A rare cause of upper GI bleed
1
*Waseem Raja Dar,
2Najeeb Ullah Sofi,
3Imtiyaz Ahmad Dar,
4Basharat Ahmad Kasana,
5
Moomin Hussain and
6Muzamil Latief
1*,2,3,4,5
Department of Medicine, Gastroenterology Division. Shere Kashmir Institute of Medical Sciences, Soura.Srinagar, J& K, India
Upper GI bleed is a common, scary and life threatening medical condition usually caused by peptic ulcer disease or oesophageal varices. Uncommon causes include neoplasms, aortoenteric fistulas, vascular lesions, Dieulafoy's lesion etc. Patients usually present with hematemesis or melena. GIST is the third most common tumor of stomach and also the most common mesenchymal tumor. GIST may be asymptomatic and discovered incidentally or they may cause nonspecific symptoms like early satiety and fullness. Although major presentation of GIST is upper GI bleed, GIST as a cause of upper GI bleed is very rare. We here present a patient admitted to us with massive upper GI bleed due to gastrointestinal stromal tumor.
Keywords: GIST, upper GI bleed, carney complex, imatinib, regorafenib.
INTRODUCTION
Upper gastrointestinal bleeding (UGIB) refers to blood loss of recent onset originating from a site proximal to the ligament of Treitz. It is a common clinical problem seen in the practice of Gastroenterology and Internal Medicine. Patients may present either with hematemesisor melena. Common causes include ulcers, varices, Mallory Weiss tears, erosions and erosive esophagitis. Rare causes include neoplasms, aortoenteric fistulas, vascular lesions, Dieulafoy's lesion, prolapse gastropathy and hemobilia or hemosuccus pancreaticus. We here present a patient admitted with upper GI bleed due to a very rare cause i.e. Gastrointestinal Stromal Tumor (GIST). A brief review of literature follows.
CASE REPORT
A 55 year old male was admitted to our Gastroenterology Department with complaints of passage of multiple black tarry foul smelling stools of two days duration. There was no history of hematemesis, abdominal pain, jaundice or bleeding from any other orifice. Patient was non-alcoholic and did not report intake of any NSAIDS. There was no previous history of hypertension, diabetes mellitus or travel outside the state. On clinical examination, patient was conscious and oriented. There was tachycardia
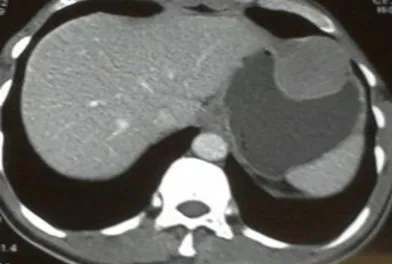
and postural drop in blood pressure. No signs of chronic liver disease were present. Respiratory, Cardiovascular and Abdominal examinations were normal. An initial diagnosis of Peptic Ulcer Disease was made and patient resuscitated with intravenous fluids, blood transfusions and pantoprazole infusions. Baseline investigations revealed microcytic anemia (Table 1). Meanwhile patient was taken to endoscopy room for urgent procedure. Endoscopy revealed a 4-5 cm sub mucosal bulge with central depression in stomach (Fig 1) with a differential diagnosis of leiomyoma or GIST. CECT abdomen showed an exoluminal homogenous enhancing mass (5 x 4.5cm) in fundus with indentation of fundic wall (Fig 2).
Patient however continued to lose blood and hence it was decided to operate patient for diagnostic and therapeutic reasons. Intraoperative findings included a 6×6 cm mass in anterior wall of stomach at junction of body and fundus and small enlarged lymph node at the root of left gastric vessels (Fig 3, 4).
*Corresponding Author: Waseem Raja Dar, Department of Medicine, Gastroenterology Division. Shere Kashmir Institute of Medical Sciences, Soura. Srinagar, J&K, India. Email: drwaseem.mw@gmail.com
Vol. 2(1), pp. 003-006. May, 2015. © www.premierpublishers.org,ISSN: XXXX-XXXXx
Dar et al. 003
Parameter Result Parameter Result ECG:- Sinus Tachycardia
X-rayChest: - Normal.
Coagulogram : Normal
USG abdomen: normal
Urine exam: normal
HIV /HBV /HCV/HAV serology: negative
Hb 5.7 g% Urea 28 mg/dl
TLC 8200/mm3 Creatinine 0.5 mg/dl
DLC Neutros=83%
Lymphos=12%
Bilirubin 0.4 mg/dl
PLT 117 lacs/mm3 ALT 15U/L
MCV 71 fL ALP 77 U/L
MCH 23 pg Total Protein 6.76 g/dl
Albumin 3.8 g/dl
Table 1.Baseline Investigations.
Figure 1. UGI endoscopy: 4 -5 cm sub mucosal bulgewith central depression in stomach.
Figure 2. CECT Abdomen: Homogenous, enhancing mass (5 x 4.5cm) in fundus.
No omental and liver metastasis were seen. Wide local excision of the tumour was carried out. Microscopic examination of the tumor was suggestive of GIST (Fig 5) while as lymph node showed reactive hyperplasia only. Tumour cells were positive for C kit, CD34 and DOG1. SMA was focally positive. Our complete diagnosis was Gastro Intestinal Stromal Tumor Stage III A (TNM Staging) presenting as massive upper GI bleed.
Patient was referred to medical oncology department where he was put on Imatinib 400mg/day. Presently
patient is on their follow up and has completed three months of treatment.
DISCUSSION
Figure 3. Intraop: 6×6 cm mass in anterior wall Figure 4. Operative Specimen of stomach
Figure 5. High Power Microscopy: Spindle cell tumour with areas of hyalinization.
characteristics of smooth muscle cells (Mazur et al 1983). Although stomach is the most common site, they may occur anywhere along the GI tract. They usually occur in middle age but may occur rarely in children as a part of Carney's triad (gastric stromal tumor, extra adrenal paraganglioma and pulmonary chordoma).
Most common mutation in GISTs is gain-of-function mutations in the KIT (c-kit) proto-oncogene, which almost occurs in all GISTs (Hirota et al 1998). These mutations lead to constitutive over expression and autophosphorylation of c-Kit, provoking a cascade of intracellular signalling that propels cells toward proliferation or away from apoptotic pathways. A small subset of patients (5%) may be KIT-negative (Corless et al 2005).In such cases, mutations of platelet-derived
growth factor receptor-alpha (PDGFA), protein kinase C, and DOG1have been detected.
Dar et al. 005
of patients present with metastatic disease (Tran et al 2005).
GISTs arise from pleuripotential mesenchymal stem cell programmed to differentiate into the interstitial cell of Cajal (Pacemaker cells of GIT).Microscopically, GIST cell morphology is usually spindle-shaped (70%), but some GISTs consist of rounded cells (epithelioid type, 20%) or a mixture, but they can also be pleomorphic.
GISTs have a fragile pseudo capsule, which may rupture during surgery which could increase the risk of peritoneal dissemination (Fletcher et al 2002).
Treatment of GISTs is medical as well as surgical. Surgery remains the treatment of choice for low risk patients’ i.e. non-metastatic local disease (Wu et al 2003). Low risk patients include those with size of tumor < 3 cm, mitotic index < 5/50 hpf and location of tumour in stomach. Lymph node metastasis is rare and routine removal of lymph nodes is typically not necessary. GIST lesions exhibit a fragile pseudo capsule, so the intraop-procedure must be optimized to minimize the risk of tumour rupture which could increase the risk of peritoneal dissemination. Laparoscopic resection has recently come as an important modality of surgical treatment (Chen et al 2012).Medical treatment is reserved for patients with advanced metastatic disease or as an adjuvant treatment in intermediate cases (Eisenbergh et al 2012). Imatinib (a tyrosine kinase inhibitor, TKI) is the drug of choice in such cases particularly in patients with mutation in Exon 11 of KIT gene (most common molecular sub type) and is started at a dose of 400mg /day, higher dose of 800 mg daily reserved for patients with KIT Exon 9 mutation. Around 15% patients may develop resistance to Imatininb manifesting as rapid progression of disease, despite Imatinib dosing that may be primary or secondary to emergence of new secondary mutation within a separate portion of KIT kinase coding sequence. Such cases may respond to other TKIs like Sunitinib (Demetri et al 2006, George et al 2009).In January 2006, the FDA approved Sunitinib as a second-line agent for patients with advanced GIST. Regorafenib received FDA approval for locally advanced, unresectable GISTs that no longer respond to Imatinib or Sunitinib. The pivotal phase III GRID trial of 199 patients with metastatic or unresectable GIST showed that Regorafenib plus best supportive care (BSC) significantly improved progression-free survival (PFS) compared to placebo plus BSC(Demetri et al 2013, Chustecka et al 2015). Conventional cytotoxic therapy is usually not used in management of GISTs.
CONCLUSION
GISTs are an uncommon form of gastric neoplasia and a very rare cause of upper GI bleed. Patients usually rebleed and need emergency care commonly in the form of surgery. Malignant potential is uncertain. Surgery is the treatment of choice for local disease. Advanced disease may be treated conservatively only.
REFERENCES
Mazur MT, Clark HB (1983). Gastric stromal tumors: Reappraisal of histogenesis. Am J Surg Pathol; 7: 507–519.
Hirota S, Isozaki K, Moriyama Y (1998). Gain-of-function mutations of c-kit inhuman gastrointestinal stromal tumors. Science; 279: 577–580.
Corless CL, Schroeder A, Griffith D (2005). PDGFRA Mutations in Gastrointestinal Stromal Tumors: Frequency, Spectrum and In Vitro Sensitivity to Imatinib. J ClinOncol; 23: 5357–5364.
Miettinen M, Sobin LH, Lasota J (2005). Gastrointestinal stromal tumors of the stomach: aclinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol; 29: 52–68.
Seya T, Tanaka N, Yokoi K, Shinji S, Oaki Y, Tajiri T (2008). Life-threatening Bleeding from Gastrointestinal Stromal Tumor of the Stomach.Journal of Nippon Medical School. 75(5): 306-311.
Singhal T, Doddi S, Leake T, Parsi S, Hussain A, Chandra A, Smedley F, Ellul J (2010). Upper gastrointestinal bleeding due to gastric stromal tumour: a case report. Cases Journal, 3:58 doi:10.1186/1757-1626-3-58.
Tran T, Davila JA, El-Serag HB (2005). The epidemiology of malignant gastrointestinalstromal tumors: an analysis of 1458 cases from 1992 to 2000. Am JGastroenterol; 100: 162–168.
Fletcher CD, Berman JJ, Corless C (2005). Diagnosis of gastrointestinal stromaltumors: A consensus approach. Hum Pathol; 33: 459–465.
Wu PC, Langerman A, Ryan CW, Hart J, Swiger S, Posner MC (2003). Surgical treatment of gastrointestinal stromal tumors in the imatinib (STI-571) era. Surgery. Oct; 134(4): 656-65; discussion 665-6.
Chen YH, Liu KH, Yeh CN (2012). Laparoscopic resection of gastrointestinal stromal tumors: safe, efficient, and comparable oncologic outcomes. J Laparoendosc AdvSurg Tech A. 22(8):758-63. Eisenberg BL, Judson I (2004). Surgery and Imatinib in
management of GIST.Emerging approach to adjuvant and neoadjuvanct therapy. Ann of Surg Onco., 11:465–475.
Demetri GD, van Oosterom AT, Garrett CR (2006). Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet., 368(9544):1329-38.
George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE (2009). Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 45(11):1959-68.
tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet., 381(9863):295-302.
Chustecka Z. Regorafenib Approved for
Gastrointestinal Stromal Tumors. Available athttp://www.medscape.com/viewarticle/779854.
Accepted 28 April, 2015.
Citation: Dar WJ, Sofi N, Dar IA, Kasana BA, Hussain M, Latief M (2015). A rare cause of upper GI bleed. World Journal of Internal Medicine, 2(1): 003-006.