkusom kar najbolj približati programskim možnostim simuliranja robnih pogo jev. Pri tem smo upoštevali dejstvo, da je model zgorevanja namenjen zgolj simuliranju procesov zgorevanja poprej pomešanih reaktantov, zato smo plamen, ki je segal od roba gorilnika v atmosfero, ogradili z adiabatniml stenami. Področje plamena, ograjeno z adiabatnimi stenami (sl. 2) smo diskre- tizirali z mrežo 25 x 20 x 20. Področje intenzivnih reakcij je v primerjavi z dolžino plamena zelo krat ko, zato smo mrežo v področju do 20 mm od roba gorilnika zgostili s faktorjem 7, kar daje lokalno mrežo 35 x 20 x 20. Preostali del območja (do 100 mm od roba gorilnika) pa je diskretiziran z mrežo 20x20x20. Tako sestavljeno mrežo smo uporabili v programskem paketu TASCflow. V modelu delne ga neposrednega simuliranja je uporabljena dvodi menzionalna mreža, ki pomeni ravnino tridimen zionalne mreže na polovični višini gorilnika, kakor prikazuje slika 2. V tej ravnini so bile izvedene tudi vse eksperimentalne meritve, v posameznih točkah z računalniško vodeno sondo.
Izvedli smo enodimenzionalno analizo rezulta tov vzdolž plamena (x=var., y=z=0, sl. 2) za dolo čen testni primer, pri čemer so rezultati lokalne sestave produktov zgorevanja podani v masnih de ležih na en kilogram produktov zgorevanja. Rela tivni razmernik zraka <2>a je 2,7 (visoka vrednost zaradi problema stabilnosti plamena), masni pre tok zraka je 10 g /s in goriva 0,11 g/s.
Main combustion product species concentra tions (Rjo, 0 2, NO) were measured with gas analysers in the laboratory for combustion cham bers at the Aerospace Department of FH-Aachen, and the results were used to validate our theore tical model. The H2 concentration was monitored with an analyser based on the detection of diffe rent gas thermal conductivities, the 0 2 concentra tion with a gas analyser of a magnetic mechanical type and NO with a chemoluminescent NO-NOx analyser. The water concentration was computed at each measuring point, applying the following balance equation:
(20) ,
where by implying the correction coefficient AfR , radical concentrations were taken into account.
Experimental techniques to measure instanta neous concentrations of H, OH and O radicals were not available. These of course would enable testing the assumed mechanism of chemical reactions by chemical analysis of the combustion products.
3 RESULTS
In order to validate the theoretical model pro perly, we tried to approach experiments as close as possible to computer simulated boundary condi tions. We respected the fact that the main object of the theoretical model was to simulate the com bustion processes of premixed reactants, and there so flame spreading from the burner edge was con fined with an adiabatic enclosure. The flame re gion within the adiabatic walls (Fig. 2) was dis cretized with a grid 25x20x20. The region of in tensive reactions is very short, compared to flame length, and the grid density within the region up to 20 mm from the burner exit edge was increased by a factor of 7, and the local grid was 35 x 20 x 20. The residual part of the domain (up to 100 mm from the burner edge) was discretized with a grid 20 X 20 X 20. The concept of this grid was used in the software package TASCflow. In the MDDS model, a 2D grid was applied, representing a sepa rate plane of a 3D grid located at the middle of burner height, as is shown in fig 2. All experi mental measurements were carried out at each grid point of this plane, and the sampling probe traversing was computer controlled.
One-dimensional analysis of experimental results traced along the flame (A'=var., y=z=0 fig. 2) was carried out for a given test example, and data of local combustion product concentra tions are given in mass fraction related to one kg of products. The equivalence air/fuel ratio was 2.7 (this high value was imposed by the flame stabili ty problem), air mass flow was 10 g /s and fuel flow was 0.11 g /s respectively.
Sl. 2. Shematski prikaz lege diskretne mreže Fig. 2. Schematic view of the discrete grid position Rezultati teoretične in eksperimentalne ana
lize so podani v diagramih na slikah 3 do 7 in iz računane vrednosti so samo zaradi primerjave na vedene za oba analitična modela.
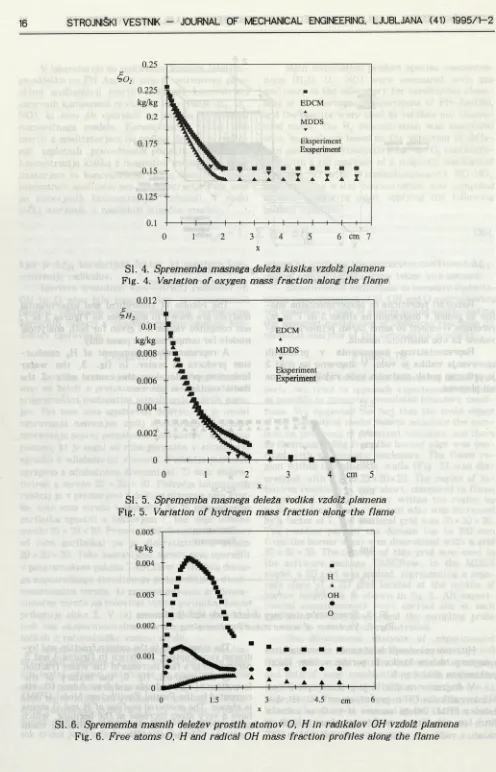
Reprezentativna komponenta v produktih zgorevanja vodika je voda. V diagramu na sliki 3 je prikazan potek nastanka vode vzdolž središčne osi plamena.
The results of theoretical and experimental analysis are shown in diagrams on Figures 3 to 7, and computed values are given for both analytical models for comparison purposes only.
A representative component of H2 combus tion products is water. In fig. 3, the water formation profile along the central axis of the flame contour is shown.
Si. 3. Sprememba masnega deleža vode vzdolž plamena Fig. 3. Variation of water mass fraction along the flame Hitremu povečanju deleža vode ustrezata hitro
manjšanje deleža kisika in poraba vodika, kakor prikazujeta sliki 4 in 5.
V diagramu na sliki 6 je prikazan potek na stanka radikalov OH in prostih atomov (0, H), do ločen s PRM. Deleža atomov H in O se začneta hitro in zelo strmo povečevati, kar je tudi v skladu z vodilnimi verižnimi reakcijami (8) do (11).
0.25
0.225
kg/kg
0.2
0.175
0.15
0.125
0.1
0 1 2 3 4 5 6 cm 7
X
Sl. 4. Sprememba masnega deleža kisika vzdolž plamena Fig. 4. Variation of oxygen mass fraction along the flame
0.012
U 0.01
kg/kg
0.008
0.006
0.004
0.002
0
0 1 2 3 4 cm 5
X
SI. 5. Sprememba masnega deleža vodika vzdolž plamena Fig. 5. Variation of hydrogen mass fraction along the flame
0.005
kg/kg
0.004
0.003
0.002
0.001
0
0 1.5 3 4.5 cm 6
X
SI. 6. Sprememba masnih deležev prostih atomov O, H in radikalov OH vzdolž plamena Fig. 6. Free atoms O, H and radical OH mass fraction profiles along the flame
1
1 m
I I
- ■
EDCM A
MDDS
m T
\ Eksperiment
m m
M m
V
Experiment
£ .
Naraščanje radikalov OH pa je dosti manj in tenzivno, kar je mogoče pripisati reakciji (11), ki deluje zaviralno na njihov nastanek. Po hitro dose ženem maksimumu deležev vodikovih in kisikovih atomov se pojavi njun prav tako hiter padec, zara di rekombinacijskih reakcij. Nastanek omenjenih vmesnih produktov se z oddaljenostjo od roba goril nika hitro ustali na precej nižjih vrednostih deležev.
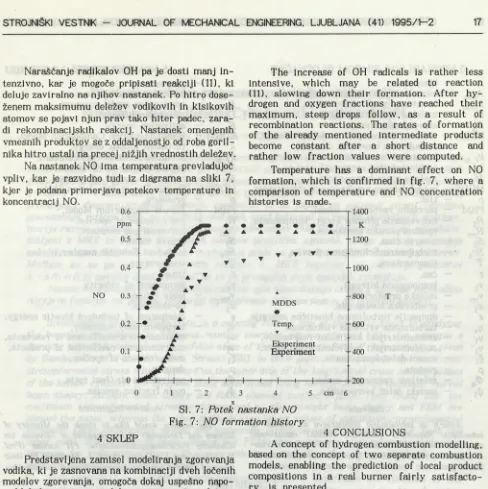
Na nastanek NO ima temperatura prevladujoč vpliv, kar je razvidno tudi iz diagrama na sliki 7, kjer je podana primerjava potekov temperature in koncentracij NO.
The increase of OH radicals is rather less intensive, which may be related to reaction (11), slowing down their formation. After hy drogen and oxygen fractions have reached their maximum, steep drops follow, as a result of recombination reactions. The rates of formation of the already mentioned intermediate products become constant after a short distance and rather low fraction values were computed.
Temperature has a dominant effect on NO formation, which is confirmed in fig. 7, where a comparison of temperature and NO concentration histories is made.
0.6 ppm
0.5
0.4
/T
f
aNO 0.3
-0.2 «
▲ A
A A
o.i
Ut
A A A ▼ ▼ ▼
-1400 K -1200
--1000
MDDS
Temp. ▼
Eksperiment
Experiment
-800
--600
-400
-t---- 1---- 1---- 1---- e -200
0 1 2 3 4 5 cm 6
SI. 7: Potek nastanka NO Fig. 7: NO formation history 4 SKLEP
Predstavljena zamisel modeliranja zgorevanja vodika, ki je zasnovana na kombinaciji dveh ločenih modelov zgorevanja, omogoča dokaj uspešno napo ved lokalne sestave produktov zgorevanja v dejan skem gorilniku.
Za določitev tokovnih in toplotnih lastnosti turbulentnega reaktivnega toka ter osnovne sesta ve produktov zgorevanja smo uporabili razpoložlji vi programski paket. Tako smo dosegli dober popis tokovnih razmer, ne pa tudi dovolj natančno napo ved lokalne sestave produktov zgorevanja, ki jih predstavljajo le osnovne komponente H20, H2, 0 2 in N2. Da bi izboljšali rezultate glede poteka na stanka posameznih komponent produktov zgoreva nja, je bil izveden prenos rezultatov, dobljenih s programom TASCflow, po vozliščih numerične mreže diskretiziranega območja. To je omogočilo uporabo enačb za neposredno simuliranje procesov zgorevanja, kar se je, glede na dokaj dobro ujema nje napovedanih (izračunanih) in eksperimentalnih vrednosti, izkazalo za upravičeno.
Model DNS omogoča tudi napoved lokalne koncentracije NO v produktih zgorevanja.
Vsekakor pa je to šele prva stopnja razvoja te zamisli modela simuliranja procesov zgorevanja, tako v teoretičnem kakor tudi v eksperimentalnem pogledu.
4 CONCLUSIONS
A concept of hydrogen combustion modelling, based on the concept of two separate combustion models, enabling the prediction of local product compositions in a real burner fairly satisfacto ry, is presented.
To compute the flow and thermodynamic pro perties of turbulent reactive flow and to determine the basic combustion product composition, an al ready known software package was explored. A fairly good approximation of flow variables is achi eved in this way. However, the prediction of local combustion product composition, including main species as H20, H2, 0 2 and N2 was not accurate enough. To improve the results of computation related to formation histories of several com bustion product species, the results of calculations obtained via TASCflow computer program, were transferred to grid points of the discretized domain. This made it possible to use equations for direct simulation of combustion processes, which proves to be correct, bearing in mind the relatively a good agreement of experimental and predicted (computed) values.
MDDS also allows the prediction of local NO concentrations in combustion products.
5 UPORABLJENI SIMBOLI IN OZNAČBE 5 NOMENCLATURE
A —konstanta, A — constant,
A. —konstanta modela zgorevanja, ‘^edc — combustion model constant,
Hgdc —konstanta modela zgorevanja, Redc — combustion model constant,
Dx — molekularna difuzlvnost, — molecular diffusivity,
Ea —energija aktiviranja, E 1
EDCM
— activation energy,
EDCM—vrtinčnl disipacijskl model zgorevanja, — Eddy Dissipation Combustion Model,
k —turbulentna kinetična energija, k — turbulent kinetic energy,
^f,b y0
—konstanta hitrosti kemičnih reakcij, ^f,b — chemical reaction rate constant, —stehiometrični razmernik zraka, 'o — stoichiometric air/fuel ratio,
MDDS_
molska masa komponente,
model delnega neposrednega simuliranja, M,MDDS molecular weight of species, Partial Direct Simulation Model,
p —tlak, P — pressure,
PRM — parcialni ravnotežni model, PRM — Partial Equilibrium Model,
Rl
R m
— individualna plinska konst, komponente, R l — gas constant of species,
— splošna plinska konstanta, R m — universal gas constant,
S \ — izvorni člen, Si — source term,
Sct —turbulentno Schmidtovo število, Sct — turbulent Schmidt number,
T — temperatura, T — temperature,
t —čas, t — time,
vi vm
— komponenta hitrosti, vi — component of velocity,
— molska prostornina, — mole volume,
x l £
— prostorska koordinata,
£
— co-ordinate,
—disipacija turbulentne kinetične energije, — dissipation of turbulent kinetic energy,
V t —turbulentna vrtinčna viskoznost, — turbulent eddy viscosity,
Vl’ —stehiometrijski koeficient reaktantov, Vj’ — stoichiometric coefficient of reactants, vi” —stehiometrijski koeficient reaktantov, V,” — stoichiometric coefficient of products,
Č1 —masni delež komponente, čl — mass fraction of species,
p —gostota, p — density,
o a —relativni razmernik zraka, — equivalence air/fuel ratio,
‘Pi molski delež komponente.
Zahvala
<pl mole fraction species.
Ackno wledgement Avtorji se zahvaljujejo M inistrstvu za znanost in
tehnologijo Republike Slovenije, Nem ški raziskovalni skupnosti (DFG), FH Aachen (prof. F. Futtrop) in Viso k i strokovni šoli, FH Köln (prof. L. Sienčnik) za f i -načno pomoč in možnost eksperimentalne raziskave. Ill
The authors would like to thank the M inistry of Science and Technology of the Republic of Slovenia, the »Deutsche Forschungsgemeinschaft«. FH Aachen (Prof. F. Futtrop) and FH Cologne (Prof. L. Sienčnik) for their financial support enabling the experimental investigation.
Ill Samec, N.: Poenostavljeni kinetični model tu r bulentnega zgorevanja homogene zmesi vodika in zraka. Magistrsko delo, TF Maribor, 1994.
[21 Naidenov, G.F.: Goreločnye ustrojstva i zaščita atmosfery ot okislov azota. Tehnika Kiev. 1979.
131 Lazarini, F.-Brenčič, J.: Splošna in anorganska kemija, DZS, Ljubljana 1984.
141 Kuo, K.K.: Principles of Combustion. John Wiley & Sons, New York 1986.
[51 Škerget, L.: Mehanika tekočin, TF Maribor, FS Ljubljana, 1994
161 Warnatz, J.-Maas, U.: Technische Verbrennung, Springer-Verlag, Berlin 1993.
171 Baulch, D.L.-Drysdale, D.D.-Horne, O.G.-Lloyd, A.C.: Evaluated Kinetic Data for High Temperature Reac tions. Voi. 1, London 1992
181 Jensen, D.E., Jones, G.A.: Reaction Rate Coefi- cients for Flame Calculations, Combustion and Flame, (32) (1978). 1-34.
Naslov avtorjev: mag. Niko Samec, dipl. inž.
prof. dr. Želimir Dobovišek, dipl. inž. prof. dr. Leopold Škerget, dipl. inž. prof. dr. Anton Černe j, dipl. inž. Fakulteta za strojništvo, Maribor Prejeto: . i 1005
Receiwed: 4119ao
6 LITERATURA
6 REFERENCES
191 Bradley, J.N.: Flame and Combustion Phenome na, London 1969
1101 Jones, W .P.-W hitelaw, J.H., Calculation Methods for Reacting Turbulent Flows: Review, Combus tion and Flame (48), (1982) 1-2.
[Ill Murray, R.G.: Hydrogen as an Energy Carrier, Hydrogen Energy (Part B), Miami 1974.
1121 Brabs, T.A.-Lezeberg, E.A.-Bittker, D.A.-Ro- bertson, T.F.: Hydrogen Oxidation Mechanism With Applications to (1) the Chaperon Efficiency of Carbon Dioxide and (2) Vitiated Air Testing, NASA Technical Memorandum 100186, Ohio, 1987.
1131 Koroll, G.W.-Kumar, R.K.-Bowles, E.M.: B ur ning Velocities of Hydrogen-Air Mixtures, Combustion and Flame, (94), (1983) 330-340.
1141 Korycinski, F.P.-Snow, D.B.: Hydrogen for the Subsonic Transport, Hydrogen Energy Conference, Miami 1974.
Authors’ Address: Prof. Mag. Niko Samec, Dipl. Ing. Prof. Dr. Želimir Dobovišek, Dipl. Ing. Prof. Dr. Leopold Škerget, Dipl. Ing. Prof. Dr. Anton Černej, Dipl. Ing. Faculty of Mechanical Engineering Smetanova 17, Maribor, Slovenia